Abstract
A number of prognostic factors affect outcome in patients with relapsed or primary refractory diffuse large B-cell lymphoma (DLBCL), including refractory disease and the second-line age-adjusted international prognostic index. In de novo DLBCL, the cell of orgin, as determined by expression microarray analysis or immunohistochemistry (IHC), predicts event-free survival (EFS). We evaluated the cell of origin, as well as other pathologic markers of outcome, on the repeat biopsy specimen of 88 transplantation-eligible patients undergoing ifosfamide, carboplatin, etoposide (ICE) second-line chemotherapy (SLT) followed by high-dose therapy (HDT) and autologous stem cell transplantation (ASCT) to see if were they prognostic in the salvage setting. Pretreatment clinical factors were well balanced between the cohorts. There was no significant difference in response to SLT, HDT, event-free or overall survival based on the cell of origin or any of the common pathologic markers examined. The cell of origin as determined by IHC does not predict outcome in transplantation-eligible patients with relapsed or primary refractory DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous disorder that can be subdivided into several subgroups by distinctive patterns of gene expression.1-3 Clinical outcomes can be divided into 2 groups by cell of origin: germinal center phenotype (GC) versus non-GC. These results have been translated into a clinically applicable approach using immunohistochemistry on tissue microarray (TMA).4 Tumors were classified as GC versus non-GC based on 3 markers, CD10, BCL6, and MUM1 (Table 1), and the outcome difference was confirmed (5-year overall survival 76% for GC vs 34% for non-GC).4
Definitions of tumor phenotype by immunohistochemistry
. | CD10 . | BCL6 . | MUM1 . |
|---|---|---|---|
| GC phenotype | |||
| 1 | + | ± | ± |
| 2 | - | + | - |
| Non-GC phenotype | |||
| 1 | - | + | + |
| 2 | - | - | ± |
. | CD10 . | BCL6 . | MUM1 . |
|---|---|---|---|
| GC phenotype | |||
| 1 | + | ± | ± |
| 2 | - | + | - |
| Non-GC phenotype | |||
| 1 | - | + | + |
| 2 | - | - | ± |
Molecular signatures associated with outcome of patients with relapsed and refractory disease have not been reported. Lack of research in this setting stems from the following: nonuniform second-line therapy (SLT); incomplete clinical databases; and lack of biopsy specimens in patients who relapse. Since 1993, we have conducted sequential studies of ifosfamide, carboplatin, etoposide (ICE) chemotherapy as SLT for relapsed and primary refractory DLBCL requiring a biopsy prior to SLT.5-9 In this analysis, we sought to determine if the cell of origin was an important factor in the outcome of patients with relapsed and refractory DLBCL. In addition, we wanted to determine if a number of other commonly reported pathologic markers in de novo DLBCL were prognostic in the second-line setting.
Study design
Patients
The study group consisted of 150 transplantation-eligible patients with relapse/primary refractory DLBCL enrolled in one of 3 institutional review board–approved clinical trials between March 1993 and April 2001; the clinical outcome has already been reported.8 All patients received ICE-based SLT; no patient received rituximab. Tissue suitable for construction of a TMA was available on 88 patients and with RNA for expression profiling on 17 cases. All patients were on an institutional review board–approved protocol and informed consent was provided according to the Declaration of Helsinki.
Methods
TMAs were constructed from the pre-ICE biopsy specimen as previously published.10 Consecutive TMA sections were stained with CD10, bcl-6, and MUM1 to determine GC versus non-GC molecular phenotype as well as with bcl-2, p53, MUC-1, MDR, and MIB-1; positive and negative cutoffs were previously reported.11 Expression profiling was performed using Affymetrix HG-U95A arrays as described previously.12 Specimens from this cohort were classified as GC versus non-GC using 18 of the 27 genes in the Wright classifier13 present on the HG-U95A array.
Statistics
The hypothesis of this study was that the cell of origin, as defined by immunohistochemistry, of the relapse/refractory biopsy specimen would be predictive of overall survival (OS) and event-free survival (EFS). An event was defined as progression of disease, secondary malignancy, death from toxicity, or death from DLBCL. OS and EFS curves were generated by the Kaplan-Meier method. Given the sample size, the probability of missing a hazard ratio more than 2.5 is less than 12%. The association of the clinical and pathologic variables on these endpoints was examined using the log-rank test. The cutoff for univariate significance was .05.
Results and discussion
In the past 10 years our group has conducted prospective intent-to-treat clinical trials using dose-dense SLT followed by high-dose therapy (HDT) and autologous stem cell transplantation (ASCT) for patients with chemosensitive DLBCL.5-9 In the context of these studies we have established ICE as a highly active regimen for SLT; shown that a complete response (CR) to ICE improves long-term EFS; reported that patients with primary refractory disease that is chemoresponsive to ICE have the same outcome as patients with chemosensitive relapsed disease; identified and validated a simple 3-factor prognostic model (stage, lactate dehydrogenase [LDH], and performance status), evaluated at the initiation of ICE chemotherapy, that predicts outcome; and lastly, showed that the addition of rituximab to the ICE regimen increases the CR rate pre-HDT.
Unfortunately, despite a large database of patients with relapsed and refractory disease the outcome of patients with identical clinical parameters varies significantly. For example, in the most favorable patient, one with relapsed disease and favorable risk factors in CR before HDT, EFS is only 66%. We wanted to test the hypothesis that markers of prognosis important in untreated DLBCL, such as cell of origin and others, would impact outcome in the second-line setting.
The median follow-up of surviving patients is 6.5 years. The actuarial EFS and OS are 29.5% and 39%, respectively; for the patients who underwent transplantation it is 39% and 54%. The patient characteristics (Table 1) and outcomes of this subset of 88 patients are similar to the entire population of patients with DLBCL (data not shown).8
The median age of the patients was 49 years, and 72% were male. Based on the second-line AAIPI, 48 patients (55%) were prognostically favorable; 40 patients (45%) had primary refractory DLBCL (Table 2). There were 65 patients (74%) who had chemosensitive disease to ICE SLT. Using the immunohistochemical model, 28 patients had GC and 60 patients had non-GC DLBCL. We were able to obtain the original biopsy specimen on 21 of the 88 patients, and there was 100% concordance with the relapsed/refractory biopsy specimen concerning cell of origin using the Hans algorithm. There were minor differences that did not affect classification: loss of BCL6 expression in 5 cases and loss of MUM1 expression in 4 cases.
Patient and disease characteristics: GC versus non-GC
. | GC cases . | . | Non-GC cases . | . | ||
|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | ||
| Total patients | 28 | 32 | 60 | 68 | ||
| AAIPI | ||||||
| Low | 3 | 11 | 12 | 20 | ||
| Low-Intermediate | 7 | 25 | 12 | 20 | ||
| High-Intermediate | 12 | 43 | 21 | 35 | ||
| High | 6 | 21 | 15 | 25 | ||
| LDH | ||||||
| Normal | 12 | 43 | 24 | 40 | ||
| Elevated | 16 | 57 | 36 | 60 | ||
| KPS | ||||||
| ≥ 80 | 18 | 64 | 41 | 68 | ||
| < 80 | 10 | 36 | 19 | 32 | ||
| Stage | ||||||
| I/II | 5 | 18 | 16 | 27 | ||
| III/IV | 23 | 82 | 44 | 73 | ||
| Age | ||||||
| ≤ 60 | 21 | 75 | 53 | 88 | ||
| > 60 | 7 | 25 | 7 | 12 | ||
| Extranodal sites | ||||||
| < 2 | 14 | 50 | 39 | 65 | ||
| ≥ 2 | 14 | 50 | 21 | 35 | ||
| Previous response | ||||||
| Relapse | 14 | 50 | 34 | 57 | ||
| Refractory | 14 | 50 | 26 | 43 | ||
| EFS (GC vs non-GC), P = .52 | 28/7 | 25 | 60/18 | 29 | ||
| OS (GC vs non-GC), P = .68 | 28/11 | 35 | 60/22 | 38 | ||
| ICE response | ||||||
| CR/PR | 22 | 79 | 43 | 72 | ||
| Failure | 6 | 21 | 17 | 28 | ||
| EFS/ASCT, P = .52 | 22/7 | 32 | 43/18 | 40 | ||
| OS/ASCT, P = .94 | 22/11 | 50 | 43/21 | 51 | ||
| Response to transplant | ||||||
| CR/PR | 15 | 54 | 27 | 45 | ||
| Failure | 4 | 14 | 10 | 17 | ||
| Relapse after transplant | 10 | 36 | 17 | 28 | ||
. | GC cases . | . | Non-GC cases . | . | ||
|---|---|---|---|---|---|---|
. | No. . | % . | No. . | % . | ||
| Total patients | 28 | 32 | 60 | 68 | ||
| AAIPI | ||||||
| Low | 3 | 11 | 12 | 20 | ||
| Low-Intermediate | 7 | 25 | 12 | 20 | ||
| High-Intermediate | 12 | 43 | 21 | 35 | ||
| High | 6 | 21 | 15 | 25 | ||
| LDH | ||||||
| Normal | 12 | 43 | 24 | 40 | ||
| Elevated | 16 | 57 | 36 | 60 | ||
| KPS | ||||||
| ≥ 80 | 18 | 64 | 41 | 68 | ||
| < 80 | 10 | 36 | 19 | 32 | ||
| Stage | ||||||
| I/II | 5 | 18 | 16 | 27 | ||
| III/IV | 23 | 82 | 44 | 73 | ||
| Age | ||||||
| ≤ 60 | 21 | 75 | 53 | 88 | ||
| > 60 | 7 | 25 | 7 | 12 | ||
| Extranodal sites | ||||||
| < 2 | 14 | 50 | 39 | 65 | ||
| ≥ 2 | 14 | 50 | 21 | 35 | ||
| Previous response | ||||||
| Relapse | 14 | 50 | 34 | 57 | ||
| Refractory | 14 | 50 | 26 | 43 | ||
| EFS (GC vs non-GC), P = .52 | 28/7 | 25 | 60/18 | 29 | ||
| OS (GC vs non-GC), P = .68 | 28/11 | 35 | 60/22 | 38 | ||
| ICE response | ||||||
| CR/PR | 22 | 79 | 43 | 72 | ||
| Failure | 6 | 21 | 17 | 28 | ||
| EFS/ASCT, P = .52 | 22/7 | 32 | 43/18 | 40 | ||
| OS/ASCT, P = .94 | 22/11 | 50 | 43/21 | 51 | ||
| Response to transplant | ||||||
| CR/PR | 15 | 54 | 27 | 45 | ||
| Failure | 4 | 14 | 10 | 17 | ||
| Relapse after transplant | 10 | 36 | 17 | 28 | ||
Expression profiling of 17 available specimens revealed that of the 10 that could be classified with at least 90% probability, 9 (4 GC and 5 non-GC) were in agreement with the classification by the immunohistochemical method. In a panel of 33 patient samples at diagnosis of DLBCL, a similar level of concordance was obtained (86%; data not shown). Pretreatment prognostic factors, including the second-line international prognostic index (IPI), age-adjusted IPI, and relapse versus primary refractory disease were well balanced between the GC and non-GC cohorts.
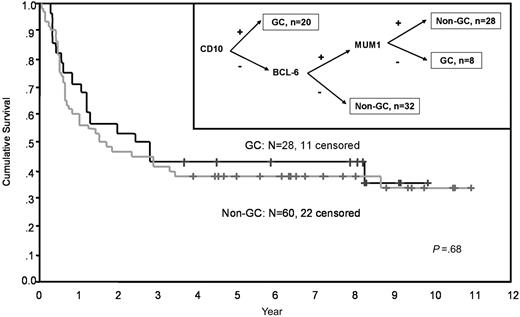
There was no significant difference based on cell of origin in response to ICE SLT or to HDT. OS and EFS were similar for the GC and non-GC patients (Figure 1). In addition, for those patients who received HDT/ASCT, the OS for the GC patients was 45% versus 54% for the non-GC patients (P = .9). We evaluated a number of markers important in untreated DLBCL to see if they had an impact on outcome in the second-line setting. As can be seen in Table 3, none of these markers had any significant impact on OS.
Prognostic markers at baseline
Marker, result . | % . | Overall survival at 5 years, % . | P . |
|---|---|---|---|
| MIB 1 | |||
| 0-2+ | 56 | 38.6 | |
| 3-4+ | 44 | 41.0 | |
| MUC 1 | .5 | ||
| Neg | 64 | 39.2 | |
| Pos | 36 | 40.6 | |
| MDR | .6 | ||
| Neg | 40 | 34.5 | |
| Pos | 60 | 40.6 | |
| P53 | .6 | ||
| Neg | 83 | 39.5 | |
| Pos | 17 | 41.0 | |
| BCL 2 | .9 | ||
| Neg | 49 | 37.1 | |
| Pos | 51 | 42.2 |
Marker, result . | % . | Overall survival at 5 years, % . | P . |
|---|---|---|---|
| MIB 1 | |||
| 0-2+ | 56 | 38.6 | |
| 3-4+ | 44 | 41.0 | |
| MUC 1 | .5 | ||
| Neg | 64 | 39.2 | |
| Pos | 36 | 40.6 | |
| MDR | .6 | ||
| Neg | 40 | 34.5 | |
| Pos | 60 | 40.6 | |
| P53 | .6 | ||
| Neg | 83 | 39.5 | |
| Pos | 17 | 41.0 | |
| BCL 2 | .9 | ||
| Neg | 49 | 37.1 | |
| Pos | 51 | 42.2 |
Overall survival of patients with relapsed or refractory DLBCL receiving SLT with ICE by cell of origin. The Kaplan-Meier estimate of overall survival based on cell of origin. (Inset) The cell of origin was determined by immunohistochemistry on TMA.
Overall survival of patients with relapsed or refractory DLBCL receiving SLT with ICE by cell of origin. The Kaplan-Meier estimate of overall survival based on cell of origin. (Inset) The cell of origin was determined by immunohistochemistry on TMA.
The current series is dominated by non-GC patients, as would be expected if patients with non-GC DLBCL have poorer outcome as reported; though the distribution of GC and non-GC patients is similar at diagnosis, the higher proportion of non-GC patients arises from inferior EFS and OS rates for non-GC patients, therefore the need for SLT.4 There are 2 potential explanations for the failure of cell of origin to predict outcome for relapsed and refractory patients. First, patients who relapse after or who are refractory to initial therapy have unfavorable characteristics that are unrelated to cell of origin. Although we have only 28 patients with GC-DLBCL in this report, the pretreatment characteristics in these cases are very similar to the non-GC patients. Additional expression profiling of patients with relapsed/refractory disease may reveal critical molecular lesions associated with outcome. Second, HDT/ASCT may overcome the poor prognostic features conferred by cell of origin. However, if true, we would expect consistent evidence that HDT/ASCT as part of initial therapy would improve outcome in DLBCL, but this is an area of controversy with no clear consensus in favor of this approach.14 Future up-front transplant studies need to evaluate outcome based on cell of origin to directly test this hypothesis.
Prepublished online as Blood First Edition Paper, August 9, 2005; DOI 10.1182/blood-2005-04-1603.
Supported by the Singer Family Lymphoma Research Fund and The Lymphoma Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal