Comment on Layez et al, page 3632
In its severe form, malaria encompasses 3 major life-threatening manifestations, cerebral malaria (CM), severe malarial anemia (SMA), and respiratory distress. Layez and colleagues shed light on the mechanisms of the least understood syndrome, malaria-induced anemia.
Plasmodium falciparum malaria is a very complex disease. Most episodes, affecting an estimated 500 million people each year, have a mild clinical outcome. Indeed, only a percentage of parasite infections lead to severe life-threatening syndromes. Yet the shear magnitude of cases still translates to 1 to 2 million fatalities per year.FIG1
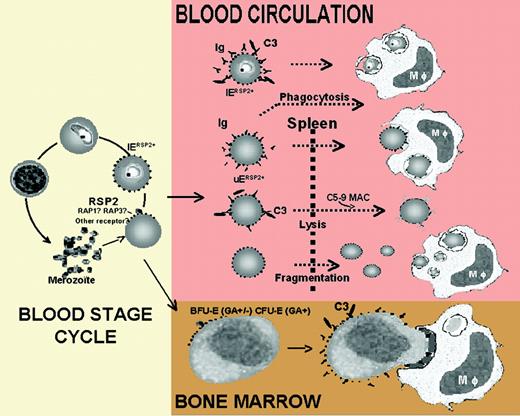
Schematic representation of the role of RSP2 in the development of severe anemia duringP falciparuminfection. See the complete figure in the article beginning on page 3632.
Schematic representation of the role of RSP2 in the development of severe anemia duringP falciparuminfection. See the complete figure in the article beginning on page 3632.
Severe clinical manifestations are mostly restricted to young children, although severe malarial anemia (SMA) is also a major factor of morbidity in pregnancy-associated malaria. In areas of high transmission, SMA occurs usually at higher rates and at earlier ages than cerebral malaria (CM), whereas in low transmission areas CM is more common than SMA and less confined to the young.
While CM is a characteristic feature of P falciparum malaria, SMA is also common to rodent malaria species, providing more convenient possibilities for study. Nevertheless, much research on SMA has painted a complicated picture, suggesting that both excessive destruction and decreased production of erythrocytes are involved. Obviously, the hemolysis of the infected erythrocyte (IE) as part of the parasite life cycle is contributing to anemic conditions in acute malaria episodes. A mathematic model of SMA however revealed that with each lysed IE, a further 8.5 uninfected erythrocytes (UEs) are destroyed.1 The thus shortened life-span of UEs is further aggravated by a dysfunctioning bone marrow. Parasite factors (hemozoin, toxins), proinflammatory cytokines, and a nonoptimal response to erythropoietin affect the erythrocyte precursors and cause dyserythropoiesis.2 Although multiple effector mechanisms contributing to SMA have been proposed, a convincing molecular explanation was lacking.
The work of Layez and colleagues suggests now a fascinating immunologic mishap elicited by a merozoite rhoptry protein, ring surface protein 2 (RSP2), which provides a convincing model for the premature destruction of erythrocytes and the reduced erythropoiesis observed in SMA. Confirming a previous report,3 Layez et al show that RSP2 is inserted into the surface of infected and uninfected erythrocytes and is left behind when the invasion of erythrocytes by merozoites fails or is delayed. The leftover RSP2 is the result of a not-so-refined invasion process and may have severe hematologic consequences.
Layez and colleagues demonstrate that cytophilic anti-RSP2 antibodies present in sera enhance phagocytosis of RSP2-tagged erythrocytes by macrophages either directly or via the activation of the classical complement pathway. As this finding may provide a clue to the high ratio of UEs destroyed in malarial anemia, it also may explain the observed decrease in erythropoiesis: in bone marrow samples from P falciparum–infected patients, RSP2 was found deposited on erythroid precursor cells, which could be destroyed by complement-mediated phagocytosis.
Layez and coworkers provide an important molecular model of anemia, but it is probably only an element of an array of interactions at the erythrocyte surface; previous findings suggest that autoantibodies, deposition of immunocomplexes, and complement factor C3b also take part in the lysis of the erythron. Most intriguingly, as is so common in parasitic infections, the new model suggests that the detrimental outcome of SMA may be a consequence of a misdirected immune response and also suggests that a vaccine including RSP2 may promote, rather than alleviate, SMA conditions.
However, some questions remain unanswered. Why is SMA restricted to the young and clearly not directly correlated to the parasitemia? Indeed, many malaria-infected individuals sustain quite high levels of parasitemia without showing clinical features of anemia? Also, hematocrit levels often continue to fall despite the clearance of parasitemia and the concomitant decrease of RSP2 depository on UEs. This suggests that other antigens also involved in merozoite entry may function as antibody targets on erythrocytes that P falciparum fails to invade. Finally, further contributing factors may include, for example, complement receptor 1, which indeed shows an age-dependent expression.4 ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal