JAK2V617F, an ostensibly myeloid lineage-specific mutation, also appears to be myeloid disease–specific according to 2 studies in the current issue of Blood, which also features a third study that identifies altered gene expression in polycythemia vera as a surrogate for JAK-STAT hyperactivation.
It all started with HOP, a JAK homolog in Drosophila, where a dominant mutation in either the Janus homology 4 (JH4) (HOPTum-1) or JH2 (HOPT42) domain resulted in constitutive kinase activity, phosphorylation of Drosophila signal transducer and activator of transcription (STAT), and leukemia-like defects.1 A similar point mutation in murine Janus kinase 2 (JAK2; JAK2E665K) also resulted in an activated protein, leading the authors to propose the leukemogenic potential of JAK2 mutations in mammals.1 Around the same time (late 1990s), other investigators demonstrated inhibition of acute lymphocytic leukemia (ALL) cell growth by a JAK2 kinase inhibitor (AG-490) and the association of TEL/JAK2 (t(9;12)(p24;p13)) with both T and pre-B ALL as well as atypical myeloproliferative disorder (MPD).2 Most recently, another JAK2 fusion mutant, PCM1/JAK2 (t(8; 9)(p21-23;p23-24)), has been associated with atypical MPD (AMPD), ALL, and acute myeloid leukemia (AML).3
However, JAK2's claim to fame came about with the description of a novel somatic point mutation (a G-C to T-A transversion, at nucleotide 1849 of exon 12, resulting in the substitution of valine to phenylalanine at codon 617; JAK2V617F) in classic, BCR/ABL-negative MPD including polycythemia vera (PV), essential thrombocythemia (ET), and myelofibrosis with myeloid metaplasia (MMM).2 However, following the initial wave of 5 studies that reported a relatively high incidence of JAK2V617F in PV (65%-97%), ET (23%-57%), and MMM (35%-57%), 2subsequent studies disclosed the occurrence of the same mutation in a spectrum of atypical MPDs as well as in myelodysplastic syndrome (MDS), albeit at a much lower mutational frequency (3%-33%).4,5 In one of these latter studies, JAK2V617F and other oncogenic kinase mutations including BCR/ABL and FIP1L1-PDGFRA were shown to be mutually exclusive events.5 FIG1
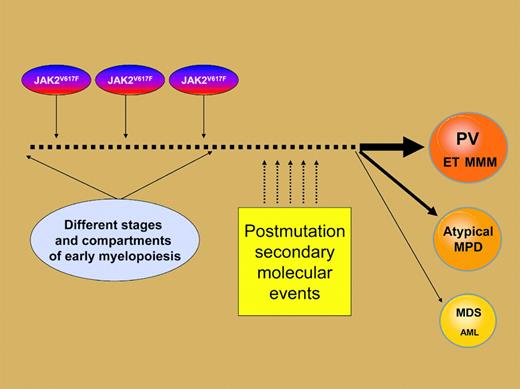
Phenotypic diversity associated with theJAK2V617F tyrosine kinase mutation might arise from a combination of the particular myeloid progenitor compartment that is affected and specific secondary mutations that occur during clonal evolution.
Phenotypic diversity associated with theJAK2V617F tyrosine kinase mutation might arise from a combination of the particular myeloid progenitor compartment that is affected and specific secondary mutations that occur during clonal evolution.
In the current issue of Blood, 2 additional studies from Levine and colleagues and Jelinek and colleagues confirm the presence of JAK2V617F mutation among a spectrum of myeloid but not lymphoid disorders. These findings are consistent with previously reported clonality studies that suggested myeloid lineage specificity for JAK2V617F. However, the incidence of the mutation in de novo, nonmegakaryocytic AML was very low and in AML with antecedent MPD was less than anticipated. The third JAK2V617F-related study in the current issue of Blood by Kralovics and colleagues suggests that currently recognized biologic markers of MPD, which include PV-derived gene expression signatures and altered polycythemia rubra vera 1 (PRV-1)/ nuclear factor erythroid-derived 2 (NF-E2) expression, are surrogates of JAK-STAT hyperactivation that are also displayed in either endogenously reactive or granulocyte colony-stimulating factor (G-CSF)–induced granulocytosis.
What are the clinical and scientific implications of these current findings? First, information regarding JAK2V617F may not be used to discriminate among myeloid disorders, although the presence of the mutation might favor MPD (classic or atypical) as opposed to MDS. Second, there are now experimental data to support the effective substitution of JAK2V617F mutation screening for other diagnostic biomarkers in PV, including endogenous erythroid colony formation and neutrophil PRV-1 transcription, each of which entails a certain level of expertise that is not widely available for routine clinical use.
Where do we go from here? The JAK2V617F mutation is self-enhancing and occurs within the enzymatically inactive JH2 domain (pseudo-kinase domain) that regulates the catalytically active C-terminal kinase domain (JH1). As a result, downstream effectors including STAT5 are constitutively activated and account for the cell-transforming property of the mutation in murine cell lines, which includes hypersensitivity to erythropoietin. Similarly, erythrocytosis has been induced in mice through JAK2V617F hematopoietic stem cell transduction.6 These laboratory observations are recapitulated by JAK2V617F-positive ET patients who display significantly higher hemoglobin levels as well as an increased rate of transformation into PV compared with their counterparts with wild-type allele.7 The particular observation is also consistent with the higher incidence of homozygous mutations in PV compared with other MPDs.
This information, combined with the incomplete as well as nonspecific association between JAK2V617F and specific categories of MPDs, implicates the mutation as a growth/survival signal that contributes to a PV-weighted myeloproliferative phenotype. One possibility is that the JAK2V617F phenotypic diversity is a function of which progenitor population is affected by the mutation during early myelopoiesis and the specific secondary molecular events that occur during clonal evolution (Figure 1). Regardless, the lack of specific disease association does not undermine the value of the mutant molecule in guiding further pathogenetic studies as well as development of rational drug therapy. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal