Abstract
Despite a lack of signaling motifs in their cytoplasmic domain, major histocompatibility complex (MHC) class II molecules trigger a variety of intracellular signals that regulate antigen-presenting cell function. They thus may use associated effector molecules as demonstrated on B cells and dendritic cells. The starting point of this study comes from our previous work, which demonstrated that the ecto-enzyme CD38 is functionally linked to MHC class II molecules. We report that CD38 and human leukocyte antigen-DR (HLA-DR) are functionally and physically associated in lipid rafts microdomains of cellsurface monocytes and that the integrity of these domains is necessary for the HLA-DR and CD38 signaling events. Moreover, we identified the tetraspanin CD9 molecule as a partner of the CD38/HLA-DR complex and demonstrated that HLA-DR, CD38, and CD9 share a common pathway of tyrosine kinase activation in human monocytes. The analysis of conjugate formation between monocytes presenting superantigen and T cells shows the active participation of CD9 and HLA-DR on the monocyte surface. Together, these observations demonstrate the presence of a CD38 and HLA-DR signaling complex within tetraspanin-containing lipid rafts and the functional impact of their molecular partner CD9 in antigen presentation.
Introduction
Reorganization of proteins at the interface between the T cell and the antigen-presenting cells (APCs) leads to the formation of the immunologic synapse.1 Once a specific T-cell receptor has recognized the major histocompatibility complex (MHC)–peptide complex, MHC class II–mediated signals influence antigen-presenting function, adhesion, apoptosis, and cytokine production of the APC.2 While MHC class II molecules do not harbor any known signaling motifs in their cytoplasmic domains, they can act as signal transducers, leading to activation of protein tyrosine kinases, phospholipase Cγ, protein kinase C, and mitogen-activated protein kinases pathways.3-9 Thus, MHC class II–mediated signals are likely to be at least partially dependent on interactions with other APC surface molecules. They also can be influenced by their compartmentalization into specialized membrane microdomains10 such as glycosphingolipid-enriched microdomains, also called lipid rafts,11 which are considered as platforms where signaling events are generated. MHC class II molecules are constitutively present in lipid rafts from murine and human B-cell lines,12 monocytic cell lines,13 human monocytes,14 and tumor cells,15 and the integrity of these microdomains is necessary for the transmission of MHC class II–mediated signals.16
We reported that engagement of CD38 or MHC class II molecules on monocytes induced phosphorylation of common cytosolic substrates with marked additive effects between the 2 activation pathways.17 CD38 plays an important role in activation and adhesion processes of monocytes.18 CD38 ligation regulates the response to respiratory-burst activators19 and enhances MHC class II and B7 expression in myeloid cell lines and leads to up-regulation of CD83 expression and interleukin 12 (IL-12) secretion in mature dendritic cells.20 Despite the fact that the cytoplasmic domain of CD38 is unable to transduce signals, numerous studies have demonstrated that CD38 engagement leads to signaling events.21-23 These data have suggested lateral associations between CD38 and other surface receptors, including MHC class II molecules on monocytes.
Since both MHC class II and CD38 are devoid of signaling motifs, we hypothesized the need of associated molecules and/or the localization in specialized surface microdomains.
Our results demonstrate that MHC class II and CD38 molecules are physically and functionally associated in lipid rafts whose integrity is necessary for their signaling. Moreover, the tetraspanin CD9 is found associated with MHC class II and CD38 both inside and outside lipid rafts and has a functional impact in the interaction between superantigen-loaded monocytes and responding T cells.
Materials and methods
Antibodies
CD38 monoclonal antibodies (mAbs) were IB424 (a gift from Pr F. Malavasi, Laboratory Immunogenetics, Dept. of Genetics, Biology, and Biochemistry, Torino, Italy) and Leu 17 (BD Bioscience, Erembodegem, Belgium). The CD9 mAbs used were Syb.1,25 ABL-6 (gifts from Dr E. Rubinstein, INSERM U602, Hôpital Paul Brousse, Villejuif, France), and PHN 200 (provided during the Fifth International Workshop).26 The anti–MHC class II (D1.12, DA6.147) and class I (W6.32) mAbs were locally produced. The CD11A mAb was HI-111 (BD Pharmingen). Leu 17, DA6.147, and Syb.1 mAbs were biotinylated with E2-link-sulfo-NHS-LC-biotin (Pierce, Rockford, IL). Fluorescein isothiocyanate (FITC)–conjugated CD9 and CD38 mAbs were from Dako (Glostrup, Denmark). The CD41 and FITC-conjugated MHC class II mAbs were from Diaclone (Besançon, France). Texas red–conjugated goat anti–mouse IgG was from Immunotech/Beckman (Marseille, France). mAbs used for negative selection of monocytes (CD3, CD19, CD20, and CD16) were from Chemicon (Hampshire, United Kingdom). The affinity-purified rabbit polyclonal Abs anti-Fgr and anti-Hck were from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell preparations
Peripheral blood mononuclear cells (PBMCs) were prepared by FicollPaque (Pharmacia, Uppsala, Sweden) density centrifugation of buffy coats obtained from the local blood bank and depleted of platelets. T cells were purified by negative selection with 2 cycles of plastic adherence followed by complement-dependent cell lysis with a cytotoxic anti–MHC class II mAb (L1.L12, locally produced).
Monocytes were purified by negative selection using a mixture of mAbs specific for T, B, and natural killer (NK) cells.17 This approach enabled obtainment of monocytes 98% pure, as assessed by CD14 expression. When indicated, purified monocytes were pretreated with diluted relevant mAbs for 1 hour at 4°C. They were then fixed by treatment with 1% paraformaldehyde for 20 minutes at room temperature, washed, and kept at least 1 week at 4°C before use in proliferation assays.
Proliferation assays
Cells were cultured in RPMI 1640 medium (Biochrom KG, Berlin, Germany) supplemented with 2 mM l-glutamine, 10 U/mL penicillin, 10 μg/mL streptomycin (Gibco, Paisley, United Kingdom), and 10% heatinactivated fetal calf serum (FCS) (Seromed, Biochrom KG). As previously described,27 purified T cells were cultured for 4 days in 96-well round bottom plates (Nunc, Roskilde, Denmark) with or without fixed autologous monocytes. Recombinant Staphylococcus enterotoxin A (SEA) (provided by Dr W. Mourad, CRRI, CHU Laval, Quebec City, Canada) was added to the cultures at 1 ng/mL. Proliferation was measured as DNA synthesis by adding 1 μCi[0.037 MBq]/well of [3H] thymidine (Amersham, Little Chalfont, United Kingdom) for the last 8 hours of culture, after which plates were harvested with a TOMTEC harvester (Tomtec, Hamden, CA), and the radioactivity was measured in a scintillation counter (1450 Microbeta Plus; Wallac, Turku, Finland). Each condition was performed in triplicate.
Immunoprecipitation and Western blotting
When indicated, purified monocytes were first biotin-labeled by incubation for 1 hour on ice with 5 mM sulfo-NHS-LC-biotin (Pierce), and unbound biotin was removed by washing with cold phosphate-buffered saline (PBS) containing 1 mM glycine. Detergent lysates of unlabeled and biotin-labeled monocytes were made by incubating cells for 30 minutes on ice in a buffer containing either 1% NP-40 or 1% CHAPS (3-[(3-cholamidopropyl)dimethylamonio]-1-propyl sulfonate) with 50 mM Tris (tris(hydroxymethyl)aminomethane), 150 mM NaCl, 5 mM EDTA (ethylenediaminetetraacetic acid), 20 μg/mL aprotinin, 10 μg/mL leupeptin, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 mM sodium pyrophosphate, and 10 mM Na3VO4. Remaining particulate material was removed by centrifugation at 15 000g for 30 minutes at 4°C.
Lysates were precleared with CD41 mAb and protein A-sepharose (Pharmacia) and subsequently incubated with specific mAbs overnight at 4°C. Immune complexes were recovered with protein A-sepharose beads. Immunoprecipitated proteins were eluted from beads by boiling in sodium dodecyl sulfate (SDS) nonreducing sample buffer, subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% polyacrylamide gel, and transferred to a nitrocellulose membrane. After blocking in PBS containing 5% milk and 0.2% Tween-20, filters were probed with either biotinylated mAbs followed by streptavidin-conjugated horseradish peroxidase (HRP-Sav) (Amersham, Little Chalfont, United Kingdom) or, when immunoprecipitations were carried out on lysates from biotinylated cells, by HRP-Sav only. Enzymatic reaction was detected with enhanced chemiluminescent reagents (ECL-kit) (Amersham).
Biochemical isolation of rafts and immunoprecipitations
Rafts were isolated by sucrose gradient equilibrium centrifugation after nonionic detergent lysis as previously described.28 Unlabeled monocytes were lysed with 0.5% TritonX-100 in 400 μL ice-cold MBS (MES-buffered solution; 5 mM MES,150 mM NaCl) (pH 6.5 1 mM Na3VO4, 1 mM PMSF, 10 mM NaF, 1 μg/mL leupeptin, 1 μg/mL pepstatin A, 4 μg/mL aprotinin) for 30 minutes on ice. Lysates were gently mixed with an equal volume of 85% sucrose (wt/vol) in MBS in polycarbonate ultracentrifuge tubes (Beckman Instruments, Palo Alto, CA) and overlaid with 2 mL 35% sucrose and 1 mL 5% sucrose in MBS. After centrifugation at 200 000g for 18 hours at 4°C in an SW55Ti rotor (Beckman Instruments), 9 fractions of 400 μL each were collected from the top to the bottom of the tube. The visible band at the 5/35% sucrose interface containing the lipid rafts corresponded to fractions 2 and 3. The presence of flotillin-1, a marker of lipid rafts, in these fractions was verified by Western blot with an anti–flotillin-1 mAb (BD Transduction Laboratory, Lexington, KY). Lipid rafts (fractions 2-3) and soluble proteins (fractions 7-9) were then collected, lysed for 30 minutes on ice with buffer containing 1% CHAPS, and immunoprecipitated as described under “Immunoprecipitation and Western blotting.”
Capping and microscopy immunofluorescence
Purified monocytes were incubated with specific mAbs (10 μg/mL) for 20 minutes on ice, washed twice in cold PBS, and subsequently incubated with Texas red–conjugated goat anti–mouse Ig for 45 minutes at 22°C to allow complete capping. Cells were then washed twice and stained with the indicated FITC-conjugated mAbs for 30 minutes on ice. After 2 washes, cells were fixed in PBS-1% paraformaldehyde for 30 minutes at 4°C. Fixed cells were kept in PBS supplemented with DABCO (1,4-diazabicyclo-2-2-2-octane) (Sigma, Saint Louis, MO) to prevent photobleaching and DAPI (4′6-diamidino-2-phenylindole 2HCl) (Sigma) to visualize nuclei. When indicated, cells were pretreated with 10 mM methyl-β-cyclodextrin (MβCD) for 30 minutes at 37°C. Cells were analyzed with a Bio-Rad MRC 1024 confocal imaging system (Bio-Rad, Hemel Hempstead, United Kingdom) equipped with an inverted Diaphot 300 Nikon microscope (Nikon France, Champigny-Sur-Marne, France) and a krypton-argon ion laser. Confocal images were acquired using the Bio-Rad Lasersharp V3.2 software and a 60×/1.4 NA oil-immersion objective lens (Nikon).
Determination of protein tyrosine phosphorylation
Purified monocytes were incubated with the indicated mAbs (10 μg/mL) for 5 minutes at 37°C and immediately lysed in 1% NP-40 lysis buffer. After centrifugation to remove nuclei, an aliquot of lysates was diluted in Laemmli sample buffer, boiled for 5 minutes, and stored at –80°C prior to SDS-PAGE. The remainder of lysates was used for either Fgr or Hck immunoprecipitations: lysates were precleared with protein A-sepharose and subsequently incubated either with anti-Fgr or with anti-Hck Abs as described under “Immunoprecipitation and Western blotting.” Immunoprecipitated material was run on a 10% SDS-PAGE and transferred to nitrocellulose membranes. Filters were probed with horseradish peroxidase–labeled 4G10, an antiphosphotyrosine mAb (UBI, Lake Placid, NY), and developed with ECL reagents (Amersham). Membranes were then stripped, blocked again with 2% to 5% nonfat dry milk in Tris-buffered saline with Tween (TBS-T), and reprobed with anti-Hck or anti-Fgr Abs.
Analysis of intracellular calcium flux
The analysis was performed as previously reported.29 Briefly, unstimulated monocytes were loaded with indo-1 by incubation with its acetomethylester (Molecular Probes, Eugene, OR). Cells were analyzed on a cytofluorograph (Epics Elite ESP Flow Cytometer, Coultronics, France), and the ratio of indo-1 violet to blue fluorescence was calculated for each individual cell. F(ab')2 preparations of the indicated mAb (5 μg/mL) were added to monocyte suspensions. When indicated, cells were pretreated with 10 mM MβCD for 30 minutes at 37°C, before loading with indo-1.
Quantification of monocytes–T-cell conjugate by flow cytometry
The Jurkat-derived human T-cell line Vβ8 +30 was incubated with the CellTrackerorange5-(and-6)-{[(4-chloromethyl)benzoyl]amino}tetramethylrhodamine (CMTMR) probe at a final concentration of 5 μM for 30 minutes at 37°C in serum-free medium, incubated for 30 minutes in fresh medium, and washed twice in serum-free medium. Purified monocytes were labeled following the same procedure with the CellTracker green CMFDA (5-chloromethylfluorescein diacetate) probe and then incubated or not for 20 minutes at 37°C with the indicated concentration of Staplylococcus enterotoxin E (SEE). Monocytes and Jurkat cells (monocytes to T-cell ratio, 1:4) were then resuspended in serum-free medium and allowed to form conjugates. When indicated, CMFDA-labeled monocytes loaded or not with SEE were incubated with specific mAbs for 20 minutes on ice before conjugate formation with CMTMR-labeled Jurkat cells. Conjugate formation was measured on an LSR cytometer using the FL-1 gate for CMFDA probe and the FL-2 gate for CMTMR probe and was characterized by a doubly stained population.
Results
CD9 mAbs inhibit T-cell proliferation induced by superantigens
We have previously shown that T-cell proliferation induced by the presentation of a superantigen (Sag) SEA by the HLA-DR molecules expressed on monocytes was inhibited by CD38 mAbs.17 We have studied other monocyte surface molecules and observed that ligation of CD9 on fixed monocytes led to strong inhibition (78%) of T-cell proliferation (Figure 1). As expected, blocking CD38 or HLA-DR also led to a clear inhibition of this T-cell response. The inhibition of proliferation observed in the presence of the CD9 mAb was not due to a toxic effect, as demonstrated by the absence of effect of CD9 mAb on phytohemagglutinin (PHA)–induced activation of PBMCs (not shown). These results suggest that CD9 may participate with MHC class II and CD38 to the Sag-induced T-cell response.
HLA-DR and CD38 molecules are associated with CD9 on monocytes
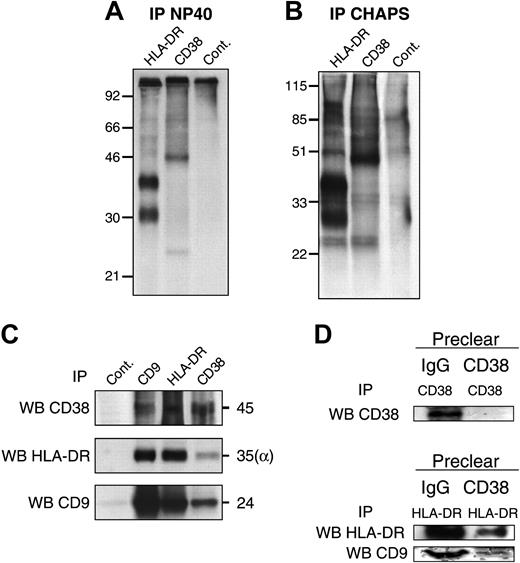
The starting point of this study was that MHC class II and CD38 molecules could be associated at the surface of human monocytes. We first analyzed proteins coprecipitated with HLA-DR or CD38 from the monocyte surface. Unlabeled or surface-biotin–labeled monocytes were solubilized in buffer containing either NP-40 or CHAPS, and the associations between different molecules were analyzed by immunoprecipitation. As controls, proteins immunoprecipitated from NP-40 monocyte lysates were analyzed. The characteristic bands of CD38 and HLA-DR (α and β chains) were precipitated by specific mAbs at 45 kDa and 28 kDa to 35 kDa, respectively (Figure 2A). After solubilization in CHAPS, a 24-kDa protein was coprecipitated with HLA-DR and CD38 (Figure 2B). Additional bands also were observed in immunoprecipitations with the isotype control mAb and are nonspecific for HLA-DR or CD38 immunoprecipitations. Western blotting of HLA-DR and CD38 precipitates with mAbs specific for the HLA-DRα chain and CD38 revealed the presence of CD38 in HLA-DR precipitates and reciprocally (Figure 2C).
CD9 mAb inhibits Sag-induced T-cell proliferation. Purified T cells (4 × 104cells/well) were cultured with SEA in the presence of fixed monocytes (8 × 103 cells/well) with mAbs (soluble or precoated to monocytes) of the indicated specificity. [3H]thymidine incorporation measured at day 4, for T cells in the presence of uncoated fixed monocytes, was 40.500 ± 2.098 cpm. Results are representative of at least 3 independent experiments. Error bars indicate standard deviation.
CD9 mAb inhibits Sag-induced T-cell proliferation. Purified T cells (4 × 104cells/well) were cultured with SEA in the presence of fixed monocytes (8 × 103 cells/well) with mAbs (soluble or precoated to monocytes) of the indicated specificity. [3H]thymidine incorporation measured at day 4, for T cells in the presence of uncoated fixed monocytes, was 40.500 ± 2.098 cpm. Results are representative of at least 3 independent experiments. Error bars indicate standard deviation.
CD9 is associated with MHC class II and CD38 on human monocytes. Monocytes were surface-labeled with biotin before lysis in NP-40 (A) or CHAPS (B-D). Immunoprecipitations were then performed with the HLA-DR (D1.12), CD38 (IB4), or isotype control mAbs. After electrophoresis under nonreducing conditions and transfer to a nitrocellulose membrane, the precipitated material was revealed by chemiluminescence. (C) The HLA-DR, CD38, and CD9 molecules were immunoprecipitated, from unlabeled monocytes, with the specific D1.12, IB4, and PHN 200 mAbs, respectively. Immunoprecipitates were analyzed by Western blot with biotinlabeled CD38 (Leu 17), CD9 (Syb.1), or anti–HLA-DR (DA6.147) mAbs. (D) Monocyte lysates were precleared twice in a period of 24 hours with CD38 (IB4) or isotype control mAb together with protein G-sepharose. HLA-DR and CD38 were then immunoprecipitated and Western blotted with biotin-labeled CD38 (Leu 17), CD9 (Syb.1), or anti–HLA-DR (DA6.147) mAbs. Supernatants of CD38 and HLA-DR precipitates obtained after Ig or CD38 preclearing were blotted with an antiactin mAb and show that equal quantities of proteins were used for immunoprecipitations (not shown). WB indicates Western blot; IP, immunoprecipitation.
CD9 is associated with MHC class II and CD38 on human monocytes. Monocytes were surface-labeled with biotin before lysis in NP-40 (A) or CHAPS (B-D). Immunoprecipitations were then performed with the HLA-DR (D1.12), CD38 (IB4), or isotype control mAbs. After electrophoresis under nonreducing conditions and transfer to a nitrocellulose membrane, the precipitated material was revealed by chemiluminescence. (C) The HLA-DR, CD38, and CD9 molecules were immunoprecipitated, from unlabeled monocytes, with the specific D1.12, IB4, and PHN 200 mAbs, respectively. Immunoprecipitates were analyzed by Western blot with biotinlabeled CD38 (Leu 17), CD9 (Syb.1), or anti–HLA-DR (DA6.147) mAbs. (D) Monocyte lysates were precleared twice in a period of 24 hours with CD38 (IB4) or isotype control mAb together with protein G-sepharose. HLA-DR and CD38 were then immunoprecipitated and Western blotted with biotin-labeled CD38 (Leu 17), CD9 (Syb.1), or anti–HLA-DR (DA6.147) mAbs. Supernatants of CD38 and HLA-DR precipitates obtained after Ig or CD38 preclearing were blotted with an antiactin mAb and show that equal quantities of proteins were used for immunoprecipitations (not shown). WB indicates Western blot; IP, immunoprecipitation.
Results from proliferation assays described in Figure 1 led us to hypothesize that the p24 protein could correspond to the tetraspanin CD9. In fact, specific tetraspanin interactions with a wide range of surface receptors might enable the generation of functional multimolecular signaling complexes, and CD9 has been described to network with MHC class II molecules. Western blotting of HLA-DR and CD38 precipitates, with the CD9 mAb Syb.1, identified the p24 protein as the tetraspanin CD9 (Figure 1C). While the 24-kDa band was weakly present in CD38 and HLA-DR immunoprecipitates from cell surface, it was clearly detected by CD9 immunoblotting in either HLA-DR or CD38 immunoprecipitates.
To confirm the association of HLA-DR and CD38 with CD9, reciprocal experiments were performed: immunoprecipitates of CD9 molecules were analyzed by Western blotting with HLA-DR and CD38 mAbs. The CD38 and HLA-DR molecules were readily detected in CD9 immunoprecipitates. Together, these results reveal that CD38 and HLA-DR are associated and also are associated with the tetraspanin CD9.
To investigate whether part of these 3 molecular pools could be present in the same molecular complex, we have performed preclearing experiments and analyzed by Western blot the remaining molecules. CHAPS lysates of purified monocytes were precleared with a CD38 mAb and subjected to immunoprecipitation with CD38 or HLA-DR mAbs. Western blotting with the specific CD38 mAb Leu17 did not detect any CD38 molecules on CD38 immunoprecipitates after CD38 preclearing, thus demonstrating that CD38 preclearing was efficient (Figure 2D). Immunoprecipitates of HLA-DR molecules after CD38 preclearing were immunoblotted with HLA-DR mAbs and revealed a clear decrease as compared with the amount detected after preclearing with an isotype control mAb. Moreover, the amount of CD9 molecules, detected by a Western blot with a CD9 mAb in those immunoprecipitates, also was significantly reduced.
This result demonstrates that a significant part of CD9 molecules associated with HLA-DR have been coprecipitated with CD38 during preclearing and shows that a significant part of CD38, HLA-DR, and CD9 molecules are associated in a same molecular complex. This experiment also shows that some HLA-DR molecules not associated with CD38 are precipitated in association with CD9.
Co-capping of HLA-DR and CD38 with CD9
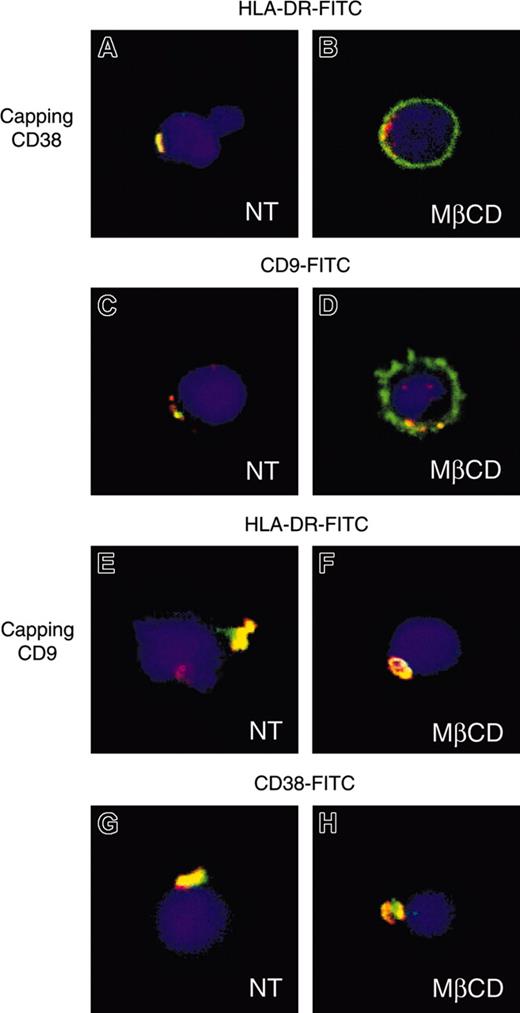
To confirm biochemical data indicating the HLA-DR/CD38/CD9 association, we directly examined the outcome of capping of one of these molecules by cross-linking and analyzed the redistribution of the others at the surface of living cells. The analysis of confocal fluorescence images showed that the cross-linking of CD38 led to its redistribution in a cap at the cell surface. A clear colocalization of HLA-DR and CD9 molecules with these CD38-induced patches was observed (Figure 3A, C). Similarly, the HLA-DR and CD38 stainings overlapped with CD9-induced caps (Figure 3E, G). No patching was observed with isotype-control antibodies.
These results indicate that CD38, CD9, and HLA-DR colocalized at the cell surface of monocytes and confirm the biochemical data.
The HLA-DR/CD38/CD9 complex is present within and outside lipid rafts
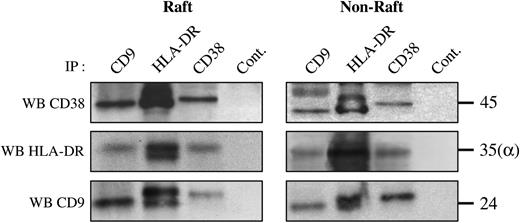
CD9,31,32 MHC class II,12,13 and CD3833 molecules have been documented individually in lipid-raft domains. We therefore examined whether the CD9/HLA-DR/CD38 association was present in rafts isolated from human monocytes. Purified monocytes were first lysed in Triton X-100 and subjected to sucrose–densitygradient ultracentrifugation. Raft (fractions 2-4) and nonraft (fractions 7-9) fractions were collected and then lysed in CHAPS before immunoprecipitation and Western blotting. Results presented in Figure 3 show that HLA-DR, CD38, and CD9 were independently detected in lipid rafts of human monocytes. Western blots of pooled raft fractions (Figure 4A) show that HLA-DR was present in CD38 and CD9 immunoprecipitates, and conversely, CD38 was detected in CD9 and HLA-DR precipitates. Western blot using the Syb.1 CD9 mAb revealed the p24 isoform of CD9 in the CD9 immunoprecipitate, the p26 isoform in CD38 immunoprecipitates, and both CD9 isoforms in MHC class II immunoprecipitates. These results demonstrate the HLA-DR/CD38/CD9 association in lipid rafts, and this was confirmed by the observation of colocalized GM1/CD38, HLA-DR, or CD9 patches by microscopy (data not shown).
Co-capping of CD9 with MHC class II and CD38. Purified monocytes untreated (A, C, E, G) or pretreated with 10 mM MβCD (B, D, F, H) were incubated with CD38 (A-D) or CD9 (E-H) mAbs (10 μg/mL) for 20 minutes on ice and subsequently with Texas red–conjugated goat anti–mouse Ig for 45 minutes at 22°C to allow complete capping. Cells were then stained with the indicated FITC-conjugated mAbs and analyzed. Confocal optic sections for the merged images of representative cells are shown.
Co-capping of CD9 with MHC class II and CD38. Purified monocytes untreated (A, C, E, G) or pretreated with 10 mM MβCD (B, D, F, H) were incubated with CD38 (A-D) or CD9 (E-H) mAbs (10 μg/mL) for 20 minutes on ice and subsequently with Texas red–conjugated goat anti–mouse Ig for 45 minutes at 22°C to allow complete capping. Cells were then stained with the indicated FITC-conjugated mAbs and analyzed. Confocal optic sections for the merged images of representative cells are shown.
It is noteworthy that the same pattern of associations could be observed on the nonraft pooled fractions: similar complexes were precipitated with mAbs to HLA-DR, CD38, or CD9 (Figure 4B). This result demonstrates that the HLA-DR/CD38/CD9 association exists both within and outside lipid rafts.
Partial depletion of cholesterol, induced by methyl-β-cyclodextrin (MβCD), disrupts cholesterol-dependent lipid rafts.34 Cocapping experiments were performed on monocytes pretreated with MβCD (Figure 3). Redistribution of HLA-DR and CD38 with CD9 molecules was still observed after cholesterol depletion, and a clear colocalization of the 3 molecules was observed (Figure 3F, H). In contrast, when CD38 was induced to cap, although a colocalization with CD9 and HLA-DR was observed, a significant staining of these molecules was observed all around the cell (Figure 3B, D). Antibody-induced redistribution of HLA-DR molecules led to the same observation.
The CD38-CD9-MHC class II complex is present in membrane rafts. Purified monocytes were lysed in 0.5% Triton X-100 and subjected to sucrose density gradient ultracentrifugation. Raft (fractions 2-4) and nonraft (fractions 7-9) fractions were collected and lysed in CHAPS before immunoprecipitation. After electrophoresis under nonreducing conditions and transfer to a nitrocellulose membrane, Western blots were performed with biotin-labeled CD38 (Leu 17), CD9 (Syb.1), or anti–HLA-DR (DA6.147) mAbs.
The CD38-CD9-MHC class II complex is present in membrane rafts. Purified monocytes were lysed in 0.5% Triton X-100 and subjected to sucrose density gradient ultracentrifugation. Raft (fractions 2-4) and nonraft (fractions 7-9) fractions were collected and lysed in CHAPS before immunoprecipitation. After electrophoresis under nonreducing conditions and transfer to a nitrocellulose membrane, Western blots were performed with biotin-labeled CD38 (Leu 17), CD9 (Syb.1), or anti–HLA-DR (DA6.147) mAbs.
These results show that a significant proportion of CD9 associates with CD38 and HLA-DR outside lipid rafts and is therefore indifferent to cholesterol depletion. This is consistent with biochemical data revealing that the HLA-DR/CD38/CD9 association is present both in raft and nonraft compartments of human monocytes.
The intracellular calcium flux mediated via CD38 or MHC class II molecules is dependent on raft integrity
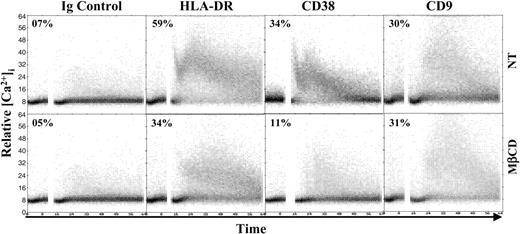
We next assessed whether localization in lipid rafts had a functional impact on signaling of each molecular partner. Monocytes were either untreated or treated with MβCD, loaded with indo-1, and the intracellular calcium flux induced by engagement with specific mAbs was analyzed (Figure 5). The analysis of untreated cells revealed that a rapid mobilization of calcium, involving 30% of the cells, could be induced following engagement of CD9. As previously described,17 HLA-DR or CD38 ligation by specific mAbs was followed by a rapid mobilization of Ca2+. Treatment with MβCD led to a marked inhibition (∼67% and 42%, respectively) of the Ca2+ flux induced by the ligation of CD38 or MHC class II molecules with specific mAbs. In contrast, the CD9-induced calcium mobilization was not altered by MβCD treatment, indicating that a substantial portion of CD9 molecules exists outside rafts and can signal independently of cholesterol-dependent lipid rafts. CD9-mediated Ca2+ signaling was unaltered in these conditions, and the inhibition of CD38/HLA-DR–mediated signaling is therefore not due to MβCD toxicity. Those results demonstrate that HLA-DR-/CD38–mediated signaling events are dependent on raft integrity, while the CD9-mediated signals may occur outside these microdomains.
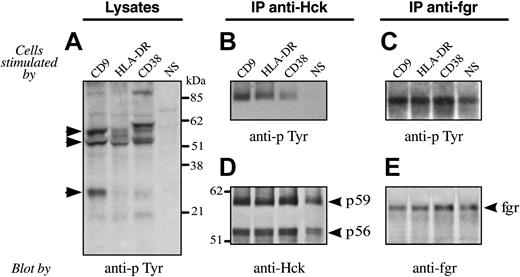
CD9 ligation induces tyrosine phosphorylation of Hck and Fgr tyrosine kinases
We have shown that CD38 and MHC class II share a common activation pathway involving the tyrosine kinases Fgr and Hck.17 We next examined the phosphorylation of cytoplasmic substrates elicited upon CD9 ligation on monocytes. A phosphotyrosine immunoblot of lysates from purified monocytes activated as indicated is shown in Figure 6A. Upon CD9 ligation, several substrates became phosphorylated, including proteins of 50 kDa to 59 kDa, similarly to CD38 and HLA-DR pathways. Additionally, a substrate at approximately 25 kDa seemed to be specific to the CD9 activation pathway. To investigate the role of Hck and Fgr in CD9-induced activation, lysates of activated monocytes were immunoprecipitated with specific anti-Hck or anti-Fgr Abs. Subsequent Western blotting with an anti-pTyr mAb (Figure 6B-C) revealed that both Fgr and the p59 isoform of Hck were tyrosine phosphorylated following CD9, CD38, or HLA-DR engagement. Reprobing immunoblots from Fgr and Hck immunoprecipitates with, respectively, an anti-Fgr or anti-Hck (Figure 6D-E) showed that Fgr and the p56 and p59 isoforms of Hck were equally immunoprecipitated from unstimulated or activated monocytes. Those results demonstrate that HLA-DR, CD38, and CD9 molecules shared a common pathway of tyrosine kinases activation in human monocytes.
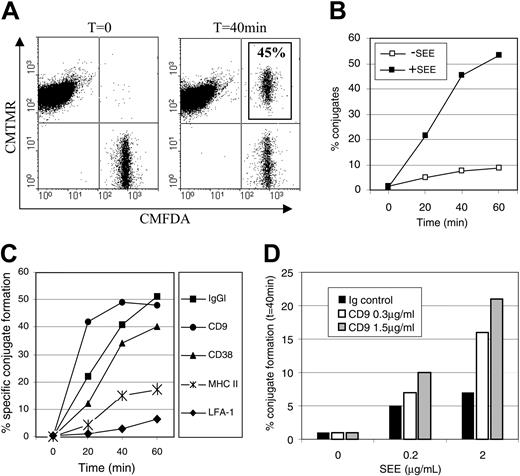
A role for CD9 in the formation of antigen-dependent monocyte/T-cell conjugates
Stimulation of a T cell by an antigen-presenting cell requires a cell-cell interaction that can be detected as conjugates. To assess the role of CD38 and CD9 in the formation of antigen-dependent conjugates between a monocyte and a T cell, we used T cells expressing Vβ8 and recognizing the SEE superantigen. Purified monocytes preloaded with SEE were incubated with Jurkat cells at 37°C over 60 minutes, and the formation of conjugates, detected as a CMFDA+CMTMR+-cell population, was monitored by flow cytometry (Figure 7A). Conjugate formation increased over the time course of the experiment, reaching a maximum after 1 hour, and was SEE dependent (Figure 7B). As expected, it could be inhibited by anti–MHC class II and anti–LFA-1 mAbs, but not by an anti–MHC class I mAb (Figure 7C, and data not shown). Ligation of CD38 at the surface of monocytes by mAbs had almost no effect on conjugate formation (Figure 7C). In contrast, a CD9 mAb was found to increase the kinetics of SEE-specific conjugate formation (Figure 7C). To further study the effect of this antibody, conjugate formation was analyzed with different concentrations of SEE or CD9 mAb (Figure 7D). An increase in the frequency of conjugate formation was observed in the presence of CD9 mAb that rose up to 3-fold with SEE at 2 μg/mL and CD9 mAb at 1.5 μg/mL. Identical data were obtained with the CD9 and ABL-6 mAbs (data not shown). Together, these data demonstrate that CD9 can influence presentation of a superantigen to T cells.
CD38 and HLA-DR–mediated intracellular calcium fluxes are dependent on raft integrity. Purified monocytes were untreated (NT) or treated with 10 mM MβCD (30 minutes, 37°C), loaded with indo-1, and tested for calcium mobilization. After measurement of basal level, the following F(ab')2 preparations of mAbs were added: CD38 mAb (IB4), HLA-DR mAb (D1.12), and CD9 mAb (PHN 200). Results are representative of at least 3 independent experiments.
CD38 and HLA-DR–mediated intracellular calcium fluxes are dependent on raft integrity. Purified monocytes were untreated (NT) or treated with 10 mM MβCD (30 minutes, 37°C), loaded with indo-1, and tested for calcium mobilization. After measurement of basal level, the following F(ab')2 preparations of mAbs were added: CD38 mAb (IB4), HLA-DR mAb (D1.12), and CD9 mAb (PHN 200). Results are representative of at least 3 independent experiments.
CD9 ligation induces tyrosine phosphorylation of the tyrosine kinases Fgr and Hck. Purified monocytes were either unstimulated or stimulated with the appropriate mAb. (A) Cells were lysed and subjected to SDS-PAGE before transferring to nitrocellulose membranes and immunoblotting with antiphosphotyrosine mAb. The same quantity of protein was loaded in each well. Molecular mass markers are indicated. (B-E) Cells were lysed and immunoprecipitates prepared using either an anti-Hck Ab (B, D) or an anti-Fgr Ab (C, E). After blotting, filters were probed with an antiphosphotyrosine mAb (B-C). The filter shown in B was stripped and reprobed with an anti-Hck Ab revealing identical amounts of Hck in all samples (D). The filter shown in C was stripped and reprobed with an anti-Fgr Ab revealing identical amounts of Fgr in all samples (E). Arrows indicate phosphorylated substrates; NS, no stimulation.
CD9 ligation induces tyrosine phosphorylation of the tyrosine kinases Fgr and Hck. Purified monocytes were either unstimulated or stimulated with the appropriate mAb. (A) Cells were lysed and subjected to SDS-PAGE before transferring to nitrocellulose membranes and immunoblotting with antiphosphotyrosine mAb. The same quantity of protein was loaded in each well. Molecular mass markers are indicated. (B-E) Cells were lysed and immunoprecipitates prepared using either an anti-Hck Ab (B, D) or an anti-Fgr Ab (C, E). After blotting, filters were probed with an antiphosphotyrosine mAb (B-C). The filter shown in B was stripped and reprobed with an anti-Hck Ab revealing identical amounts of Hck in all samples (D). The filter shown in C was stripped and reprobed with an anti-Fgr Ab revealing identical amounts of Fgr in all samples (E). Arrows indicate phosphorylated substrates; NS, no stimulation.
Discussion
The idea that MHC class II molecules and CD38 can function through association with other membrane molecules is widely accepted. We have determined whether the HLA-DR–or CD38-mediated signaling events in human monocytes could be due to specific interactions with other surface proteins and/or their localization in specific membrane microdomains. Our results provide clear evidence that CD38 and HLA-DR molecules are associated with each other and with the tetraspanin CD9 at the surface of human monocytes. These molecules are detected to be associated within and outside lipid rafts. In addition, raft integrity is necessary for MHC class II and CD38 signaling, but not for CD9, which is still able to transduce signals after cholesterol depletion. The physical association between these molecules was established by a biochemical approach, which shows that similar molecules can be immunoprecipitated with mAbs specific for CD38, CD9, and HLA-DR. Moreover, preclearing experiments show that a significant part of CD38, HLA-DR, and CD9 molecules are associated in a same molecular complex. Secondly, this physical association has been documented by cell-surface localization experiments that demonstrate the codistribution of the 3 molecules and directly confirm the physical proximity of HLA-DR, CD38, and CD9 at the cell surface. Tetraspanins molecules involved in several cellular functions35 are known to associate with each other and to be involved in a network of molecular interactions called the tetraspanin web,36-39 which includes additional cell-surface molecules such as MHC class II molecules.40 Thus, the participation of other tetraspanin, but also of integrins, in the molecular association between CD38, CD9, and MHC class II molecules is highly probable. This study demonstrates that CD38 and HLA-DR molecules are associated with the tetraspanin molecule CD9 at the surface of human monocytes, but this does not exclude the association of other cell-surface proteins with CD38, MHC II, and CD9 in a larger molecular network.
The functional association and the physical proximity of CD38 with BCR/CD19/CD21, TCR/CD3, and CD16 previously have been demonstrated on B, T, and NK cells, respectively.41-43 The present observation that CD38 is associated with HLA-DR at the surface of human monocytes extends our initial study demonstrating that CD38-mediated activation is linked to the MHC class II signaling pathway.17 In the same way, the HLA-DR/CD9 association at the surface of human monocytes is consistent with results from previous studies reporting such an association on B cells and immature dendritic cells.44
Because CD38, MHC class II, and CD912,32,33 have been found independently in lipid rafts on various cells, we have analyzed the association between these molecules in such microdomains. Such microdomains have been shown to be implicated in various cellular processes such as membrane trafficking, signal transduction, and cell adhesion.11,45 We provide evidence that HLA-DR, CD38, and CD9 are constitutively present and associated in plasma-membrane lipid rafts since they are coprecipitated from low-density membrane fractions in sucrose gradient as demonstrated by Western blots performed on raft-pooled fractions after lysis in CHAPS. This association also is found outside lipid rafts, results consistent with previous studies that have shown tetraspanin complexes within and outside lipid-raft domains.31,32,46 Since the proportion of total cellular protein, which is concentrated in lipid-rich microdomains, is estimated at 2% to 5%,47 the localization of these molecules to these domains represents a significant partitioning.
The association of HLA-DR and CD38 with the tetraspanin CD9 molecule associated with signaling enzymes as phosphoinositide 4-kinase and the protein kinase C α and βII48,49 and the presence of these molecules in lipid rafts are 2 observations that are relevant to the signal transduction events mediated through HLA-DR and CD38 on human monocytes. Lipid rafts function as platforms for initiating and sustaining signaling via various immunoreceptors.50-52 Our results demonstrate that disruption of these microdomains inhibited the MHC class II and CD38-mediated intracellular calcium flux in monocytes. Such treatment did not modify the CD9-induced calcium flux. These results demonstrate that the signals mediated through MHC class II and CD38 on human monocytes are dependent on the integrity of lipid rafts, whereas signaling via CD9 is independent of lipid rafts. Despite the potential of tetraspanins to associate with ganglioside and cholesterol, the tetraspanin-enriched microdomains consistently remain distinct from lipid rafts and are resistant to cholesterol depletion.53 Results of Charrin et al demonstrate that the tetraspanin-associated cholesterol might not be reached by MβCD on living cells,54 and this would explain the results we obtained on living monocytes as well in co-capping experiments and intracellular calcium flux analysis.
A role for CD9 in the formation of antigen-dependent monocyte/T-cell conjugates. CMFDA-labeled monocytes loaded or not with SEE were incubated for various periods of time with CMTMR-labeled Jurkat cells before flow cytometry. (A) Conjugate formation at t = 0 minute and t = 40 minutes of incubation between monocytes loaded with 10 μg/mL of SEE and Jurkat cells are detected as CMFDA+ CMTMR+ events. (B) Kinetic analysis of conjugate formation between CMFDA-labeled monocytes loaded or not with SEE (10 μg/mL) and CMTMR-labeled Jurkat cells. (C) CMFDA-labeled monocytes loaded or not with SEE (10 μg/mL) were incubated with CD9 (PHN200), CD38 (IB4), anti–HLA-DR (D1.12), CD11A (HI-111), or isotype control mAbs (5 μg/mL) before conjugate formation with Jurkat cells. The percent of SEE-specific conjugates are indicated for different times. (D) Formation of conjugates was analyzed for different concentrations of SEE and different concentrations of CD9 mAbs.
A role for CD9 in the formation of antigen-dependent monocyte/T-cell conjugates. CMFDA-labeled monocytes loaded or not with SEE were incubated for various periods of time with CMTMR-labeled Jurkat cells before flow cytometry. (A) Conjugate formation at t = 0 minute and t = 40 minutes of incubation between monocytes loaded with 10 μg/mL of SEE and Jurkat cells are detected as CMFDA+ CMTMR+ events. (B) Kinetic analysis of conjugate formation between CMFDA-labeled monocytes loaded or not with SEE (10 μg/mL) and CMTMR-labeled Jurkat cells. (C) CMFDA-labeled monocytes loaded or not with SEE (10 μg/mL) were incubated with CD9 (PHN200), CD38 (IB4), anti–HLA-DR (D1.12), CD11A (HI-111), or isotype control mAbs (5 μg/mL) before conjugate formation with Jurkat cells. The percent of SEE-specific conjugates are indicated for different times. (D) Formation of conjugates was analyzed for different concentrations of SEE and different concentrations of CD9 mAbs.
MHC class II molecules may populate the surface of the APC in an organized manner that comprises at least 2 types of membrane domains: lipids rafts in which MHC class II molecules are concentrated irrespectively of their antigen loading, and tetraspan domains that are enriched in MHC class II molecules characterized by the CDw78 determinant and carrying a relatively homogeneous population of antigenic peptides. However, results obtained by Kropshofer et al suggest that at least one other type of MHC class II tetraspan cluster may exist unrelated to the CDw78 cluster.46 As CDw78 microdomains are mostly absent from the surface of monocytes and immature dendritic cells, we can hypothesize that, on monocytes, the HLA-DR, CD38, and CD9 molecules may exist both within GM1-enriched microdomains and in microdomains different from both CDw78 clusters and lipid rafts.
Our initial observation was that blocking CD38, CD9, or HLA-DR molecules with specific mAbs at the surface of fixed monocytes inhibited Sag-induced proliferation, and this result suggested that CD9, CD38, and MHC class II molecules may participate to presentation of a Sag to T cell. We demonstrate here that CD38, CD9, and HLA-DR molecules share a common activation pathway. CD9-induced Ca2+ mobilization occurred with timing and distribution similar to the flux observed following HLA-DR or CD38 engagement, and we also have observed that CD9 engagement induced tyrosine phosphorylation of intracellular substrates common with CD38 and the MHC class II pathway. These observations lead to the conclusion that these 3 molecules are functionally linked. To document the implication of these molecules at the surface of living cells in an antigen-dependent response, we have analyzed the formation of Sag-specific monocyte/T-cell conjugates. Results obtained show that engagement of CD9 enhances both the frequency with which monocytes presenting SEE interact specifically with T cells, the kinetics of this interaction, and, therefore, that CD9 can play a role in antigen presentation by MHC class II molecules.
Redistribution of surface molecules is a phenomenon observed at the surface of T cells and APCs during the formation of the immunologic synapse.1 HLA-DR, CD38, and CD9 are preassociated on monocytes without any interaction with T cells. MHC class II molecules present in lipid rafts are concentrated at the synapse to facilitate antigen presentation,55 and tetraspans also are dynamically redistributed during synapse formation.56 Our data demonstrate that HLA-DR, CD38, and CD9, which are associated at the surface of monocytes, are implicated in antigen-presentation to T cells and that the MHC class II and CD38-mediated signaling consequences on the APCs are lipid raft dependent. Dynamic changes in this molecular association, such as recruitment of other surface and/or signaling molecules, may occur during monocyte differentiation to either dendritic cells or macrophages and the formation and establishment of the relevant synapse(s). Further studies will indicate how this complex evolves during differentiation/activation of APCs and antigen presentation.
Prepublished online as Blood First Edition Paper, June 7, 2005; DOI 10.1182/blood-2004-10-4094.
Supported by Association pour la Recherche sur le Cancer (Villejuif, France), Institut National de la Santé Et de la Recherche Médicale (Paris, France), Assistance Publique-Hôpitaux de Paris (Paris, France), and European Union grants alk3-CT-2002-02026 (ALL DNA VAC) and FP6#503319 (Allostem).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Marie-Claude Gendron (Institut J. Monod, Paris) for intracellular calcium measurements and to the members of the Service d'Infographie (Institut d'Hématologie, Paris).

![Figure 1. CD9 mAb inhibits Sag-induced T-cell proliferation. Purified T cells (4 × 104cells/well) were cultured with SEA in the presence of fixed monocytes (8 × 103 cells/well) with mAbs (soluble or precoated to monocytes) of the indicated specificity. [3H]thymidine incorporation measured at day 4, for T cells in the presence of uncoated fixed monocytes, was 40.500 ± 2.098 cpm. Results are representative of at least 3 independent experiments. Error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/9/10.1182_blood-2004-10-4094/6/m_zh80200585210001.jpeg?Expires=1769112699&Signature=eSmpXf8ypfM8e84vmH7GRKtA-jg1yPlVanePg5emOT7Ui-yW22NpfQei-zKoWWrLrbYB4tWGkYFwM3taOjrULDF7n8TwdoLrvXU1pClwc-lJ89kFg5EXG3BUVJPZac2LcbIuTjL2Dt2rQ~R9ozLPGUZNVg296~uqoiiRFs2RbuGzcAUm6fkdKqH6vwNR-BidfA5TlM-qmpXUlLEHBJeHf9O2cICxg2FF3rKXbodlB5yYLRpJss0Gd4ASFFWX1-xHf~lf6EgjP6JYzkKqCF4ow50FQtadeiBBYgg3Tpkr1zs7OUi46tPcGoF-~lgLsiEtlx5KeQdHXWeZ74mjRyUq9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal