Heparin-induced thrombocytopenia (HIT) is caused by platelet-activating IgG antibodies that recognize platelet factor 4 (PF4) bound to heparin. Immunogenicity of heparins differs in that unfractionated heparin (UFH) induces more anti–PF4/heparin antibodies than low-molecular-weight heparin (LMWH) and UFH also causes more HIT. Fondaparinux, a synthetic anticoagulant modeled after the antithrombin-binding pentasaccharide, is believed to be nonimmunogenic. We tested 2726 patients for anti–PF4/heparin antibodies after they were randomized to receive antithrombotic prophylaxis with fondaparinux or LMWH (enoxaparin) following hip or knee surgery. We also evaluated in vitro cross-reactivity of the IgG antibodies generated against PF4 in the presence of UFH, LMWH, danaparoid, or fondaparinux. We found that anti–PF4/heparin antibodies were generated at similar frequencies in patients treated with fondaparinux or enoxaparin. Although antibodies reacted equally well in vitro against PF4/UFH and PF4/LMWH, and sometimes weakly against PF4/danaparoid, none reacted against PF4/fondaparinux, including even those sera obtained from patients who formed antibodies during fondaparinux treatment. At high concentrations, however, fondaparinux inhibited binding of HIT antibodies to PF4/polysaccharide, indicating that PF4/fondaparinux interactions occur. No patient developed HIT. We conclude that despite similar immunogenicity of fondaparinux and LMWH, PF4/fondaparinux, but not PF4/LMWH, is recognized poorly by the antibodies generated, suggesting that the risk of HIT with fondaparinux likely is very low.
Introduction
Fondaparinux (Arixtra; Sanofi-Synthelabo, Paris, France, and Organon, Oss, The Netherlands) is a novel anticoagulant that catalyzes inhibition of factor Xa (but not thrombin) by antithrombin, resulting in the inhibition of thrombin generation.1 Its structure closely resembles the pentasaccharide sequence within heparin that binds to antithrombin. In large clinical trials, fondaparinux has been shown to be at least as effective as a low-molecular-weight heparin (LMWH), enoxaparin (Lovenox; Aventis Pharma, Bridgewater, NJ), in preventing postoperative deep vein thrombosis (DVT) following orthopedic surgery,2 and in the treatment of venous thromboembolism.3,4
Additionally, fondaparinux could have a reduced risk of causing a syndrome resembling heparin-induced thrombocytopenia (HIT), a prothrombotic adverse drug reaction caused by platelet-activating antibodies of IgG class that recognize multimolecular complexes of platelet factor 4 (PF4) bound to heparin.5,6 The frequency of HIT is about 3% to 5% in orthopedic surgery patients treated with unfractionated heparin (UFH) but is less than 1% in patients receiving LMWH.7,8 The reduced risk of HIT could be because LMWH forms smaller, and presumably less immunogenic, complexes with PF4, compared with UFH.9 Although the pentasaccharide, fondaparinux, may bind to PF4 (based on evidence that PF4 binds to sulfated oligosaccharides as small as a tetrasaccharide10 ), its length is shorter than the 10 to 12 saccharides reported for binding to PF4 to result in strong reactivity with HIT antibodies.11,12 Thus, fondaparinux was expected to be nonimmunogenic and unable to cause thrombocytopenia.13
Recently, 2 orthopedic surgery trials compared fondaparinux to the LMWH, enoxaparin, for the prevention of thrombosis after elective knee replacement surgery14 or elective hip replacement surgery.15 The prospective measurement of platelet counts and the serologic assessment of anti–PF4/heparin antibodies in these patients permitted us to determine the frequency and the antigen reaction profiles of anti–PF4/heparin antibodies in these study patients. The findings of our study suggest that fondaparinux may be associated with formation of anti–PF4/heparin antibodies but, in contrast to LMWH, it is unlikely to cause HIT because of the poor reactivity of antibodies against PF4/fondaparinux.
Patients, materials, and methods
Patient study populations
We tested patient sera from 2 randomized, double-blind clinical trials that compared the LMWH, enoxaparin, with fondaparinux, for the prevention of DVT following orthopedic surgery, either elective knee replacement (PENTAMAKS [Pentasaccharide in Major Knee Surgery] trial)14 or elective hip replacement (PENTATHLON [Pentasaccharide in elective hip replacement] 2000 trial).15 Table 1 indicates the number of patients in whom serologic investigations for anti–PF4/heparin antibodies were performed and provides other information such as the scheduling of drug administration, median time from surgery to first study drug dose, and median time from first study drug dose to blood sampling.
Two randomized, double-blind clinical trials comparing enoxaparin and fondaparinux started after orthopedic surgery
Trial and study drug . | No. study patients tested for anti-PF4/heparin antibodies (%)* . | Dosing regimen (timing of first dose after surgery) . | Median time from surgery to blood sampling for anti-PF4/heparin antibodies, d (IQR) . | Median time from first injection of study drug to blood sampling for anti-PF4/heparin antibodies, d†(IQR) . |
|---|---|---|---|---|
| Knee (PENTAMAKS)14 | ||||
| Enoxaparin | 365 (70.6) | 30 mg bid (12-24 h) | 5.72 (3.93, 6.78) | 4.82 (2.92, 5.88) |
| Fondaparinux | 388 (75.0) | 2.5 mg od (4-8 h) | 5.71 (3.93, 6.74) | 5.34 (3.55, 6.38) |
| Hip (PENTATHLON)15 | ||||
| Enoxaparin | 984 (87.2) | 30 mg bid (12-24 h) | 5.77 (4.61, 6.82) | 4.88 (3.77, 5.88) |
| Fondaparinux | 989 (87.7) | 2.5 mg od (4-8 h) | 5.81 (4.65, 6.84) | 5.42 (4.29, 6.47) |
Trial and study drug . | No. study patients tested for anti-PF4/heparin antibodies (%)* . | Dosing regimen (timing of first dose after surgery) . | Median time from surgery to blood sampling for anti-PF4/heparin antibodies, d (IQR) . | Median time from first injection of study drug to blood sampling for anti-PF4/heparin antibodies, d†(IQR) . |
|---|---|---|---|---|
| Knee (PENTAMAKS)14 | ||||
| Enoxaparin | 365 (70.6) | 30 mg bid (12-24 h) | 5.72 (3.93, 6.78) | 4.82 (2.92, 5.88) |
| Fondaparinux | 388 (75.0) | 2.5 mg od (4-8 h) | 5.71 (3.93, 6.74) | 5.34 (3.55, 6.38) |
| Hip (PENTATHLON)15 | ||||
| Enoxaparin | 984 (87.2) | 30 mg bid (12-24 h) | 5.77 (4.61, 6.82) | 4.88 (3.77, 5.88) |
| Fondaparinux | 989 (87.7) | 2.5 mg od (4-8 h) | 5.81 (4.65, 6.84) | 5.42 (4.29, 6.47) |
IQR indicates interquartile range; bid, twice daily; od, once daily.
Percent of study patients given at least one dose of study drug.
The difference in the median number of days between first injection of anticoagulant to blood sampling for anti—PF4/heparin antibodies differs between patients receiving enoxaparin and fondaparinux (right column) because of the trial design, in which fondaparinux was started 4 to 8 hours after surgery, whereas enoxaparin was started 12 to 24 hours after surgery.
As a control for anti–PF4/heparin antibody formation in patients not receiving heparin after orthopedic surgery, we also tested plasma obtained from 112 patients who participated in clinical trials in which the recombinant hirudin, desirudin (Revasc; Aventis, Frankfurt, Germany), was given for the prevention of DVT following elective hip replacement surgery.16,17 The plasma samples for assessment of anti–PF4/heparin antibodies were obtained between postoperative days 5 to 9 (median, day 6).
Laboratory testing for anti–PF4/heparin antibodies
Screening for anti–PF4/heparin antibodies was performed using a commercially available solid-phase enzyme immunoassay (EIA) that detects IgG, IgA, and IgM antibodies (GTI-PF4 ELISA; GTI, Waukesha, WI).12 Sera giving positive results in the screening assay were then tested for each of these 3 immunoglobulin classes, as described.18 Each well in the plate was coated overnight with PF4 (50 μL of 100 μg/mL, assayed by a bicinchoninic acid [BCA; Pierce, Rockford, IL] method using an albumin standard; this corresponds to 20 μg/mL using A280 absorption) and UFH (50 μLof 1.0 IU/mL). Positive and negative controls were included with every plate. Samples with readings greater than or equal to 0.45 absorbance units were considered positive (mean + 3 SD of 100 normal sera).
In addition, we performed a platelet activation assay, the platelet 14C-serotonin release assay, as described.19 We used 10% serotonin release as the cutoff in the assay to detect those samples with anti–PF4/heparin antibodies that had weak platelet-activating properties. This cutoff is more than 3 SD above the mean serotonin release of control sera at all heparin concentrations. We considered as a positive result any sample that gave the following reaction pattern: (1) greater than a mean of 10% serotonin release among the following 4 reaction conditions: 0.1 IU/mL UFH, 0.1 IU/mL UFH plus hirudin 8 U/mL, 0.3 IU/mL UFH, and 0.2 IU/mL enoxaparin (all concentrations final); (2) less than 10% serotonin release at 100 IU/mL UFH; and (3) less than 10% serotonin release in the presence of 0.3 IU/mL UFH and Fc receptor–blocking monoclonal antibody. Hirudin was used to rule out thrombin as the explanation for platelet activation. UFH used for these experiments was Heparin LEO (LEO Pharma, Thornhill, ON, Canada). Enoxaparin was obtained from Aventis Pharma.
Blood samples were usually available on postoperative day 5 or later for testing, along with the prestudy sample, and were stored at –70°C until testing. Studies were performed by personnel blinded to all clinical information, including which study drug the patients received.
The protocol was approved by independent local institutional review boards, and written informed consent was obtained from all patients, according to the Declaration of Helsinki.
Preparation of PF4
PF4 was purified from washed human platelets. In brief, the washed platelets were lysed by 4 freeze-thaw cycles. The lysate was centrifuged at 10 000g at 10°C for 30 minutes. The supernatant was applied to heparin Sepharose 6 fast flow (Amersham Pharmacia Biotech, Uppsala, Sweden). The Sepharose was washed and the bound PF4 was eluted using an increasing salt gradient. The fractions containing PF4 were pooled and the concentration was determined using a BCA protein assay kit. The purified PF4 was determined to be more than 95% pure by analytical sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Biotinylation of the purified PF4 for use in the fluid-phase EIA was performed, as described,20 except that biotin-NHS-ester (Roche Diagnostics, Laval, QC, Canada) was used to label the PF4.
Cross-reactivity of antibodies for PF4/polysaccharide complexes
Samples that tested positive for IgG anti–PF4/heparin antibodies in the solid-phase EIA were tested for cross-reactivity against PF4 bound to either UFH, LMWH, danaparoid, or fondaparinux, using the fluid-phase EIA. IgG antibodies that had been detected in the solid-phase EIA were investigated further in the fluid-phase EIA because the Sepharose beads used in the fluid-phase EIA capture only IgG antibodies.20 Further, clinical HIT is most likely to be caused by antibodies of IgG class.21 Danaparoid sodium was obtained from Organon (Toronto, ON, Canada).
The fluid-phase EIA was performed as described elsewhere,20 with modifications, as follows. A total of 100 μL diluted patient serum (1:10 in phosphate-buffered saline [PBS]–Tween containing 1% bovine serum albumin [BSA]) was mixed with 100 μL purified human PF4 with or without drug for 1 hour at room temperature. All concentrations (final) were based on the 200-μL reaction mixture, consisting of PF4 10 μg/mL (20% biotinylated) and one of the following drugs (or buffer): UFH, 0.6 IU/mL; enoxaparin, 0.5 anti–factor Xa U/mL; danaparoid, 0.1 anti–factor Xa U/mL; fondaparinux, 0.1 μg/mL, 0.4 μg/mL, 1.2 μg/mL, and 10 μg/mL. These drug concentrations are pharmacologically relevant, including those for fondaparinux given during antithrombotic prophylaxis (about 0.3 μg/mL) or therapy (about 1.4 μg/mL).22 These concentrations of PF4, drug, and patient serum were used because preliminary experiments using positive HIT sera showed maximal reactivity at these concentrations of UFH, LMWH, and danaparoid.
The IgG complexes were captured by incubation of the 200-μL reaction mixture with 50 μL (settled volume) protein G Sepharose beads (Amersham Pharmacia Biotech) for 1 hour at room temperature. The Sepharose beads were washed 5 times with 1 mL PBS-Tween buffer. Next, 500 μL streptavidin-horseradish peroxidase (HRP; 1:5000 in PBS-Tween/BSA buffer; Amersham Pharmacia Biotech) was added to the Sepharose beads for 30 minutes. The Sepharose was washed as before and 400 μL K-Blue Max Substrate (Neogen, Lexington, KY) was added, and mixed for 5 minutes. The Sepharose was pelleted and an aliquot of supernatant was added to an equal volume of 1 M HCl to stop the color reaction. The absorbance was read at A450 nm using a Microplate Reader (model 550; Bio-Rad, Mississauga, ON, Canada). Known positive and negative HIT sera were used as controls. Danaparoid was included as a positive control for detection of weak cross-reactivity (which occurs in up to 15%-40% of HIT sera).20
Interpretation of antibody studies
For patients in the fondaparinux versus enoxaparin trials, we classified the patients who formed anti–PF4/heparin antibodies into 2 groups. Group 1 included patients who formed anti–PF4/heparin antibodies of IgG class and had a negative baseline. Group 2 included all other patients who either formed only IgM or IgA or both (but not IgG) anti–PF4/heparin antibodies or who tested positive at baseline, but subsequently developed at least a 2-fold increase (by change in optical density [OD]) in any EIA. All sera from groups 1 and 2 were tested in the platelet activation assay.
The primary serologic end point (determined a priori) consisted of sera classified as group 1, that is, those patients who formed anti–PF4/heparin antibodies of the IgG class. This end point was chosen because IgG class antibodies are more likely to cause HIT than antibodies of other classes.21 All other end points were secondary end points.
Inhibition of anti–PF4/polysaccharide reactivity of HIT sera by fondaparinux in the EIA
We investigated whether increasing concentrations of fondaparinux (100, 200, 400, and 1000 μg/mL, final) inhibited reactivity of sera from patients with clinical HIT (n = 5) in the solid-phase EIA and in the fluid-phase EIA. Buffer and high concentrations of UFH (100 IU/mL) or enoxaparin (100 anti-Xa U/mL) served as controls. For both assays, the PF4/polysaccharide (UFH or enoxaparin) antigen complexes were prepared at concentrations that reacted optimally in preliminary experiments (described in “Cross-reactivity of antibodies for PF4/polysaccharide complexes”). After overnight incubation (solid-phase EIA) or after a minimum of 1 hour (fluidphase EIA), the high (inhibitory) concentrations of polysaccharide (fondaparinux, UFH, or enoxaparin) were added together with patient serum, and the assay was performed as described in “Cross-reactivity of antibodies for PF4/polysaccharide complexes”).
Definition of HIT
HIT was defined as a 50% or greater fall in platelet count from the postoperative peak platelet count (usually after postoperative day 4) in association with formation of anti–PF4/heparin antibodies (by EIA) and a positive platelet activation assay.23
Incidental heparin exposure
We obtained information on potential perioperative heparin exposures from the medical records of patients identified as having formed anti–PF4/heparin antibodies in the hip replacement trial. (Information on perioperative heparin exposures was not available for the knee replacement trial.)
Statistics
Because blood samples for serologic testing were taken at variable times following initiation of study drug, it was important to consider this when estimating the distribution of seroconversion. We used “current status” methods for analysis. This method was used because a negative test can result either from no seroconversion or testing before seroconversion. The current status method generates a Kaplan-Meier type of estimate of the proportion of subjects experiencing the event of interest (antibody formation) over time. Log-rank tests (modified for the current status data set) were conducted for comparisons between groups.24,25 In addition, crude analyses were conducted based on the proportions of patients seroconverting using a 2-sided Fisher exact test at the 5% level.26 The times between first study drug injection, surgery, and blood testing were summarized using medians and quartiles. Hypothesis tests regarding seroconversion rates were carried out based on Fisher exact test for 2-sample comparisons (eg, when seroconversion rates between patients receiving fondaparinux and enoxaparin are compared) and the McNemar test27 when estimated rates were correlated because the classifications were based on multiple designations for each subject in a single sample (ie, when different test results are compared within a particular sample). One-sample t tests (2-sided) were performed on the ratio (null hypothesis, ratio = 1.0) of the OD value for binding of antibodies to PF4 in the presence of drug divided by baseline OD value (binding to PF4 in the presence of buffer). Paired t tests (2-sided) were performed to assess significance of inhibition of fondaparinux in comparison with buffer control.
Results
Table 2 shows the results of antibody investigations (categorical analysis) for the 2 clinical trials comparing fondaparinux and enoxaparin. The proportion of patients forming anti–PF4/heparin antibodies was similar for both enoxaparin and fondaparinux treatment groups, including the primary serologic end point of IgG antibody formation (group 1) and total antibody formation (groups 1 and 2 combined). Sera from 4 patients in group 1 (3 of whom had received fondaparinux) tested positive for heparin-dependent platelet-activating antibodies by platelet activation assay. None of these patients had a fall in platelet count.
Categorical analysis of anti–PF4/heparin antibody seroconversion events
Study and study drug . | No. patients . | Group 1, no. (%)* . | P . | Group 2, no. (%)† . | P . | Combined, no. (%) . | P . |
|---|---|---|---|---|---|---|---|
| Knee (PENTAMAKS)14 | > .999 | .07 | .13 | ||||
| Enoxaparin | 365 | 5 (1.4) | 14 (3.8) | 19 (5.2) | |||
| Fondaparinux | 388 | 5 (1.3) | 6 (1.5) | 11 (2.8) | |||
| Hip (PENTATHLON)15 | .37 | > .999 | .55 | ||||
| Enoxaparin | 984 | 1 (0.1) | 10 (1.0) | 11 (1.1) | |||
| Fondaparinux | 989 | 4 (0.4) | 11 (1.1) | 15 (1.5) |
Study and study drug . | No. patients . | Group 1, no. (%)* . | P . | Group 2, no. (%)† . | P . | Combined, no. (%) . | P . |
|---|---|---|---|---|---|---|---|
| Knee (PENTAMAKS)14 | > .999 | .07 | .13 | ||||
| Enoxaparin | 365 | 5 (1.4) | 14 (3.8) | 19 (5.2) | |||
| Fondaparinux | 388 | 5 (1.3) | 6 (1.5) | 11 (2.8) | |||
| Hip (PENTATHLON)15 | .37 | > .999 | .55 | ||||
| Enoxaparin | 984 | 1 (0.1) | 10 (1.0) | 11 (1.1) | |||
| Fondaparinux | 989 | 4 (0.4) | 11 (1.1) | 15 (1.5) |
Group 1 defined as formation of anti-PF4/heparin antibodies of IgG class from a negative baseline. Four patients in group 1 tested positive for heparin-dependent platelet-activating antibodies in the serotonin release assay (knee study: 1 in enoxaparin group, 2 in fondaparinux group; hip study: 1 in fondaparinux group).
Group 2 consisted of all non—group 1 patients who had evidence for an immune response, including the formation of IgM or IgA or both (but not IgG) anti—PF4/heparin antibodies or who had a positive test at baseline, but subsequently developed at least a 2-fold increase in reactivity in the EIA (by change in OD).
Fifty patients (25 each receiving enoxaparin or fondaparinux) formed anti–PF4/heparin antibodies from a negative baseline. (Another 6 patients included within group 2 who developed at least a 2-fold increase in reactivity from a positive baseline in the EIA are not included in this analysis of antibody class distribution.) Among these, 15 (30%) formed antibodies of the IgG class (enoxaparin, n = 6; fondaparinux, n = 9; group 1 in Table 2). Whereas 4 (26.7%) of these 15 sera exhibited heparin-dependent, platelet-activating properties, none of the 35 antibody-positive sera that did not contain IgG class antibodies had heparin-dependent platelet-activating properties (P = .006). IgA and IgM class antibodies (alone or with IgG) were generated in the same number of patients (IgA, n = 15; IgM, n = 15; both IgA and IgM, n = 12), with no significant differences between patients receiving enoxaparin or fondaparinux.
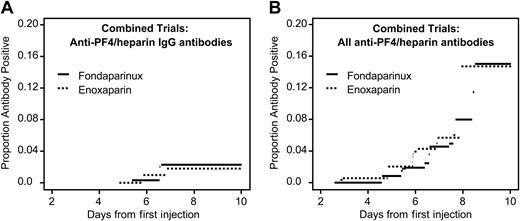
Anti–PF4/heparin antibody formation in patients receiving fondaparinux or enoxaparin after orthopedic surgery (current status analysis). Data are combined for patients undergoing knee and hip replacement. (A) Anti–PF4/heparin antibodies of IgG class. There is no significant difference between the groups (P = .86). (B) All anti–PF4/heparin antibodies. There is no significant difference between the study drug groups (P = .24).
Anti–PF4/heparin antibody formation in patients receiving fondaparinux or enoxaparin after orthopedic surgery (current status analysis). Data are combined for patients undergoing knee and hip replacement. (A) Anti–PF4/heparin antibodies of IgG class. There is no significant difference between the groups (P = .86). (B) All anti–PF4/heparin antibodies. There is no significant difference between the study drug groups (P = .24).
Figure 1 shows the seroconversion rates over time (current status analysis). No significant differences between fondaparinux and enoxaparin were observed for any of the serologic end points. In only one subgroup analysis (knee replacement surgery) was a nonsignificant trend for lower frequency of total anti–PF4/heparin antibodies observed in patients receiving fondaparinux (P = .058) (not shown).
None of the 112 patients who received desirudin for antithrombotic prophylaxis following hip replacement surgery developed anti–PF4/heparin antibodies, consistent with the hypothesis that the antibodies detected in the clinical trials were related at least in part to postoperative administration of enoxaparin or fondaparinux, rather than because of a nonspecific effect of orthopedic surgery.
Incidental heparin exposure
Twenty-six patients in the hip replacement trial developed anti–PF4/heparin antibodies (15 receiving fondaparinux, 11 receiving enoxaparin). Of these, 19 (73.1%) had received no incidental exposure to heparin. The 7 remaining patients (4 receiving fondaparinux, 3 receiving enoxaparin) had possible incidental exposure to small amounts of UFH (1 arterial line, 1 central venous line, 2 cell saver devices, and 3 with a combination of these). These observations suggest that incidental heparin exposure was unlikely to account for the majority of anti–PF4/heparin antibody seroconversion events. Information regarding incidental heparin exposure in the knee replacement trial was not available.
Antibody reactivity against PF4/polysaccharides
Fifteen patient samples (group 1) tested positive for anti–PF4/heparin antibodies of IgG class by solid-phase EIA in comparison with a negative baseline testing. In 9 of these patients, antibody formation was associated with fondaparinux treatment, whereas in 6, enoxaparin treatment had been given. By fluid-phase EIA, no differences in anti–PF4/polysaccharide reactivity profiles were seen regardless of whether the antibodies were generated during treatment with fondaparinux or enoxaparin (Figure 2). All 15 sera reacted against PF4/enoxaparin, whereas none reacted against PF4/fondaparinux. Notably, even those 9 patients whose antibodies were formed while receiving fondaparinux did not react against PF4/fondaparinux, even though they reacted against PF4/heparin, including 3 that showed heparin-dependent platelet activation in the serotonin release assay.
Inhibition of anti–PF4/polysaccharide reactivity of HIT sera by fondaparinux in the EIA
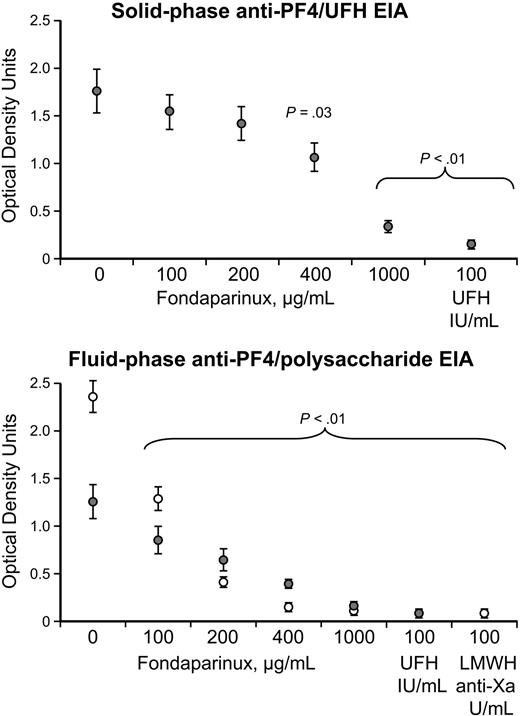
Figure 3 shows that increasing concentrations of fondaparinux inhibited reactivity of anti–PF4/heparin reactivity of HIT sera in the solid-phase anti–PF4/heparin EIA, as well as in the fluid-phase anti–PF4/polysaccharide (UFH or enoxaparin) EIAs. Significant inhibition was observed at a fondaparinux concentration of 400 μg/mL (or greater) in the solid-phase EIA and was seen at fondaparinux concentrations of 100 μg/mL (or greater) in the fluid-phase EIA.
Discussion
LMWH carries a lower risk of HIT than does UFH.7,8,28 A lower frequency of immunization7,8,29,30 is believed to be a major factor that explains a lower risk of HIT with LMWH, particularly because both UFH and LMWH preparations yield similar magnitude of reactivity in vitro in assays used to detect the antibodies that cause HIT.7,20,31 This corresponds to the known risk of persisting or recurring thrombocytopenia if LMWH is used to treat HIT caused by UFH.32
In this report, we describe our studies comparing the frequency and reactivity profiles of anti–PF4/heparin antibodies in 2 large clinical trials of patients receiving fondaparinux or LMWH after orthopedic surgery. Our hypothesis was that treatment with the pentasaccharide, fondaparinux, would not be associated with formation of anti–PF4/heparin antibodies. In contrast, we expected some patients receiving LMWH to develop anti–PF4/heparin antibodies,7,8,29,30 and potentially even HIT. This hypothesis was based on previous observations indicating that although heparin molecules of tetrasaccharide length or greater bind to PF4,10 they do not create the antigens recognized by HIT antibodies unless they are at least about 8 to 10 saccharides in length or greater.11,12 LMWH (mean, 15 saccharide units33 ) but not the pentasaccharide exceeds this minimum length. Further, previous studies have shown that serum obtained from patients with HIT contain anti–PF4/heparin antibodies that consistently fail to react against PF4 in the presence of fondaparinux.34,35 For these reasons, it seemed probable that fondaparinux would not be associated with antibody formation, or at least would show a significantly lower frequency of antibody formation than LMWH.
Ratio of antibody binding to PF4/polysaccharide complexes compared to PF4 alone by fluid-phase EIA. Results of fluid-phase EIA testing for sera from 15 patients who formed anti–PF4/heparin-IgG antibodies (detected using solid-phase EIA) while receiving enoxaparin (n = 6, •) or fondaparinux (n = 9, ○). The data are expressed as ratios of binding to PF4 in the presence of polysaccharide (UFH, 0.6 IU/mL; LMWH, 0.5 anti-Xa U/mL; danaparoid, 0.1 anti-Xa U/mL; and fondaparinux, 0.1, 0.4, 1.2, and 10.0 μg/mL) over the baseline (buffer). Horizontal bars indicate medians. * indicate the 4 samples that tested positive (in the presence of UFH) in the platelet activation assay. For comparison, results are also shown for 15 patients with clinical HIT (▪). Statistically significant increases in reactivity (null hypothesis, mean ratio of OD [presence of drug]/OD [presence of buffer] = 1) for the 15 sera obtained from patients in the orthopedic trials were observed for UFH (P = .003), LMWH (P < .001), danaparoid (P = .002), but not with fondaparinux at any concentration (P > .05). Whereas 14 of 15 sera from patients in the orthopedic trials exhibited more than 2-fold greater reactivity than baseline against PF4/LMWH, none reacted similarly against PF4/fondaparinux (P < .001 by the McNemar test, 2-tailed).
Ratio of antibody binding to PF4/polysaccharide complexes compared to PF4 alone by fluid-phase EIA. Results of fluid-phase EIA testing for sera from 15 patients who formed anti–PF4/heparin-IgG antibodies (detected using solid-phase EIA) while receiving enoxaparin (n = 6, •) or fondaparinux (n = 9, ○). The data are expressed as ratios of binding to PF4 in the presence of polysaccharide (UFH, 0.6 IU/mL; LMWH, 0.5 anti-Xa U/mL; danaparoid, 0.1 anti-Xa U/mL; and fondaparinux, 0.1, 0.4, 1.2, and 10.0 μg/mL) over the baseline (buffer). Horizontal bars indicate medians. * indicate the 4 samples that tested positive (in the presence of UFH) in the platelet activation assay. For comparison, results are also shown for 15 patients with clinical HIT (▪). Statistically significant increases in reactivity (null hypothesis, mean ratio of OD [presence of drug]/OD [presence of buffer] = 1) for the 15 sera obtained from patients in the orthopedic trials were observed for UFH (P = .003), LMWH (P < .001), danaparoid (P = .002), but not with fondaparinux at any concentration (P > .05). Whereas 14 of 15 sera from patients in the orthopedic trials exhibited more than 2-fold greater reactivity than baseline against PF4/LMWH, none reacted similarly against PF4/fondaparinux (P < .001 by the McNemar test, 2-tailed).
Inhibition of anti–PF4/polysaccharide reactivity by fondaparinux. (Top) Solid-phase anti–PF4/UFH EIA. Mean (± SEM) reactivity of 5 HIT sera is shown in the absence (buffer) and presence of increasing concentrations of fondaparinux (100, 200, 400, or 1000 μg/mL, final). Progressive inhibition of reactivity is seen that is significant at all concentrations of fondaparinux. As expected, high heparin (100 IU/mL) also inhibited reactivity. (Bottom) Fluid-phase anti–PF4/UFH (•) and anti–PF4/LMWH (○) EIAs. Mean (± SEM) reactivity of 5 HIT sera is shown in the absence (buffer) and presence of increasing concentrations of fondaparinux (100, 200, 400, or 1000 μg/mL, final). Progressive inhibition of reactivity is seen that is significant at all concentrations of fondaparinux. High heparin (100 IU/mL) and enoxaparin (100 IU/mL) concentrations also inhibited reactivity.
Inhibition of anti–PF4/polysaccharide reactivity by fondaparinux. (Top) Solid-phase anti–PF4/UFH EIA. Mean (± SEM) reactivity of 5 HIT sera is shown in the absence (buffer) and presence of increasing concentrations of fondaparinux (100, 200, 400, or 1000 μg/mL, final). Progressive inhibition of reactivity is seen that is significant at all concentrations of fondaparinux. As expected, high heparin (100 IU/mL) also inhibited reactivity. (Bottom) Fluid-phase anti–PF4/UFH (•) and anti–PF4/LMWH (○) EIAs. Mean (± SEM) reactivity of 5 HIT sera is shown in the absence (buffer) and presence of increasing concentrations of fondaparinux (100, 200, 400, or 1000 μg/mL, final). Progressive inhibition of reactivity is seen that is significant at all concentrations of fondaparinux. High heparin (100 IU/mL) and enoxaparin (100 IU/mL) concentrations also inhibited reactivity.
In contrast to our hypothesis, we found that the frequency of forming anti–PF4/heparin antibodies was the same for patients receiving fondaparinux or enoxaparin. This was true regardless of how the immune response was measured, for example, as any antibody class capable of binding to PF4/heparin, or as IgG-mediated, heparin-dependent platelet activation.
As a nonheparin control, we assessed 112 patients who received the recombinant protein, desirudin, for anticoagulation after orthopedic surgery. None of these patients developed anti–PF4/heparin antibodies. Nevertheless, we cannot exclude the possibility that in some patients, major surgery itself could trigger antibodies reactive against PF4/polysaccharide complexes. This would require serologic investigation of large numbers of patients undergoing major surgery without any heparin exposure.
The immunologic basis of antibody formation in HIT remains poorly understood. It is known that one or more neoepitopes on PF418,36,37 are created when PF4 forms complexes with heparin (or certain other polyanions36,38-40 ) at an optimal PF4-heparin stoichiometric ratio. However, unlike these other molecules, our studies show fondaparinux is unique in that although its administration can be associated with formation of anti–PF4/heparin antibodies that are indistinguishable from those generated during LMWH therapy, these antibodies do not bind well to PF4/fondaparinux, at least under the physicochemical conditions we used. Presumably, the antigens must be expressed on PF4 in the presence of fondaparinux (so as to effect immunization) despite the difficulty in demonstrating this binding in vitro by ourselves (using the fluid-phase EIA) and others (using solid-phase EIA34,35 and platelet activation assays35 ). However, evidence that fondaparinux does interact with PF4 in a way that influences antigen expression is suggested by our observation that very high (suprapharmacologic) concentrations of fondaparinux inhibit binding of anti–PF4/polysaccharide antibodies within HIT sera in both solid-phase and fluid-phase EIAs (Figure 3). Thus, despite a similar frequency of inducing immunization, fondaparinux and enoxaparin differ considerably in their capacity to form the antigens in vitro. As discussed subsequently, these in vitro differences in cross-reactivity appear to correspond with differing in vivo risks of inducing acute thrombocytopenia depending on whether LMWH or fondaparinux is used to treat a patient with clinical HIT.
The poor binding of anti–PF4/heparin antibodies to PF4/fondaparinux in a purified in vitro system does not rule out other more complex interactions that could occur in vivo, such as clustering of PF4 by fondaparinux in the presence of endothelial cell heparan sulfate. Such interactions could produce alterations of PF4 structure sufficient to induce an immune response. Indeed, a preliminary report41 provides evidence that close approximation of PF4 molecules due to charge neutralization that occurs when negatively charged polysaccharide binds to positively charged PF4 is an important step in the formation of the antigens recognized by HIT antibodies.
Our observations have certain clinical implications. First, our study suggests that a syndrome resembling HIT will occur even less frequently (or not at all) with fondaparinux compared with LMWH. This hypothesis is based on the concept that even if anti–PF4/heparin antibodies are generated in a few patients during treatment with fondaparinux, these antibodies are not able to bind well to PF4/fondaparinux complexes, and thus would not be able to activate platelets in the presence of fondaparinux. Indeed, 3 patients who received fondaparinux did develop anti–PF4/heparin antibodies of IgG class, together with a positive heparin-dependent platelet activation assay (Table 2). However, none of these patients developed thrombocytopenia despite receiving fondaparinux for 5 to 7 days. Potentially, such positive testing for antibodies could cause diagnostic confusion if a patient developed thrombocytopenia from another cause.
Second, our findings also suggest that fondaparinux might even be a safe anticoagulant for patients with HIT induced by UFH or LMWH, as suggested by anecdotal reports.42-44 This contrasts with the situation of LMWH being used to treat HIT caused by UFH, in which at least half the patients treated develop recurrent thrombocytopenia or thrombosis.32,45 However, prospective clinical studies need to be done to confirm this hypothesis.
Third, because the antibodies generated during treatment with fondaparinux are otherwise indistinguishable in their biologic characteristics from those generated during treatment with heparin, it also follows that a patient who forms potent heparin-dependent platelet-activating antibodies during treatment with fondaparinux could develop acute HIT if UFH or LMWH is subsequently given.
The discrepancy between apparent immunogenicity of fondaparinux and its poor cross-reactivity with anti–PF4/polysaccharide antibodies differs from UFH, LMWH, and other polyanions that can initiate an immune response against conformationally altered PF4. The low capacity of fondaparinux to form the antigens on PF4 may contribute to reduce further, or perhaps even avoid, the most frequent immune-mediated adverse drug reaction associated with anticoagulant therapy.
Prepublished online as Blood First Edition Paper, August 18, 2005; DOI 10.1182/blood-2005-05-1938.
Supported by Sanofi-Synthelabo and Organon, by the Heart and Stroke Foundation of Ontario (operating grants T-4502 and T-5207), and by the National Heart, Lung and Blood Institute of the National Institutes of Health (RO-1 HL 074051). All investigators performed the serologic studies and data analyses and made the decision to submit the manuscript for publication.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. Ratio of antibody binding to PF4/polysaccharide complexes compared to PF4 alone by fluid-phase EIA. Results of fluid-phase EIA testing for sera from 15 patients who formed anti–PF4/heparin-IgG antibodies (detected using solid-phase EIA) while receiving enoxaparin (n = 6, •) or fondaparinux (n = 9, ○). The data are expressed as ratios of binding to PF4 in the presence of polysaccharide (UFH, 0.6 IU/mL; LMWH, 0.5 anti-Xa U/mL; danaparoid, 0.1 anti-Xa U/mL; and fondaparinux, 0.1, 0.4, 1.2, and 10.0 μg/mL) over the baseline (buffer). Horizontal bars indicate medians. * indicate the 4 samples that tested positive (in the presence of UFH) in the platelet activation assay. For comparison, results are also shown for 15 patients with clinical HIT (▪). Statistically significant increases in reactivity (null hypothesis, mean ratio of OD [presence of drug]/OD [presence of buffer] = 1) for the 15 sera obtained from patients in the orthopedic trials were observed for UFH (P = .003), LMWH (P < .001), danaparoid (P = .002), but not with fondaparinux at any concentration (P > .05). Whereas 14 of 15 sera from patients in the orthopedic trials exhibited more than 2-fold greater reactivity than baseline against PF4/LMWH, none reacted similarly against PF4/fondaparinux (P < .001 by the McNemar test, 2-tailed).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/12/10.1182_blood-2005-05-1938/2/m_zh80240587400002.jpeg?Expires=1767745426&Signature=5Bj8p5SovxjOP1MOvHzAaA5nhK7kBk8XGq2F57PIe2lLYckI19Lyx2pzS~Y9pr6V-YybY~f62M~8nA5OXAKPjB88cT0ADSVYejTsjh9W2FvSsJ3oE72QGFPR0Jed1h16fOt-Q0CIivAmk0oETfKSpONTT9GM9M6CcHY~2g-fjTAfYXSEb6e5C1D2Or6FGC~hU-YVnlx9XgY6j2J0O2tHo0J~h1YT8dlJOGQmBALQPBppX8KK4hi7gXUt-qhrFh6MKUoME3FjYA0XUi~XThi5nVfLVnAcYKquhcHnWpnQgI6QZJm5YoMFCEp8Zj6iPwLwJFZ6OAzUYf-~yYoWyQSnEQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal