Comment on Hardtke et al, page 1924
In this issue, Hardtke and colleagues conclusively demonstrate that expression on T cells of the B-cell–associated chemokine receptor, CXCR5, is essential for migration into B follicles (follicular B helper T cells [TFH]) and, further, that T cells are excluded from B follicles by the T cells' expression of the T-zone–associated chemokine, CCR7.
Band T cells are normally segregated within lymphoid tissue by chemokine gradients that are established by local populations of lymphoid stromal cells. CXCR5+ B cells are attracted to the areas forming B follicles, whereas both CCR7+ T cells and antigen-presenting dendritic cells are attracted to sites that form T zones. This isolation of B cells from T-cell areas is essential for the production of high-affinity antibodies, as it excludes T cells of irrelevant specificities from the B follicle in which B-cell selection by “licensed” TFH occurs.
As others have found,1 induction of CXCR5 on T cells occurs rapidly following priming and identifies cells destined to provide help in B follicles but not inflammatory responses. Although CXCR5 is expressed on a substantial fraction of primed T cells, many such cells continue to coexpress CCR7, an expression motif that localizes them at the B–T interface where the chemokine gradients establishing the B follicle and T zone compete for influence. Since activated CXCR5-expressing B cells mirror the situation in T cells by up-regulating CCR7,2 they too localize at the B–T interface, an arrangement likely to optimize the efficiency of B–T collaboration not only for primary antibody production but also for memory responses. Only a minority of CXCR5+CCR7+-expressing T cells go on to down-regulate CCR7 and become mature TFH where they foster the development of B-cell germinal centers, the structures within which affinity maturation of the B-cell response occurs. It is not certain which signals induce T cells to do this; perhaps the signals arise from some as-yet-unidentified interaction with antigen-activated B cells.
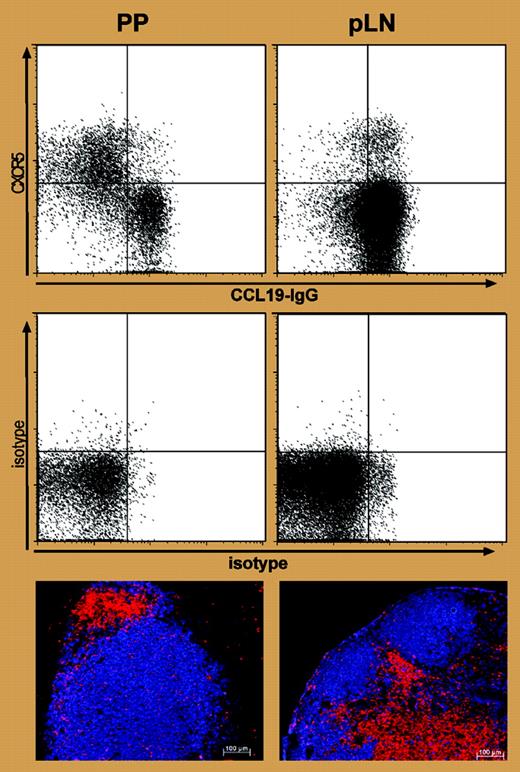
One very interesting observation from Hardtke et al is that dual CXCR5+CCR7+-expressing T cells appear in lymph nodes where there is no obvious ongoing B-cell response (see figure), suggesting that these T cells might have migrated there following priming elsewhere. This possibility is supported by the fact that these T cells are found in blood.3 I think that these are recirculating memory cells primed to provide B-cell help and that their dual expression of CXCR5+CCR7+ guides them to a CD4+CD3- non–dendritic cell located at the B–T interface.4 My colleagues and I have found that the development and persistence of T memory cells that provide help to B cells depend on OX40 (CD134) and CD30 survival signals from these cells.5 The location of the T memory cells at the B–T interface puts them in pole position to provide rapid help to B cells at the earliest sign of reinfection, but the theory remains to be tested. ▪
Expression of CXCR5 and CCR7 and positioning of T cells to B-cell follicles in LNs and PPs. See the complete figure in the article beginning on page 1924.
Expression of CXCR5 and CCR7 and positioning of T cells to B-cell follicles in LNs and PPs. See the complete figure in the article beginning on page 1924.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal