Comment on Thomas et al, page 3559
Thomas and colleagues demonstrate that RNAi targeted to the MLL-AF4 leukemia fusion can effectively induce apoptosis as well as inhibit clonogenicity, proliferation, and engraftment of MLL-AF4 leukemia cell lines in a xenotransplantation model.
Translocations involving the MLL gene on chromosome 11 cause a variety of leukemias, with particular MLL fusions resulting in different phenotypes of disease.1 The most common MLL translocation is the t(4;11), which produces the mixed-lineage leukemia (MLL)–AF4 fusion protein. This particular translocation accounts for about 40% of all MLL translocations, including the majority of infant leukemias.2 Patients with MLL-AF4 leukemia almost always develop acute lymphoblastic leukemia (ALL), usually pro-B ALL, and have a particularly poor prognosis with current therapy regimens.3 Therefore, effective novel treatment strategies for this type of leukemia would be important to develop.FIG1
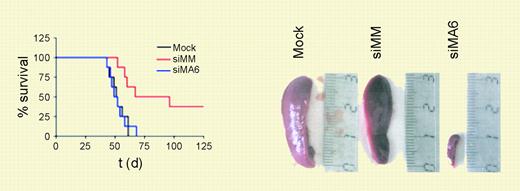
MLL-AF4 suppression diminishes leukemic engraftment. See the complete figure in the article beginning on page 3559.
MLL-AF4 suppression diminishes leukemic engraftment. See the complete figure in the article beginning on page 3559.
Recently, much interest has focused on targeting cancer-specific RNAs as a potential cancer therapy.4 This primarily has to do with the success of preclinical studies that harness an inherent cellular process for the posttranscriptional degradation of RNAs, RNA interference (RNAi). In RNAi, double-stranded RNA molecules are processed into short (approximately 21 nucleotide) fragments called short-interfering RNAs (siRNAs). The siRNAs are processed by the RNA-induced silencing complex (RISC), and ultimately cause the cleavage of RNAs homologous to the original targeting sequence.5 The resultant decreased expression is often referred to as “knockdown” of the specific target.
In this issue of Blood, Thomas and colleagues assessed the effect of using siRNAs to knockdown expression of MLL-AF4 on several properties of MLL-AF4 leukemia cell lines. The ability to grow as colonies in methylcellulose was significantly diminished after treatment with relevant siRNAs, whereas normal primary human CD34+ hematopoietic progenitors were not adversely affected by this same treatment. In vitro, specific siRNA treatment also inhibited proliferation, increased the percentage of cells that undergo apoptosis, and decreased expression of genes known to be up-regulated by MLL fusion proteins, HOXA7, HOXA9, and MEIS1. The authors found decreased CD133 expression in treated cells, and suggest that this marker of hematopoietic stem and progenitor cells might also be a target of MLL and MLL fusion protein regulation. Importantly, cells targeted with MLL-AF4–specific siRNA had significantly decreased leukemic engraftment in a severe-combined immunodeficient (SCID) mouse model. This suggested that MLL-AF4 expression was critical for persistence of the leukemia-initiating cells.
It is exciting that even short-term knockdown expression of the MLL-AF4 fusion was sufficient to push these leukemia cell lines, which have presumably acquired additional mutations, to differentiation and apoptosis. This absolute requirement for continuous expression of MLL-AF4 suggests that if indeed the MLL fusion can be effectively targeted, it should prove invaluable as a treatment strategy. However promising this may be, one must consider the problem of how this potentially effective therapeutic could be delivered with high enough efficiency to improve patient survival. As with other previous methods to target nucleic acids, a major obstacle remains efficient delivery in vivo. In vitro, multiple transfections are used to increase siRNA uptake. This task becomes even more difficult in vivo. But if these obstacles can be overcome, specific siRNA-based therapeutics may improve the treatment out come for many patients. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal