The cell type requiring HFE for normal body iron homeostasis is not known. Makui and colleagues demonstrate that wild-type macrophages are capable of partially normalizing the iron homeostasis abnormalities in HFE knock-out mice.
The mechanism by which loss of HFE leads to hemochromatosis remains enigmatic.1 The difficulty lies in part with identifying the cell type that requires HFE for normal body iron homeostasis, as HFE is broadly expressed. An early hypothesis focused on the crypt enterocyte, where HFE was proposed to participate in sensing circulating iron and modulating the iron absorptive capacity of daughter enterocytes. It has since become clear that the pathogenesis of HFE-related hemochromatosis involves underexpression of the liver peptide hepcidin.2 The demonstration that HFE knock-out mice have normalization of their iron parameters when crossed with mice overexpressing hepcidin3 has turned attention turned away from crypt cell HFE and toward liver HFE.
Within the liver, HFE is expressed by hepatocytes and Kupffer cells. Hepatocytes also express a number of other genes participating in iron homeostasis including hepcidin, transferrin receptor 2, and hemojuvelin. Functional loss of any of these genes results in inappropriately low hepcidin and hemochromatosis, suggesting that hepatocytes function both in sensing and modulating body iron status. However, isolated hepatocytes in culture fail to demonstrate an increase in hepcidin expression when exposed to iron. One possible explanation is that another cell type senses iron status and communicates a signal to hepatocytes. Because Kupffer cells (resident macrophages in the liver) participate in the up-regulation of hepcidin during inflammation, attention has been given toward a possible additional role in the up-regulation of hepcidin in response to iron.
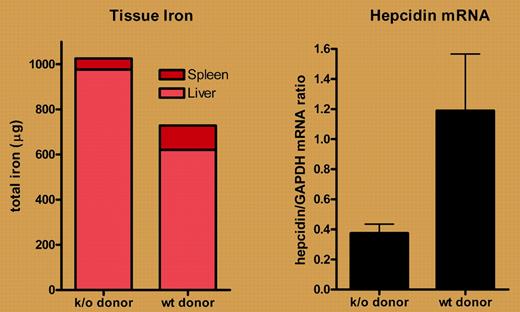
In this issue of Blood, Makui and colleagues examine the participation of macrophage HFE in iron homeostasis. Using irradiation followed by transplantation, they replaced marrow-derived cells (including resident macrophages) from HFE knock-out mice with wild-type cells. Of interest, they found that transplanting wild-type cells partially abrogated the hepatocellular iron loading of HFE knock-out mice and partially offset the iron sparing from splenic macrophages. Of most importance, these changes were associated with an increase in liver hepcidin mRNA (see figure). These results strongly suggest that macrophage HFE influences liver hepcidin expression. However, when the investigators performed the converse experiment, replacing wild-type with HFE knock-out macrophages, they observed no major effects on hepcidin expression or iron parameters. The authors attribute this latter observation to the low efficiency (∼ 30%-40%) of macrophage replacement and suggest that a relatively small number of HFE-expressing Kupffer cells are sufficient to transduce a signal between iron status and hepcidin.
Transplantation of marrow from wild-type (wt) but not HFE knock-out (k/o) mice partially normalizes tissue iron concentrations (left panel) and up-regulates liver hepcidin expression (right panel) in HFE knock-out mice. Data derived from Makui et al (see the article beginning on page 2189).
Transplantation of marrow from wild-type (wt) but not HFE knock-out (k/o) mice partially normalizes tissue iron concentrations (left panel) and up-regulates liver hepcidin expression (right panel) in HFE knock-out mice. Data derived from Makui et al (see the article beginning on page 2189).
Two recent studies used a different approach to examine the role of Kupffer cells in iron metabolism. Lou et al4 and Montosi et al5 depleted Kupffer cells using chemical agents and observed that the ability of iron administration to enhance hepcidin expression was retained. They each interpreted their results to indicate that hepatocytes, rather than Kupffer cells, play the key role in sensing body iron status. Two explanations may reconcile these conclusions with those of Makui et al. One possibility is that residual intact Kupffer cells or extrahepatic resident macrophages provided adequate iron sensing in the chemical depletion studies. Another possibility is that both hepatocytes and Kupffer cells are capable of sensing iron and influencing hepcidin expression. In this situation, the hepatocyte sensor adequately maintained normal iron homeostasis in the face of either Kupffer cell depletion or transplantation with HFE knockout macrophages.
The transplantation studies of Makui et al led to reconstitution of not only Kupffer cells but also splenic macrophages. As such, the authors raise the possibility that macrophage HFE might affect the iron status of these cells directly, rather than acting through hepcidin. The latter mechanism is supported by data presented by Rivera and colleagues, in this issue of Blood, demonstrating that exogenously administered hepcidin peptide accumulates in the spleen. Moreover, the time course and dose response of the associated hypoferremia in these studies are consistent with hepcidin inhibiting ferroportin-mediated iron efflux from splenic macrophages.6
Taken together, these observations suggest that Kupffer cells may elaborate a novel paracrine factor that influences expression of hepcidin in proximate hepatocytes. An analogous situation occurs during inflammation, with the elaboration of interleukin-6 by Kupffer cells and up-regulation of hepatocellular hepcidin expression.7 Additional studies—including the cell-type–specific knock out of HFE in macrophages and hepatocytes—will help to determine the contribution of HFE in each cell type to the regulation of hepcidin. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal