Comment on Fauriat et al, page 2186
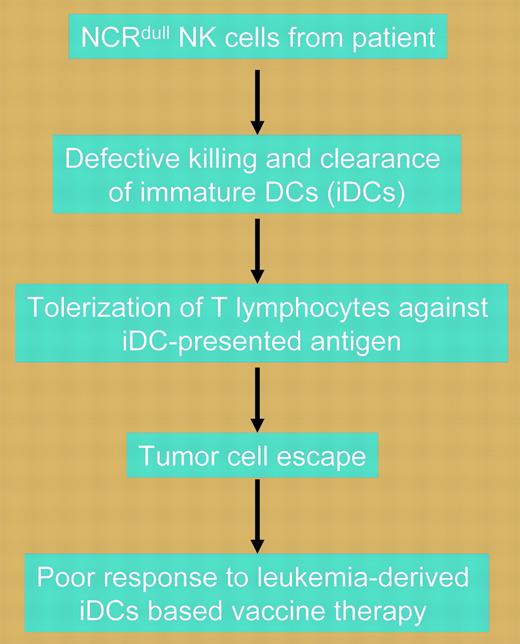
Natural killer cells from the majority of AML patients show defective killing of immature dendritic cells due to low expression of the natural cytotoxicity receptor NKp30. Defective killing of immature DCs leads to the emergence of tolerogenic T cells and tumor escape.
Over the past decade, there has been increasing interest in immunotherapy of cancer using dendritic cells (DCs). Most of the research to date has focused on optimizing the methods for generating and culturing DCs, antigen presentation, and route of adminstration.1 While much remains to be unraveled, a more fundamental question remains: which patient is more likely to respond to DC-based immunotherapy?FIG1
In this issue of Blood, Fauriat and colleagues describe a novel mechanism by which tumor cells can escape adaptive immunity and hypothesize that such knowledge may assist in the identification of patients most suited for DC-based treatment. Investigating acute myeloid leukemia (AML), the investigators show that the majority of patients with the disease have natural killer (NK) cells characterized by a defect in killing either normal or leukemia-derived immature DCs (iDCs) but not mature DCs. Excessive numbers of iDCs due to inefficient clearance induce tolerization of T lymphocytes against iDC-presented tumor antigens. Unlike the situation for mature DCs, iDC killing appears to depend on the expression of the natural cytotoxicity receptor (NCR), NKp30, by NK cells. In an elegant set of experiments, the investigators demonstrate that in the 80% of AML patients in whom defective killing of iDCs is observed, NK cells have low or no expression of NCR, including NKp30 (NCRdull). Furthermore, in redirected killing assays, the NCRdull NK cells retained normal cytolytic potential compared with NK cells obtained from healthy donors, indicating that the reduced ability to kill iDCs was due only to low expression of NKp30. The dependence on NKp30 for NK cell-mediated killing of iDCs was demonstrated by the ability of immunoglobulin M (IgM) anti-NKp30 blocking antibodies to inhibit lysis. In contrast, the minority of AML patients with the NCRbright NK phenotype showed normal killing of iDCs, as did healthy controls. Although confirming these results requires prospective evaluation in clinical trials, the implications of this important work are that leukemia-derived iDC vaccines may be effective only in the minority of AML patients with NCRbright NK cells and that NCR expression phenotype should be considered at baseline in patients enrolled in such trials.
The work by Fauriat and colleagues raises a number of questions. First, why do NK cells from the majority of AML patients have an aberrant NCR phenotype if such cells are not part of the malignant clone? Can such a defect be reversed using activating cytokines or, potentially, other agents that may de-repress expression of NCR? Finally, is the NK cell abnormality also present in patients with other tumor types? If so, what is it about the environment of the tumor-bearing host that results in aberrant acquisition of such receptors? Answers to such questions will not only permit the better selection of patients for future trials using DCs, but will also open new avenues for investigating ways to optimize such therapy. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal