Although Notch receptor expression on malignant B cells is widespread, the effect of Notch signaling in these cells is poorly understood. To investigate Notch signaling in B-cell malignancy, we assayed the effect of Notch activation in multiple murine and human B-cell tumors, representing both immature and mature subtypes. Expression of constitutively active, truncated forms of the 4 mammalian Notch receptors (ICN1-4) inhibited growth and induced apoptosis in both murine and human B-cell lines but not T-cell lines. Similar results were obtained in human precursor B-cell acute lymphoblastic leukemia lines when Notch activation was achieved by coculture with fibroblasts expressing the Notch ligands Jagged1 or Jagged2. All 4 truncated Notch receptors, as well as the Jagged ligands, induced Hes1 transcription. Retroviral expression of Hairy/Enhancer of Split-1 (Hes1) recapitulated the Notch effects, suggesting that Hes1 is an important mediator of Notch-induced growth arrest and apoptosis in B cells. Among the B-cell malignancies that were susceptible to Notch-mediated growth inhibition/apoptosis were mature B-cell and therapy-resistant B-cell malignancies, including Hodgkin, myeloma, and mixed-lineage leukemia (MLL)–translocated cell lines. These results suggest that therapies capable of activating Notch/Hes1 signaling may have therapeutic potential in a wide range of human B-cell malignancies.
Introduction
Notch receptors, of which there are 4 mammalian family members (Notch1-4), are transmembrane proteins with large extracellular epidermal growth factor (EGF)–like domains that mediate ligand binding and intracellular regions that mediate signaling.1,2 Notch signaling initiates when ligand, from either the Delta or Serrate/Jagged family, binds the receptor and causes successive proteolytic cleavages that result in the release and nuclear translocation of the intracellular domain of Notch (ICN). Within the nucleus, ICN assembles a large transcriptional activation complex that interacts with the conserved transcription factor CSL (CBF-1 [c-promoter binding factor 1], Su(H) [suppressor of hairless], Lag-2 [Lin-12 and Glp-1 phenotype 2], RBP-Jk [recombining binding protein-J kappa]), leading to de-repression/activation of CSL-dependent downstream targets.3-5 Many potential targets of Notch signaling have been identified in various cell contexts, and several members of the Hairy/Enhancer of Split (Hes) family of basic helix-loop-helix (bHLH) proteins are direct Notch/CSL targets, including Hes1.6,7
Notch signaling has multiple functions during normal hematolymphoid development. These include regulation of T-cell commitment from a multipotential precursor, regulation of αβ T-cell development, and marginal zone B-cell development.8 Notch signaling is tightly controlled, and failure to maintain this regulation can lead to transformation.9 Within the hematolymphoid compartment, constitutive Notch signaling causes T-cell malignancy in multiple species, and recent data identified Notch-activating mutations as the most frequent event in human T-cell leukemia.10 In malignant T cells, Notch signaling influences proliferation, differentiation, and survival.
Although Notch receptor expression is widespread throughout the hematolymphoid compartment, its transforming potential seems to be restricted to developing T cells. This raises the question of what is the consequence of dysregulated Notch signaling in other lineages? Several studies have explored this issue in malignant B cells, with conflicting results. Three studies suggest that constitutive Notch signaling in malignant B cells leads to growth inhibition and/or apoptosis. Morimura et al11 demonstrated Notch-induced growth arrest and apoptosis in an avian B-cell lymphoma line. Expression of constitutively active chicken Notch1 in DT40 cells induced growth inhibition with accumulation in the G1 phase of the cell cycle. The investigators also noted a 3- to 5-fold increase in apoptotic cells compared with controls. Furthermore, expression of Hes1, a downstream target of Notch signaling, induced apoptosis, with less dramatic effects on the cell cycle. In a second study, Romer et al12 showed that Notch signaling enhanced apoptosis in a murine B-cell lymphoma line after B-cell antigen receptor (BCR) crosslinking. In the third study, Nefedova et al13 found that Notch activation induced growth arrest in 4 human myeloma-derived cell lines.
In contrast, 3 studies suggest that Notch signaling promotes proliferation of malignant B cells. Hubmann et al14 reported that Notch2 induced CD23a expression in a pre-B acute lymphoblastic leukemia (ALL) cell line, and both Notch2 and CD23a are expressed at high levels in chronic lymphocytic leukemia (CLL) samples, suggesting a potential role for Notch2 in CD23a-mediated proliferation. In 2 publications that used Jagged1-expressing cells to induce Notch signaling, Jundt et al15,16 demonstrated that 5 Hodgkin and 3 myeloma-derived cell lines increased thymidine incorporation after 48 hours of ligand signaling, suggesting a proliferative effect on these cell lines. This result is in contrast to that of Nefedova et al13 who observed decreased thymidine incorporation in 2 of the same cell lines tested.
These conflicting results reveal the need to reevaluate the effects of Notch signaling in B-cell malignancies. Given the variety of effects in a range of different B-cell malignancies, we sought to address 3 topics: (1) whether Notch signaling had a consistent effect on different types of B-cell malignancies, (2) whether different Notch receptors differentially affected the B-cell response, and (3) to determine the transcriptional targets of Notch signaling that mediate the B-cell effect. To establish the effects of Notch signaling in B cells, we investigated the effects of constitutive Notch signaling in transformed murine B cells. We found that, in contrast to T cells, murine B-cell lines underwent caspase-dependent apoptosis and growth inhibition in response to Notch signaling. Unlike T cells whereby Notch1-3 but not Notch4 induces T-ALL (J.C.A. and W.S.P., in preparation), all 4 Notch receptors induced B-cell growth arrest and apoptosis. In all cases, Notch-mediated cell death was associated with up-regulation of Hes1 expression. Retroviral expression of Hes1 was sufficient to induce B-cell growth arrest and apoptosis, suggesting that Hes1 mediates the Notch signal. From these observations, we assayed the effect of Notch signaling in malignant human B cells. Initially, we assessed the effects of Notch signaling in human precursor B-cell acutelymphoblastic leukemia (pre-B ALL) cell lines, because these represent the most common B-cell malignancy in children. We induced Notch signaling through expression of constitutively active Notch1, the downstream Notch target Hes1, or coculture with fibroblasts expressing the Notch ligands, Jagged1 or Jagged2. All 3 approaches induced growth inhibition and apoptosis in the human pre-B ALL cell lines. Furthermore, expression of constitutively active Notch1 induced growth inhibition and apoptosis in Hodgkin, myeloma, and biphenotypic mixed-lineage leukemia (MLL)–translocated B ALL lines, which represent both mature and therapy-resistant B-cell malignancies. Together, these results demonstrate a generalized phenomenon of Notch-mediated growth inhibition and apoptosis in murine and human malignant B cells. Of significance is the consistent finding of Notch-induced growth inhibition and apoptosis in both immature and mature types of human B-cell malignancy, including therapy-resistant subtypes, suggesting that Notch signaling may have a potentially broad therapeutic role in the treatment of a range of B-cell malignancies.
Materials and methods
Cell culture
Murine cell lines. Pre–B-cell lines V917 and 70Z/318,19 and the BCR-ABL–transformed CD4+CD8+ T-cell lymphoma-derived cell line G4A220 were cultured in RPMI 1640, supplemented with 10% fetal calf serum, penicillin-streptomycin, l-glutamine, and 2-mercaptoethanol.
Human B-cell lines. Four cell lines derived from human pre-B ALLs were obtained, namely JM-1 (CRL-10423), Nalm-6 (ACC 128; Deutsche Sammlung von Mikroorganismen und Zellkulturen [DSMZ], Braunschweig, Germany;), JIM, 697 (CC 42; DSMZ). Three myeloma-derived lines, RPMI-8226 (CCL-155; American Type Culture Collection [ATCC], Manassas, VA;), U266BL (TIB-196; ATCC), NCI-H929 (CRL-9068; ATCC), 1 Hodgkin line L428,21 and 2 MLL-translocated B-myeloid biphenotypic lines SEM-K2,22 RS4;1123 were also obtained (see Table 1). The T-cell leukemia-derived Jurkat cell line was transduced with an ecotropic virus receptor (JurkatE).30 All human cell lines were grown in C10 lymphocyte media.31
B-cell malignancy-derived cell lines
. | Oncogenic events . | Phenotype/genotype . | References . |
|---|---|---|---|
| Precursor B ALL | |||
| V9 (murine) | v-ABL | NA | Gurish et al, 199517 |
| 70Z (murine) | Nitrosourea-induced | NA | Paige et al, 197818 |
| WEHI231 (murine) | Mineral oil–induced | NA | Ralph, 197924 |
| 697 | E2A-PBX1, BCL2, MYC | Relapsed, t(1;19), del(6)(q21), CALLA | Findley et al, 198225 |
| Nalm-6 | TEL-PDGFRB | Relapsed, t(5;12), CALLA | Hurwitz et al, 197926 |
| JM-1 | BCL2 | CALLA | NA |
| JIM | NA | NA | NA |
| Hodgkin | |||
| L428 | NA | Refractory, hypertetraploid | Schaadt et al, 198021 |
| Myeloma | |||
| RPMI-8226 | NA | Triploid, 14q+, 22q-, lambda+, EBV- | Matsuoka et al, 196727 |
| NCI-H929 | cellular MYC, RAS | Relapsed, 8q+, kappa+, EBV- | Gazdar et al, 198028 |
| U266 | BCL2 | Refractory, 11q13, IgE, lambda, IL6+ | Nilsson et al, 197029 |
| Biphenotypic MLL | |||
| SEM-K2 | MLL-AF4 | Relapsed, t(4;11), CD19+/CD13+ | Pocock et al, 199522 |
| RS4;11 | MLL-AF4 | Relapsed, t(4;11), i(7q) | Stong et al, 198523 |
. | Oncogenic events . | Phenotype/genotype . | References . |
|---|---|---|---|
| Precursor B ALL | |||
| V9 (murine) | v-ABL | NA | Gurish et al, 199517 |
| 70Z (murine) | Nitrosourea-induced | NA | Paige et al, 197818 |
| WEHI231 (murine) | Mineral oil–induced | NA | Ralph, 197924 |
| 697 | E2A-PBX1, BCL2, MYC | Relapsed, t(1;19), del(6)(q21), CALLA | Findley et al, 198225 |
| Nalm-6 | TEL-PDGFRB | Relapsed, t(5;12), CALLA | Hurwitz et al, 197926 |
| JM-1 | BCL2 | CALLA | NA |
| JIM | NA | NA | NA |
| Hodgkin | |||
| L428 | NA | Refractory, hypertetraploid | Schaadt et al, 198021 |
| Myeloma | |||
| RPMI-8226 | NA | Triploid, 14q+, 22q-, lambda+, EBV- | Matsuoka et al, 196727 |
| NCI-H929 | cellular MYC, RAS | Relapsed, 8q+, kappa+, EBV- | Gazdar et al, 198028 |
| U266 | BCL2 | Refractory, 11q13, IgE, lambda, IL6+ | Nilsson et al, 197029 |
| Biphenotypic MLL | |||
| SEM-K2 | MLL-AF4 | Relapsed, t(4;11), CD19+/CD13+ | Pocock et al, 199522 |
| RS4;11 | MLL-AF4 | Relapsed, t(4;11), i(7q) | Stong et al, 198523 |
E2A-PBX1 indicates early region 2a-pre-B-cell leukemia transcription factor 1; MYC, pre-B-cell leukemia transcription factor 1; CALLA, common acute lymphoblastic leukemia antigen; TEL-PDGFRB, translocated ets leukemia-platelet-derived growth factor receptor β; EBV, Epstein-Barr virus; IgE, immunoglobulin E; IL6, interleukin-6; MLL-AF4, mixed-lineage leukemia–ALL1 fused gene from chromosome 4; and NA, not available.
Plasmids and retroviral constructs
The intracellular domain of human Notch1 (ICN1, aa1760-2555) or full-length human Hes1 was inserted into the MigR1 (murine stem cell virus–based) retroviral vector that coexpresses green fluorescent protein (GFP) or truncated human nerve growth factor receptor (NGFR) as surrogate expression markers.32,33 The analogous intracellular constructs for ICN2, ICN3, and ICN4 were similarly generated. A tamoxifen-inducible ICN1 construct was created with a mutated portion of the murine estrogen receptor (ER) hormone binding domain34 fused to the N-terminus of ICN1 with a 20 amino acid linker (ER-ICN1).
Retrovirus supernatants and transduction
Retroviral supernatants were prepared following transient transfection of HEK293 cells and titered in NIH3T3 cells.20,35,36 The titers of the retroviral supernatants were normalized prior to use, based on either GFP or hNGFR expression by flow cytometry.37 Retroviral envelopes with ecotropic (pHIT123, for murine cells) or amphotropic (pHIT456 or vesicular stomatitis virus glycoprotein, for human cells) host ranges were used. Transduction protocols have been published previously.38 After transduction of the human leukemia cell lines with the ER-ICN1, single-cell clones were isolated by sorting for GFP expression. These clones were exposed to 1 μM tamoxifen or ethanol control for time point experiments.
Antibodies
Cell cycle and apoptosis
For cell-cycle analysis, cells were fixed in ethanol and stained with 50 μg/mL propidium iodide (PI) in saline for more than 30 minutes; alternatively DRAQ5 (Alexis Biochemicals, San Diego, CA) was added to live cells to a final concentration of 5 μM for more than 5 minutes. Cells with less than 2N DNA content (subdiploid) were considered apoptotic. Cells with greater than 2N DNA content were considered to be cycling (non-G0/G1). To measure apoptosis, fresh cells were labeled with 1:500 annexin V–biotin (556417; Pharmingen, San Diego, CA) followed by 1:1000 streptavidin-cytochrome C (Pharmingen). PI, DRAQ5, and annexin V–CytochromeC were measured by flow cytometry (FACScan; Becton Dickinson, San Jose, CA) and analyzed with FlowJo Software (TreeStar, Ashland, OR).
RT-PCR analysis
Standard TriZol (Invitrogen, Carlsbad, CA) RNA isolation, reverse transcriptase–polymerase chain reaction (RT-PCR) techniques were used.39 Murine primers for mHes1 are sense GCCAGTGTCAACACGACACCGG and antisense TCACCTCGTCATGCACTCG. The primers used for the murine hypoxanthine phosphoribosyltransferase (mHPRT) control have been described previously.40 Human primers included the following: hNotch1 sense, CAGCTGCACTTCATGTACGTG; hNotch1 antisense, GGCAGACACAGCCGCATGCAGC; hNotch2 sense, CCACCAGGCACTCGGGGCCTA; hNotch2 antisense, GGAGTAATAAGGAGGCTGGCG; hNotch3 sense, AAGCGGCTAAAGGTAGAGGAG; Notch3 antisense, GCATCGGCTGTGACAGCTGTG; hNotch4 sense, TGGGTATCTCTGCCAGTGTGC; hNotch4 antisense, CAGTGGCAGATGAAACCCAGG; hHes1 sense, AAAATGCCAGCTGATATAATGGAG; and hHes1 antisense, GGTCTGTGCTCAGCGCAGCCGTCA. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. All amplifications crossed intron-exon boundaries to exclude genomic DNA amplification.
Statistics
All experiments were conducted in triplicate, unless otherwise noted. Mean, standard deviation (SD), and P values based on the 2-tailed t test and repeated-measures analysis of variance (ANOVA) were calculated with Excel X (Microsoft, Redman, WA) or InStat3 (GraphPad Software, San Diego, CA) where appropriate.
Results
Activated Notch1 inhibits B-cell but not T-cell growth
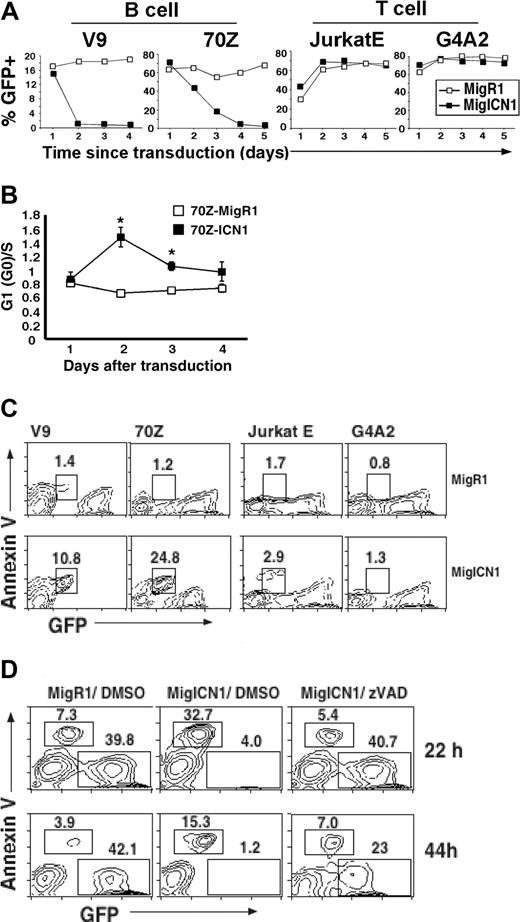
To determine the effect of Notch signaling in malignant murine B cells, we transduced several cell lines with retroviral constructs expressing either a constitutively active truncated intracellular portion of the human Notch1 receptor (MigICN1) and GFP or GFP only, from the vector control (MigR1). Titer-matched retroviral supernatants were used to transduce 2 different murine pre–B-cell lines, v-abl–transformed V9 cells17 and chemically transformed 70Z cells.18,19 MigR1-transduced GFP+ cells proliferated at a rate similar to the untransduced GFP- cells, as indicated by the stable percentage of GFP+ cells at different times after transduction (Figure 1A, B-cell, open squares). In contrast, the percentage of MigICN1 GFP+ cells dramatically declined, indicating growth arrest and/or cell death (Figure 1A, B-cell, black squares). The decline in the GFP+ ICN1 population occurred more rapidly in V9 cells than in 70Z cells, although by 5 days after transduction, there were very few GFP+ cells present in either cell population (Figure 1A). Similar results were obtained in the murine B-cell lymphoma line WEHI-23124 (data not shown).
To determine whether the ICN1-induced decline in cell number was cell-type specific, we examined the effect of ICN1 expression in 2 T-cell lines, JurkatE, a subline of the human T lymphoma Jurkat cell line expressing the retroviral ecotropic receptor,30 and G4A2, a CD4+CD8+ T-cell line derived from a BCR-ABL–induced murine T-cell lymphoma.20 Unlike the effects in the assayed B-cell lines, ICN1 did not inhibit the growth of either T-cell line (Figure 1A, T-cell). In fact, Notch signaling is required to maintain proliferation and viability of many murine and human T-ALL cell lines.9,10
To assess cell-cycle perturbations, MigR1- or ICN1-transduced GFP+ cells (70Z cells) were sorted 24 hours after transduction and analyzed for DNA content by PI staining. Comparison of the G1/S ratio between MigR1 and ICN1-transduced 70Z cells showed a significant increase in this ratio at early times after transduction (Figure 1B). These data suggest that ICN1 induced growth inhibition in these transformed murine B-cell lines.
Activated Notch1 induces B-cell but not T-cell apoptosis
Because the small but significant growth disadvantage observed in the ICN1-expressing B-cell lines was unlikely to account for the rapid decrease in the percentage of GFP+ V9 cells, apoptotic rates and DNA content were determined in various cell populations. Markedly increased annexin V positivity was found in MigICN1-transduced B cells relative to MigR1-GFP+ cells (10.8% and 24.8% versus 1.4% and 1.2%, respectively) (Figure 1C, left). The average cell size of the ICN1 GFP+ population was also significantly smaller, another finding consistent with apoptosis (data not shown).41 By using the same experimental protocol, 2 ICN1-transduced T-cell lines showed no increase in annexin V positivity (2.9% and 1.3% versus 1.7% and 0.8%, respectively) (Figure 1C, right) or changes in cell size (data not shown).
To determine whether ICN1-induced cell death was caspase dependent, ICN1-transduced V9 cells were cultured with either the caspase inhibitor, zVAD (50 μM in DMSO) or DMSO alone (Figure 1D). When cultured in control media (no zVAD), the great majority of GFP+ cells became annexin V+ by 22 hours after transduction (89% of GFP+ cells), and few GFP+ cells remained by 44 hours (Figure 1D, middle). In contrast, only a minority of ICN1-transduced cells cultured in the presence of 50 μM zVAD became annexin V+ at 22 hours (12%), and the percentage of annexin V+ cells did not differ significantly from V9 cells transduced with the control vector cultured in the presence of DMSO (15%). These results indicate that caspase activation is involved in ICN1-induced B-cell death. Thus, constitutive Notch1 signals induce both apoptosis and growth arrest in transformed murine B cell lines.
ICN1 inhibits B-cell but not T-cell growth. (A) V9 pre–B-cell line, 70Z pre–B-cell line, JurkatE human T-cell line, and G4A2 T-cell lines were transduced with retrovirus expressing either GFP only (MigR1) or ICN1 and GFP (MigICN1) and analyzed by flow cytometry. Transduced cells were identified as GFP+, and the percentage of GFP+ cells was plotted against time. (B) ICN1 induces cell cycle arrest. 70Z cells were transduced with the indicated retrovirus, and GFP+ cells were sorted 24 hours later. Sorted cells were then plated out (5 × 104 cells/mL). Cells were harvested at 4 time points: 30, 48, 60, and 72 hours after transduction and fixed for PI staining and FACS analysis to determine the cell-cycle profile. The ratio of G0(G1)/S is shown. Results are from 3 independent experiments; mean and standard deviation are shown. P < .05 (ANOVA). (C) ICN1 induces apoptosis in pre–B-cell lines but not in T-cell lines. The transduced cell lines described in Figure 1A were assayed for annexin V expression by FACS. The GFP intensity of the annexin V+ cells decreased because of apoptosis-induced GFP leakage. (D) ICN1-induced apoptosis is caspase dependent. V9 cells were transduced with MigR1 or MigICN1 as described in Figure 1A. After transduction, ICN1-transduced cells were divided into aliquots into 2 wells in parallel and incubated in the presence of either 50 μM caspase inhibitor z-val-Ala-DL-Asp-fluoromethylketone (zVAD) or dimethyl sulfoxide (DMSO). Cells were collected at 22 hours and 44 hours after transduction and stained for annexin V prior to analysis.
ICN1 inhibits B-cell but not T-cell growth. (A) V9 pre–B-cell line, 70Z pre–B-cell line, JurkatE human T-cell line, and G4A2 T-cell lines were transduced with retrovirus expressing either GFP only (MigR1) or ICN1 and GFP (MigICN1) and analyzed by flow cytometry. Transduced cells were identified as GFP+, and the percentage of GFP+ cells was plotted against time. (B) ICN1 induces cell cycle arrest. 70Z cells were transduced with the indicated retrovirus, and GFP+ cells were sorted 24 hours later. Sorted cells were then plated out (5 × 104 cells/mL). Cells were harvested at 4 time points: 30, 48, 60, and 72 hours after transduction and fixed for PI staining and FACS analysis to determine the cell-cycle profile. The ratio of G0(G1)/S is shown. Results are from 3 independent experiments; mean and standard deviation are shown. P < .05 (ANOVA). (C) ICN1 induces apoptosis in pre–B-cell lines but not in T-cell lines. The transduced cell lines described in Figure 1A were assayed for annexin V expression by FACS. The GFP intensity of the annexin V+ cells decreased because of apoptosis-induced GFP leakage. (D) ICN1-induced apoptosis is caspase dependent. V9 cells were transduced with MigR1 or MigICN1 as described in Figure 1A. After transduction, ICN1-transduced cells were divided into aliquots into 2 wells in parallel and incubated in the presence of either 50 μM caspase inhibitor z-val-Ala-DL-Asp-fluoromethylketone (zVAD) or dimethyl sulfoxide (DMSO). Cells were collected at 22 hours and 44 hours after transduction and stained for annexin V prior to analysis.
All 4 Notch receptor homologs induce B-cell death
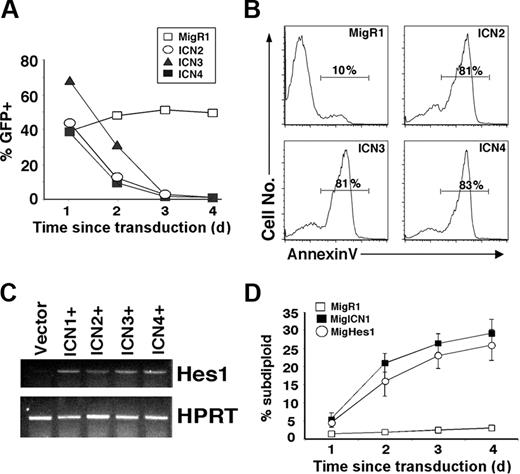
To determine whether constitutive signaling by the 3 other Notch receptor homologs (collectively referred to as ICN2-4) also induce B-cell death, the intracellular domain of each of these homologs was retrovirally expressed in malignant B-cell lines. Similar to ICN1, ICN2-4 blocked the growth of GFP+ V9 cells (Figure 2A) and 70Z cells (data not shown). To confirm the presence of apoptosis, annexin V staining was performed on purified GFP+ V9 cells transduced with ICN2-4. At 72 hours after transduction, the majority (81%-83%) of ICN2-4–transduced cells were annexin V+, whereas, only a small minority (10%) of MigR1-transduced cells were annexin V+ (Figure 2B). Thus, the intracellular domains of all 4 mammalian Notch receptors have the capacity to induce B-cell apoptosis. When Hes1 expression was measured by RT-PCR, all intracellular Notch homologs, ICN1-4, induced Hes1 expression compared with the control vector (Figure 2C).
Hes1 is sufficient to induce B-cell apoptosis
Although the important targets of Notch signaling in B cells are not yet known, Hes1 is a documented Notch target in lymphocytes that is specifically induced by ICN1-4 expression (Figure 2C). Because ICN1-4 all induce Hes1 expression, we wondered whether Hes1 expression was sufficient to induce B-cell death. The human Hes1 homologue, whose amino acid sequence is 98% identical to murine Hes1, was cloned into MigR1 and retrovirally expressed in 70Z cells. The ability of Hes1 to induce 70Z cell death, as measured by the percentage of subdiploid cells at several time points after transduction, was very similar to that of ICN1 (26% and 29% versus 3% for control at 4 days) (Figure 2D). In contrast, retroviral-directed Hes1 expression in Jurkat T cells did not affect cell growth or survival (data not shown). Thus, Hes1 is a potent inducer of B-cell death, suggesting that it plays an important role in mediating Notch signaling in B cells.
Activated Notch2, Notch3, and Notch4 and the downstream target Hes1 are capable of inducing B-cell death. (A) V9 cells were transduced with the indicated retroviral supernatant, and the percentage of GFP+ cells was determined as described in Figure 1. (B) Transduced GFP+ V9 cells were purified by FACS at 20 hours after transduction. The GFP+ cells were cultured for 24 hours, stained for annexin V, and analyzed by FACS. The histograms of annexin V staining are shown. (C) 70Z cells were transduced with the indicated retrovirus, and NGFR+ cells were purified on a MACS column at 48 hours after transduction. RNA was isolated, and RT-PCR to detect Hes1 transcription was performed. HPRT RT-PCR was performed as an internal control. (D) 70Z cells were transduced with the indicated retrovirus, and GFP+ cells were purified by FACS 24 hours later. The GFP+ cells were harvested, and the percentage of subdiploid (apoptotic) cells was determined by PI staining and FACS analysis. Mean and standard deviation are shown.
Activated Notch2, Notch3, and Notch4 and the downstream target Hes1 are capable of inducing B-cell death. (A) V9 cells were transduced with the indicated retroviral supernatant, and the percentage of GFP+ cells was determined as described in Figure 1. (B) Transduced GFP+ V9 cells were purified by FACS at 20 hours after transduction. The GFP+ cells were cultured for 24 hours, stained for annexin V, and analyzed by FACS. The histograms of annexin V staining are shown. (C) 70Z cells were transduced with the indicated retrovirus, and NGFR+ cells were purified on a MACS column at 48 hours after transduction. RNA was isolated, and RT-PCR to detect Hes1 transcription was performed. HPRT RT-PCR was performed as an internal control. (D) 70Z cells were transduced with the indicated retrovirus, and GFP+ cells were purified by FACS 24 hours later. The GFP+ cells were harvested, and the percentage of subdiploid (apoptotic) cells was determined by PI staining and FACS analysis. Mean and standard deviation are shown.
ICN1 expression leads to growth inhibition in human precursor B-cell leukemia lines
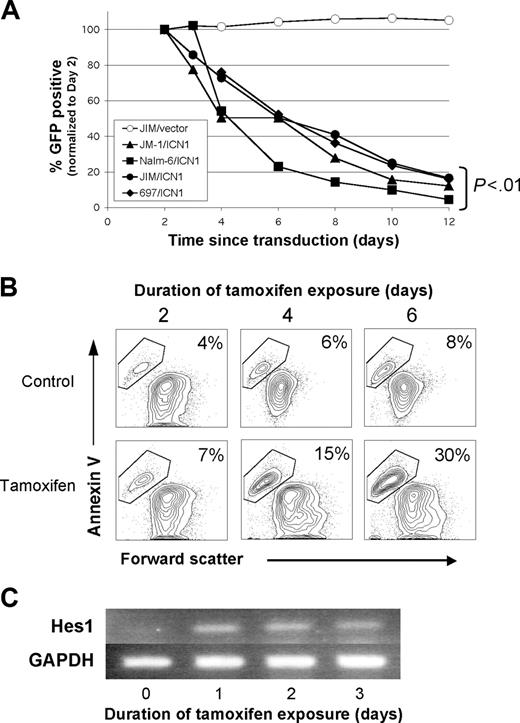
We next assayed a variety of human B-cell malignancies to determine whether human B-cell malignancies exhibited a similar sensitivity to Notch signaling as the murine B cell lines. We initially studied precursor B-cell acute lymphoblastic leukemia (pre-B ALL) cell lines because these are the predominant B-cell malignancy in children. As in the murine B cells, ICN1 expression led to significant growth inhibition in 4 pre-B ALL cell lines (Figure 3A). All 4 cell lines showed a progressive decrease in transduced (GFP+) cells over a 12-day period, ultimately leading to less than 20% of the original GFP ratios (P < .01, all values). Absolute cell counts demonstrated a decrease in GFP+ cell number compared with expanding nontransduced and MigR1 control-transduced populations, suggesting both growth inhibition and cell death (data not shown).
Notch-mediated growth inhibition/apoptosis occurs in human pre-B ALL cell lines. (A) Growth inhibition in 4 pre-B ALL cell lines is seen after retroviral transduction with intracellular Notch1 (ICN1). This graph shows the percentage of GFP+ (ICN1 transduced) cells versus days from transduction (normalized to day 2). All cell lines were also transduced with the control vector MigR1 (representative control shown). The P values were from repeated-measures ANOVA. (B) By using a subclone of the JM-1 pre-B ALL cell line stably transduced with tamoxifen-inducible ER-ICN1 retrovirus, apoptosis is shown by annexin V staining, comparing tamoxifen exposure to ethanol control. (C) Hes1 expression was measured by RT-PCR in the tamoxifen-induced cells from panel B. GAPDH was used as a cDNA control. A representative image from 2 experiments is shown.
Notch-mediated growth inhibition/apoptosis occurs in human pre-B ALL cell lines. (A) Growth inhibition in 4 pre-B ALL cell lines is seen after retroviral transduction with intracellular Notch1 (ICN1). This graph shows the percentage of GFP+ (ICN1 transduced) cells versus days from transduction (normalized to day 2). All cell lines were also transduced with the control vector MigR1 (representative control shown). The P values were from repeated-measures ANOVA. (B) By using a subclone of the JM-1 pre-B ALL cell line stably transduced with tamoxifen-inducible ER-ICN1 retrovirus, apoptosis is shown by annexin V staining, comparing tamoxifen exposure to ethanol control. (C) Hes1 expression was measured by RT-PCR in the tamoxifen-induced cells from panel B. GAPDH was used as a cDNA control. A representative image from 2 experiments is shown.
To better evaluate the contributions of growth inhibition and apoptosis in the human cell lines, we generated a tamoxifen-inducible ICN1 retroviral vector (ER-ICN1). The JM-1 pre-B ALL cell line was transduced with ER-ICN1, and single cell clones were analyzed to measure the effect of Notch signaling in pure populations which express uniform levels of ICN1. Following addition of tamoxifen, apoptosis, as measured by annexin V binding, increased steadily over a 6-day period (30% versus 8% in controls) (Figure 3B). In addition, Notch signaling was confirmed by transcriptional up-regulation of the Notch target Hes1 (∼ 5-fold) by 24 hours after induction (Figure 3C). Hes1 up-regulation remained constant over 3 days. Similar results were obtained with the Nalm-6 cell line (data not shown).
Notch ligands lead to growth inhibition in coculture assays
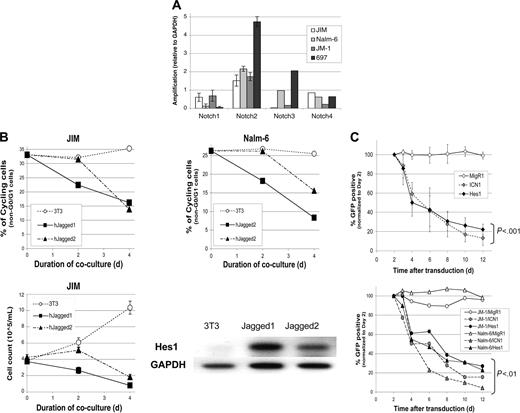
Because ligand-mediated signaling may be a feasible therapeutic approach, we assessed the effect of using Notch ligands to induce Notch signaling in these B-cell malignancies. Using RT-PCR, all 4 Notch receptors were detected in the human pre-B ALL cell lines (Figure 4A). All samples expressed Notch2 transcripts at similar levels, whereas Notch1 and Notch3 were detected at lower levels with more variability. Notch4 transcripts were also detected in all samples. Expression of Notch receptors in all 4 of the pre-B ALLs assayed provides the potential for ligand-mediated Notch signaling. We next cultured JM-1 or Nalm-6 cells with murine NIH3T3 fibroblasts that had been engineered to express the human Notch ligands, Jagged1 or Jagged2. Both pre-B ALL cell lines underwent significant growth inhibition (40%-70% decrease in cycling cells versus < 6% in controls; Figure 4B). Cell death was observed through decreasing cell numbers in the JIM cell line (Figure 4B). Hes1 was up-regulated dramatically (5- to 8-fold compared with controls) within 24 hours of coculture with either ligand in the Nalm-6 cell line (Figure 4B).
Hes1 also leads to human B-cell leukemia growth inhibition/apoptosis
Because Hes1 expression correlated with Notch activation by either ligand or ICN transduction, we directly tested the effect of Hes1 expression in the human pre-B ALL lines. We found that Hes1 was able to induce growth inhibition/apoptosis in all 4 human pre-B ALL cell lines tested (Figure 4C). This effect was comparable in potency and time course to ICN1 (P < .01). Thus, Hes1 functions similarly to ICN in both human and murine transformed B-cell lines.
Constitutive Notch signaling induces growth inhibition in mature human B-cell leukemias
The effect of Notch activation in mature human B-cell tumors is controversial because studies have shown both proliferation and growth arrest.13,16 To determine the effect of Notch signaling in mature B-cell subtypes, we selected 1 Hodgkin and 3 myeloma-derived cell lines that were previously used to suggest that Notch signaling promotes their growth. We transduced these cell lines with the inducible ER-ICN1 retrovirus. Following addition of tamoxifen, all 4 cell lines responded to Notch signaling with varying degrees of growth inhibition, compared with ethanol controls (Figure 5A). Two myeloma lines, RPMI-8226 and NCI-H929, demonstrated growth inhibition to a similar degree and time course as the pre-B ALL lines (-74% and -88%, P <.001 and P < .001). One myeloma line, U266BL, showed an intermediate effect (-56%, P < .001). The Hodgkin lymphoma line, L428, did not show any growth difference between the tamoxifen-treated and control cells in the first 2 to 4 days. Thereafter, we observed a reproducible and significant inhibitory effect (-20%, P = .01). None of these lines increased their proliferation in response to Notch signaling.
Notch ligands and Hes1 induce Notch-mediated growth arrest/cell death in human B-cell leukemia lines. (A) RNA expression levels of the 4 Notch homologs were measured in 4 pre-B ALL cell lines via RT-PCR. GAPDH was used as a control. The data for Notch1 and Notch2 are in triplicate with standard deviation. Data for Notch3 and Notch4 are averages of duplicate samples. (B) Growth inhibition and cell death in pre-B ALL cell lines during coculture with Notch ligand-expressing fibroblasts (Jagged1 or Jagged2 NIH3T3s) was measured by staining for DNA content (PI) and analysis by flow cytometry. Comparison was made to coculture with untransduced NIH3T3 controls. The top graph represents the percentage of non-G0/G1 cells versus days of coculture for 2 different pre-B ALL cell lines (JIM, Nalm-6) (duplicate experiments gave similar results). Bottom graph represents the cell count versus days of coculture (counted in triplicate). Up-regulation of Hes1 by RT-PCR was measured at 24 hours after initiation of coculture with either Jagged1- or Jagged2-expressing fibroblasts. GAPDH was used as the cDNA control. (C) Hes1 expression leads to growth arrest/cell death that is equivalent to intracellular Notch1. The graph shows the percentage of GFP+ (transduced) cells versus days from transduction (normalized to day 2) for the 697 pre-B ALL cell line. The second graph shows similar data from 2 other cell lines, JM-1 and Nalm-6. The P values are from repeated-measures ANOVA. In panels B and C, means and standard deviation are shown.
Notch ligands and Hes1 induce Notch-mediated growth arrest/cell death in human B-cell leukemia lines. (A) RNA expression levels of the 4 Notch homologs were measured in 4 pre-B ALL cell lines via RT-PCR. GAPDH was used as a control. The data for Notch1 and Notch2 are in triplicate with standard deviation. Data for Notch3 and Notch4 are averages of duplicate samples. (B) Growth inhibition and cell death in pre-B ALL cell lines during coculture with Notch ligand-expressing fibroblasts (Jagged1 or Jagged2 NIH3T3s) was measured by staining for DNA content (PI) and analysis by flow cytometry. Comparison was made to coculture with untransduced NIH3T3 controls. The top graph represents the percentage of non-G0/G1 cells versus days of coculture for 2 different pre-B ALL cell lines (JIM, Nalm-6) (duplicate experiments gave similar results). Bottom graph represents the cell count versus days of coculture (counted in triplicate). Up-regulation of Hes1 by RT-PCR was measured at 24 hours after initiation of coculture with either Jagged1- or Jagged2-expressing fibroblasts. GAPDH was used as the cDNA control. (C) Hes1 expression leads to growth arrest/cell death that is equivalent to intracellular Notch1. The graph shows the percentage of GFP+ (transduced) cells versus days from transduction (normalized to day 2) for the 697 pre-B ALL cell line. The second graph shows similar data from 2 other cell lines, JM-1 and Nalm-6. The P values are from repeated-measures ANOVA. In panels B and C, means and standard deviation are shown.
Constitutive Notch signaling induces growth inhibition in biphenotypic human B-cell leukemias harboring the MLL t(4;11) translocation
Several subtypes of B-cell ALL have been described through chromosomal aberrations and array analysis.42 A distinct category of ALL that is particularly divergent from the others and carries a uniformly dismal clinical prognosis comprises ALLs harboring the t(4;11) MLL translocation.43 Translocations of the MLL gene, or mixed lineage leukemia, are found in both ALL and AML, and these leukemias often express markers and phenotypes of both myeloid and lymphoid lineages. To determine whether our observed Notch effect would extend to these therapy-resistant leukemias, we assayed 2 previously characterized biphenotypic t(4;11) MLL-translocated cell lines, SEM-K2 and RS4;11. Both cell lines underwent dramatic growth inhibition and death, because GFP+ cells were nearly undetectable by day 8 following transduction (< 3%, P < .001) (Figure 5B). Growth inhibition was confirmed through cell-cycle analysis. SEM-K2 cells showed an approximate 50% decrease in cycling cells by day 4 following transduction (Figure 5C, histograms), and similar decreases in both S and G2/M phases of the cell cycle demonstrate a rapid G0/G1 growth arrest (Figure 5D). These are the most sensitive human cell lines we have tested to date.
Discussion
The functional consequences of Notch signaling in malignant B cells are poorly understood. Previous studies have shown both promotion and inhibition of growth and/or survival in both murine and human B-cell neoplasms.11-16 As reported here, our comprehensive survey of the functional consequences of Notch activation in 13 lines representing multiple subclasses of B-cell neoplasia showed a consistent induction of growth arrest and/or apoptosis. This effect was observed by both expression of constitutively active intracellular Notch, as well as by ligand-induced activation of Notch signaling. Unlike the effect of constitutive Notch signaling in T-ALL in which Notch inhibits differentiation while promoting survival and proliferation,9 Notch signaling failed to promote either survival or proliferation in any of the cell lines assayed. All 4 Notch receptors were capable of inducing B-cell growth inhibition/apoptosis. This is in contrast to T-cell transformation whereby Notch1-3, but not Notch4, induce T-cell leukemia in murine models (J.C.A. and W.S.P., in preparation). Furthermore, the mechanism of this phenomenon appears to depend on HES signaling, as constitutive expression of Hes1 is sufficient to mimic the effect of Notch on B-cell growth inhibition/apoptosis.
Notch-mediated growth inhibition and apoptosis in a range of human B-cell malignancies. (A) Notch1-induced growth inhibition in human Hodgkin and myeloma cell lines. Growth inhibition in Hodgkin (L428) and myeloma (RPMI 8226, U266, NCI-H929) cell lines stably transduced with tamoxifen-inducible ER-ICN1 retrovirus. We measured the percentage of GFP+ cells following exposure to tamoxifen or ethanol control by flow cytometry. This graph shows the ratio of tamoxifen-treated to control EtOH-treated GFP percentage for each sample. Average and standard deviation of triplicate experiments are shown. (B) Notch-induced growth inhibition in 2 human biphenotypic MLL-translocated ALL cell lines. Graph represents mean and standard deviation from triplicate experiments. The P values are from repeated measures ANOVA. (C) Decrease in cycling cells by DRAQ5 staining for DNA content in ICN1 and vector-transduced SEM-K2 cells on days 2, 4, 6, and 8 after transduction. Dotted lines represent untransduced (GFP-) cells, solid lines represent transduced (GFP+) cells. (D) Percentages of cells in stages of cell cycle (G0/G1, S, G2/M) from panel C. Day 0 is pretransduction. Untransduced cells (GFP-) are shown in open symbols. Transduced cells (GFP+) are shown in solid symbols. Duplicate experiments were performed with similar results.
Notch-mediated growth inhibition and apoptosis in a range of human B-cell malignancies. (A) Notch1-induced growth inhibition in human Hodgkin and myeloma cell lines. Growth inhibition in Hodgkin (L428) and myeloma (RPMI 8226, U266, NCI-H929) cell lines stably transduced with tamoxifen-inducible ER-ICN1 retrovirus. We measured the percentage of GFP+ cells following exposure to tamoxifen or ethanol control by flow cytometry. This graph shows the ratio of tamoxifen-treated to control EtOH-treated GFP percentage for each sample. Average and standard deviation of triplicate experiments are shown. (B) Notch-induced growth inhibition in 2 human biphenotypic MLL-translocated ALL cell lines. Graph represents mean and standard deviation from triplicate experiments. The P values are from repeated measures ANOVA. (C) Decrease in cycling cells by DRAQ5 staining for DNA content in ICN1 and vector-transduced SEM-K2 cells on days 2, 4, 6, and 8 after transduction. Dotted lines represent untransduced (GFP-) cells, solid lines represent transduced (GFP+) cells. (D) Percentages of cells in stages of cell cycle (G0/G1, S, G2/M) from panel C. Day 0 is pretransduction. Untransduced cells (GFP-) are shown in open symbols. Transduced cells (GFP+) are shown in solid symbols. Duplicate experiments were performed with similar results.
Nine of 10 human cell lines showed a greater than 50% decrease in relative cell number in a 1-week period using this single intervention. Most strikingly, both of the therapy-resistant MLL-translocated lines underwent greater than 75% decrease in 4 days and greater than 95% after 1 week of treatment. This is of particular significance because the t(4;11) MLL translocation is associated with aggressive leukemias in infants and as second cancers following prior chemotherapy.44 In both cases, these are difficult to cure and often lead to early treatment failure, bone marrow transplantation, and use of experimental therapies. New adjuvant therapies would clearly have significant benefit in treating MLL-translocated leukemias and myelomas. This consistent phenomenon, seen in a variety of malignant human, mouse, and avian B cells, suggests a conserved pathway of Notch-mediated growth inhibition and apoptosis, which may be used as a novel therapeutic approach in the treatment of a wide range of B-cell malignancies.
Our current data and previous studies show that there are multiple ways in which Notch signaling may be induced to cause B-cell growth arrest/apoptosis. Although ICN expression is effective, if expression is not B-cell specific, T-cell transformation may ensue. Ligand-induced Notch activation was also effective for inducing B-cell growth arrest/apoptosis; however, 2 studies described lymphoproliferative disorders and leukemia in mice receiving hematopoietic stem cells (HSCs) transduced with the Notch-ligand Delta-like 4 (DLL4).45,46 These lymphoproliferative disorders were dependent on continuous ligand expression. Nevertheless, Notch receptor expression, especially Notch2, is widespread on malignant B cells, suggesting that pulsed treatments via ligand may be effective. Constitutive Hes1 expression seemed to be equivalent to ICN expression in causing B-cell growth arrest/apoptosis. In contrast to ICN expression, constitutive Hes1 expression in murine HSCs has not been associated with malignancy.47 Thus, small molecules that mimic Hes1 signals or its downstream targets may be efficacious.
The mechanism by which Hes1 promotes B-cell growth arrest/apoptosis is not understood. Hes1 is a bHLH transcriptional repressor. Thus, the mechanism of this effect may be through direct repression of specific genes, although few direct targets have been identified. Alternatively, Hes1 may function independently of its transcriptional activity. For example, Hes1 has been shown to facilitate the interaction of Janus kinase 2 (JAK2) and signal transducer activator of transcription 3 (STAT3), inducing STAT3 phosphorylation,48 suggesting a possible mechanism for altering cytokine signaling. Also, Hes1 has been shown to inhibit E2A reporter activity, suggesting that inhibition of E2A may be a potential mechanism of Notch/Hes1-mediated effects.47 E2A proteins are critical for B-cell development, and inhibition of E2A function can lead to B-cell growth arrest and apoptosis. Several theories have arisen to explain this potential mechanism. Hes1 might compete for E box binding directly, or Hes1 may heterodimerize with E2A proteins through the bHLH domain. Finally, Hes1, like ICN1, may up-regulate Inhibitor of DNA binding (ID) proteins which heterodimerize with and inhibit E2A function. Although inhibition of E2A by Notch/Hes1 is a tempting explanation of our results, we have not consistently seen repression of E2A target gene expression, evidence of heterodimerization on gel shift assays, or up-regulation of ID genes (data not shown). Therefore, it is not clear to us that E2A plays a critical role in this phenomenon, although further evaluation is warranted.
Our findings of Notch-mediated B-cell growth inhibition and apoptosis contrast with several published reports on B-cell malignancies. In human B-cell chronic lymphocytic leukemias (B-CLL), Hubmann et al14 suggested a role for Notch2 in CD23a-mediated proliferation of B-CLLs. However, the outcome of Notch signaling in B-CLL cells was not evaluated in their experiments, and CLL cell lines were not tested in our panel. Because ICN2 was equally able to induce apoptosis in our studies, it is not clear whether Notch2 would provide a proliferative signal through CD23a expression in CLL cells and remains to be tested. In contrast to our findings, Jundt et al15,16 have published 2 studies in which they demonstrated a proliferative effect of exposure to Notch ligand Jagged1 in several Hodgkin-derived cell lines (including the L428 line), and in 3 of 5 myeloma-derived cell lines (including RPMI-8226), with no effect on 2 myeloma lines (U266 and NCI-H929). Four of these cell lines were tested in our study. In comparison to our assays, their findings were based on thymidine incorporation and apoptotic cell counts following 48 hours of Jagged1 exposure. Our findings in the L428 Hodgkin line demonstrate no early proliferative effect and after 96 hours of retrovirally expressed ICN1, a modest decrease in relative proliferation (-20%, P < .01). The myeloma lines U266, NCI-H929, and RPMI-8226 all demonstrated a statistically significant decrease in relative proliferation by 48 hours of ICN1 expression (P < .004) and eventually led to a greater than 50% relative reduction in ICN1-expressing cells (P < .002) (Figure 5A). In support of our findings, Nefedova et al13 demonstrated that Notch signaling decreased thymidine incorporation in the RPMI-8226 and NCI-929 cell lines following 48 hours of coculture with Jagged1-expressing fibroblasts. Ultimately, whether there is an initial increase in DNA replication over the first 48 hours of Notch signaling in these myeloma-derived cells as reported by Jundt et al,16 we observed a uniform pattern of significant growth inhibition in all lines by 48 to 96 hours of ICN1 expression. To assess the potential effect of growth arrest in myeloma cells, Nefedova et al13 demonstrated that induction of Notch signaling in myeloma cell lines led to decreased sensitivity to in vitro single-agent melphalan or mitoxantrone. They hypothesize that this may explain why myelomas are therapy resistant. This warrants further exploration in an in vivo setting.
In summary, we have demonstrated that Notch signaling in a wide variety of malignant murine and human B-cell lines induces growth inhibition and apoptosis. This effect can be mediated either by retroviral expression of ICN1 or Hes1 or through ligand interaction. We have shown that both therapy-responsive (pre-B ALL, Hodgkin) and therapy-resistant (myeloma and MLL-translocated) cell lines undergo Notch-mediated growth inhibition and apoptosis. Thus, Notch/Hes1 signaling has a potential novel therapeutic role in the treatment of a wide range of human B-cell malignancies.
Prepublished online as Blood First Edition Paper, August 23, 2005; DOI 10.1182/blood-2005-01-0355.
Supported by grants from the National Institutes of Health (J.C.A., D.A., and W.S.P.) and a Specialized Center of Research (SCOR) grant from the Leukemia and Lymphoma Society, and the National Cancer Institute (K08CA101934) (P.A.Z.-M.).
P.A.Z.-M. designed and performed the research, analyzed the data, and wrote the manuscript; Y.H. designed and performed the research, analyzed the data, and contributed to the manuscript; W.S.P. contributed to the design of the research and wrote the manuscript; L.X. contributed vital new reagents and performed the research; A.C.C. and J.C.A. contributed vital new reagents; C.G.R., D.A., and F.G.K. performed the research.
P.A.Z.-M. and Y.H. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Stephan Grupp for providing the pre-B ALL lines, Carolyn Felix for the MLL-translocated cell lines, and Jim Riley for the L428 Hodgkin line. We thank members of the Pear Lab for their technical support and critical reading of the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal