Abstract
The Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) 0496 protocol, through the central handling of bone marrow samples at presentation, allowed us to combine cytogenetic and molecular information on a large series of adults with acute lymphoblastic leukemia (ALL) treated homogeneously, enabling us to define as broadly as possible their genetic profile and to determine the impact on outcome of the cytogenetic-molecular signature. Of 414 patients centrally processed, 325 were considered for the categorization into the following cytogenetic-molecular subgroups: normal, t(9;22)/BCR-ABL, t(4;11)/MLL-AF4, t(1;19)/E2A-PBX1, 9p/p15-p16 deletions, 6q deletions, miscellaneous structural abnormalities, and hyperdiploid. The inclusion into each subgroup was based on a hierarchical approach: molecular abnormalities with adverse prognosis had precedence over karyotypic changes with less-defined prognosis and the latter over ploidy. Patients without abnormalities and those with isolated 9p/p15-p16 deletions showed a relatively favorable outcome (median disease-free survival [DFS], > 3 years). The t(9;22)/BCR-ABL, t(4;11)/MLL-AF4, t(1; 19)/E2A-PBX1 defined a group with dismal prognosis (median DFS, 7 months), whereas 6q deletions, miscellaneous aberrations, and hyperdiploidy predicted an intermediate prognosis (median DFS, 19 months). This study highlights the importance of a combined cytogenetic-molecular profiling of adult ALL at presentation as a critical independent determinant of their outcome, providing further evidence of the necessity of a risk-adapted therapeutic algorithm for an optimal management of these patients.
Introduction
The analysis of the chromosome and genetic abnormalities that occur in acute leukemias has provided relevant insights into the prognostic significance of these genetic lesions, which, in turn, become of potential importance for the design of risk-adapted therapeutic strategies for newly diagnosed cases. In the last decade, only a few studies have analyzed the prognostic value of cytogenetic abnormalities in large series of adult patients with acute lymphoblastic leukemia (ALL).1-5 Although there is general agreement on the unfavorable clinical outcome of patients with ALL with t(9;22) or t(4;11) abnormalities, the prognostic significance of other cytogenetic abnormalities is still debated. This may be attributed to the well-known technical difficulties associated with chromosome analysis in ALL, the frequent overlap of numerical and structural changes confounding the prognostic significance of the single anomaly, and the low frequency of certain karyotypic changes.6,7 In addition, it should be considered that most studies have focused on patients undergoing different treatment modalities.1,3,5
Over the last 2 decades, it has become evident that molecular investigations are a useful integration of cytogenetic analysis, allowing the detection of molecular alterations in cases with apparently normal or failed karyotype.8,9 Therefore, an integrated cytogenetic-molecular approach seems the most appropriate tool for an accurate genetic characterization of patients with ALL at the onset of the disease.
Based on these considerations, the prospective Italian multicenter Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) 0496 study, started in 1996, provided for all adult patients with ALL entering the protocol a multiparametric diagnostic approach including conventional cytogenetics and molecular analysis for the following gene rearrangements: BCR-ABL, MLL-AF4, E2A-PBX1, TEL-AML1, MLL, and p15 and p16 deletion. This effort aimed at a careful characterization of the genetic lesions occurring in adult ALL and at evaluating their prognostic significance in a large cohort of patients treated homogeneously.
Patients, materials, and methods
The GIMEMA ALL 0496 protocol, which began in October 1996 and was closed in September 2000, contemplated a central handling and biologic investigation of bone marrow (BM) or peripheral blood (PB) samples of the enrolled patients at presentation including conventional cytogenetics, Southern blot and reverse transcription-polymerase chain reaction (RT-PCR), and multidrug resistance (MDR1) expression. Samples were sent by overnight dispatch to the laboratories of the Department of Cellular Biotechnologies and Hematology, University “La Sapienza” of Rome; the specific investigations were performed at the same center, at the Department of Clinical and Biological Sciences, “S. L. Gonzaga” Hospital, Orbassano, University of Turin, at the Hematology and Bone Marrow Transplantation Unit, University of Perugia, at the CEINGE and Department of Biochemistry and Medical Biotechnologies, University “Federico II” of Naples, and at the Department of Biomedical Science, Hematology Unit, University of Ferrara, all in Italy.
All patients were treated according to the ALL 0496 protocol, which is derived from the ALLVR589 regimen10 and included patients aged 16 to 60 years with a diagnosis of ALL, with the exclusion of L3 B-cell ALL (B-ALL). The study was approved by the Institutional Review Board of the Department of Cellular Biotechnologies and Hematology, University “La Sapienza” of Rome. Informed consent was provided according to the Declaration of Helsinki. The protocol design has been described elsewhere11 ; in summary it comprised an induction period including high-dose daunorubicin, a consolidation with high-dose ara-C, chemoprophylaxis and radioprophylaxis of central nervous system involvement, and periodic reinductions over a 3-year maintenance period. Patients positive for Philadelphia chromosome (Ph+) achieving complete remission (CR) were treated according to the HAM protocol that included high-dose ara-C (3 g/m2/12 h for 4 days) and mitoxantrone (10 mg/m2/24 h for 3 days) followed by allogeneic or autologous hemopoietic stem cell transplantation based on the availability of an HLA-identical sibling.12
Cytology and immunology
The immunophenotype of ALL patients was performed by the referring centers on BM or PB samples at diagnosis. The reactivity with monoclonal antibodies directed against lymphoid and myeloid-associated antigens (TdT, HLA-DR, CD19, CD10, CD20, CyIg, CD2, CD5, CD7; CD3, CD13, CD33, CD14, CD34) was evaluated by flow cytometry. Fixed cytocentrifuge preparations were used for the detection of TdT and CyIgμ chains by immunocytochemical staining. The results were reviewed by the GIMEMA biologic committee. The surface markers were considered positive when 20% or more of the blasts expressed the antigen; intracytoplasmic positiveness was based on 10% or more reactive blasts. The immunologic definition in B- and T-lineage ALL was based on the criteria defined by the European Group for the Immunological Characterization of Leukemias.13
Cytogenetic analysis
Cytogenetic analyses were performed in 3 referral centers (Rome, Ferrara, and Perugia) on direct and 24-hour cultured preparations of BM or PB cells (or both) without stimulation. GTG-banding chromosomes were classified according to the International System for Human Cytogenetic Nomenclature.14 A successful cytogenetic analysis required the detection of at least 2 or more cells with the same structural change or chromosomal gain, 3 or more cells with the same chromosomal loss, or at least 20 metaphases without clonal changes. However, in less than 10% of cases classified as normal, 10 to 19 mitotic figures could be analyzed. A central karyotype review was also performed in difficult cases. Each patient was classified hierarchically in different subgroups on the basis of the cytogenetic abnormality; in particular, karyotypic aberrations with known adverse prognostic significance as t(9;22), t(4;11), t(1;19) took precedence over others with less defined prognostic value, as del(6q) and del(9p). If none of these was identified, patients having structural aberrations with or without numerical changes not exceeding one chromosome gain or loss were assigned to the miscellaneous group, whereas patients carrying numerical aberrations only were assigned to the hyperdiploid group. Patients without karyotypic anomalies were included in the normal group.
DNA analysis
High-molecular-weight DNA was extracted from Ficoll-Hypaque–isolated mononuclear cells, digested to completion with EcoRI, HindIII, or BglII restriction endonucleases, size-fractionated by electrophoresis through 0.8% agarose gels, denatured, and transferred to nitrocellulose membranes. These DNAs were prehybridized for 2 to 4 hours at 42°C in a solution containing 50% deionized formamide, 5 × Denhardt solution, and 100 μg/mL denatured salmon sperm and then hybridized at 42°C for 16 to 18 hours in the same solution with the denatured p16 exon 2 probe previously labeled with 32P by the random priming technique.11 Filters were washed at room temperature for 10 minutes in 0.2 × standard saline citrate/0.1% sodium dodecyl sulfate and at 50°C for 15 to 20 minutes in the same solution. Filters were then exposed for 2 to 8 days at -80°C for autoradiography using intensifying screens.
The B859 probe previously described, which explores the MLL breakpoint region at chromosome 11q23, was also used to study the configuration of this gene in ALL patients.15 In addition to the detection of rearrangements at this locus, this probe served as control for the assessment of the DNA amount present in the filters. The genomic deletions of the p15 and p16 genes were evaluated in 211 patients.
RT-PCR
Total RNA was extracted from BM or PB mononuclear cells collected at diagnosis and cryopreserved in guanidinium isothiocyanate, according to the method of Chomczynski and Sacchi.16 To identify the most frequent fusion genes in ALL—namely, MLL-AF4, MLL-ENL, the BCR-ABL p190 (e2a2) and p210 (b2a2, b3a2) isoforms, E2A-PBX1, TEL-AML1—we used a multiplex RT-PCR strategy17 in 60 cases and the standardized RT-PCR protocol defined by the Biomed-1 Concerted Action in the remaining.18 Analyses were conducted in 3 referral centers (Rome, Turin, and Naples) and centrally reviewed. Because the therapeutic program contemplated a different postremission strategy for patients with BCR-ABL+ ALL, the BCR-ABL fusion product detection was performed in all patients. The presence of the MLL-AF4, E2A-PBX, and TEL-AML1 fusion transcripts was investigated in 287, 162, and 162 cases, respectively.
MDR1 expression
MDR1 expression was assessed on BM or PB samples by depicting the leukemic cells with the MRK16 antibody (Kamiya Biomedical, Seattle, WA), which reacts with a cell surface epitope of the P-glycoprotein (P-gp) 170, as previously reported.19 Flow cytometry was used to measure the fluorescence intensity of the leukemic samples and of the MDR1+ cell lines used as controls in the analysis standardization. Differences in fluorescence intensity were evaluated using the Kolmogorov-Smirnov statistic test (D).20 According to the study priority, 250 cases were analyzed in one referral laboratory in Rome.
Statistical analyses
Comparison of pretreatment characteristics on CR rate was carried out using the χ2 test or Fisher exact test. The relationship of the outcome with age, white blood cells (WBCs), phenotype, MDR, and cytogenetic-molecular classification was analyzed using the logistic and Cox regression models.21 In the multivariate analysis, the classification in 2 cytogenetic-molecular risk groups was tested with the following variables: age and
Results
Cytogenetics
Conventional cytogenetics was performed on 378 of 414 cases centrally processed, due to inadequate samples for cytogenetic analysis (6 cases) or missing cytogenetic samples (30 cases). An evaluable karyotype was obtained in 282 cases (75%) and an abnormal clone was detected in 168 (60%). Cases with a defined karyotype were classified in different subgroups following the hierarchical approach indicated (Table 1). Among cases with structural changes, the subgroup with the t(9;22) translocation was the largest (64 of 378; 16.9%). In this group, additional chromosomal changes were identified in 55% of the cases and included an additional Ph (8 cases), 9p deletion (3 cases), and chromosomal gain resulting in hyperdiploidy (3 cases). Patients with t(9;22) had B-lineage ALL in all cases but one (a T-ALL) with expression of the CD34 and CD10 antigens in more than 90 of cases, a WBC count more than 10 × 109/L in more than 60%, and an age older than 30 years in 78% of cases. A t(4;11) translocation was identified in 18 patients (4.8%); 5 had additional structural anomalies that included i(7q) (1 case) and del(6q) (1 case). A t(1;19) translocation was detected in 3 cases (0.8%); 2 had the unbalanced form and 1 the balanced form of the translocation; all cases showed additional changes including in one instance a del(6q). Partial deletions of the long arm of chromosome 6 were identified in 15 cases (4%); of these, 1 had the presence of a BCR-ABL rearrangement at the molecular level. A T-cell phenotype was found in 8 (57%) of 14 cases, and in 6 of them the anomaly was present as isolated change. An isolated del(9p) was identified in 2 cases, both showing a B-cell phenotype. The other 41 cases (10.8%) with an abnormal karyotype were combined to form the miscellaneous group. The most common structural changes included in this group were del(7q) (3 cases), del(5q) (2 cases), del(12p) or t(12p) (2 cases), t(10;14)(q24;q11) (2 cases), t(4;11)(q21;p15) (1 case), and 14q abnormalities (5 cases). Numerical aberrations consisting in low hyperdiploidy (47-50 chromosomes, 16 cases) and in high hyperdiploidy (> 50 chromosomes, 9 cases) were identified in 25 patients (6.6%). Finally, 114 cases (30.2%) without apparent structural or numerical changes were recorded.
Distribution by cytogenetic hierarchical classification
Groups . | No. cases . | % of cases . |
|---|---|---|
| Normal | 114 | 30.2 |
| del(9p) | 2 | 0.5 |
| del(6q) | 15 | 4.0 |
| Hyperdiploid | 25 | 6.6 |
| Miscellaneous structural abnormalities | 41 | 10.8 |
| t(9;22) | 64 | 16.9 |
| t(4;11) | 18 | 4.8 |
| t(1;19) | 3 | 0.8 |
| Failed | 96 | 25.4 |
| Total | 378 | 100.0 |
Groups . | No. cases . | % of cases . |
|---|---|---|
| Normal | 114 | 30.2 |
| del(9p) | 2 | 0.5 |
| del(6q) | 15 | 4.0 |
| Hyperdiploid | 25 | 6.6 |
| Miscellaneous structural abnormalities | 41 | 10.8 |
| t(9;22) | 64 | 16.9 |
| t(4;11) | 18 | 4.8 |
| t(1;19) | 3 | 0.8 |
| Failed | 96 | 25.4 |
| Total | 378 | 100.0 |
Molecular analysis
Molecular analyses were performed on 386 of 414 cases centrally collected, owing to inadequate samples for molecular investigation (6 cases) or absence of molecular abnormalities in cases with missing cytogenetic samples (22 cases). BCR-ABL fusion was detected in 102 of 386 cases (26.4%; Table 2). Of these, 58 (57%) showed a p190, 23 (23%) a p210, 19 (19%) a p190/210 fusion protein, and 1 a rare e1a3 fusion. AF4-MLL, E2A-PBX1, and TEL-AML1 fusion genes were identified in 23 (8%) of 287, 6 (3.7%) of 162, and 1 (0.6%) of 162 of the investigated cases, respectively. A homozygous or hemizygous loss of p15 or p16 genes (or both) was documented in 52 (24.6%) of 211 analyzed cases. Among the 52 deleted cases, 30 showed the deletion as an isolated anomaly. BCR-ABL (11 cases), E2A-PBX1 (1 case), and AF4-MLL (1 case) fusions emerged as the molecular changes more frequently associated with p15/p16 deletion. Of the 386 cases molecularly investigated, 61 with molecular analysis negative for the presence of fusion genes or p15-p16 deletion and inadequate cytogenetic results (no mitosis or < 10 analyzed cells with normal karyotype) were considered as not evaluable.
Distribution by molecular abnormalities
Type of transcript or gene rearrangement . | No. abnormal cases/no. analyzed cases (%) . |
|---|---|
| BCR-ABL | 101/386 (26.2) |
| MLL-AF4 | 23/287 (8.0) |
| TEL-AML1 | 1/162 (0.6) |
| E2A-PBX1 | 6/162 (3.7) |
| p15/p16 deletion | 52/211 (24.6) |
Type of transcript or gene rearrangement . | No. abnormal cases/no. analyzed cases (%) . |
|---|---|
| BCR-ABL | 101/386 (26.2) |
| MLL-AF4 | 23/287 (8.0) |
| TEL-AML1 | 1/162 (0.6) |
| E2A-PBX1 | 6/162 (3.7) |
| p15/p16 deletion | 52/211 (24.6) |
Integrated cytogenetic and molecular classification
Considering the cases having both conventional cytogenetics (282 cases) or molecular analysis (325 cases) successfully performed, a total of 325 patients with a defined genetic profile were hierarchically classified in the following subgroups: normal, BCR-ABL/t(9; 22), MLL-AF4/t(4;11), E2A-PBX1/t(1;19), 6q deletions, miscellaneous, hyperdiploid, 9p and/or p15 and/or p16 deletions.
This integrated cytogenetic-molecular approach ensures a more precise categorization of patients with respect to the presence or absence of distinct genetic alterations.
The frequency of each cytogenetic-molecular subgroup and their associated biologic and clinical features are presented in Tables 3 and 4. With respect to previously cytogenetic-based classifications, this classification system allowed us to delineate, in particular, 3 subgroups of patients: namely, normal, 9p/p15-p16 deletions, and hyperdiploid, as much as possible free from confounding genetic abnormalities. Compared with the other cytogenetic-molecular subgroups, patients included in the normal subgroup showed more frequently an age younger than 30 years (59.3% versus 40.6%; P = .003) and a WBC count lower than 30 × 103/L (72.1% versus 56.1; P = .009). As for the del(6q) subgroup, patients included in the group with isolated cytogenetic or molecular 9p/p15-p16 abnormalities had more frequently a T-cell lineage ALL than patients of the other subgroups (57.1% versus 36.7%, P = < .001). The hyperdiploid group showed a CD10- B-lineage ALL more frequently than the other groups (58.3% versus 32.2%; P = .009) and tended to have more frequently a WBC count lower than 30 × 103/L (76% versus 59%), as in the normal subgroup.
Distribution of 386 ALL cases according to conventional cytogenetic and cytogenetic-molecular classification
. | . | Cytogenetic-molecular groups, no. . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytogenetic group . | Normal . | t(9;22)/BCR-ABL . | t(4;11)/AF4-MLL . | t(1;19)/E2A-PB XI . | del(6q) . | Hyperdiploid . | Miscellaneous . | del(9p)/p15-p16 . | Not evaluable . | Total . | ||||||||
| Normal | 86 | 4 | 3 | 2 | 0 | 0 | 1* | 18 | 0 | 114 | ||||||||
| t(9;22) | 0 | 64† | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 64 | ||||||||
| t(4;11) | 0 | 0 | 18‡ | 0 | 0 | 0 | 0 | 0 | 0 | 18 | ||||||||
| t(1;19) | 0 | 0 | 0 | 3‡ | 0 | 0 | 0 | 0 | 0 | 3 | ||||||||
| del(6q) | 0 | 1 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 15 | ||||||||
| Hyperdiploid | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 25 | ||||||||
| Miscellaneous | 0 | 2 | 2 | 1 | 0 | 0 | 36 | 0 | 0 | 41 | ||||||||
| del(9p) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | ||||||||
| Not evaluable | 0 | 25 | 1 | 1 | 0 | 0 | 0 | 8 | 61 | 96 | ||||||||
| Not done | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 8 | ||||||||
| Total | 86 | 102 | 24 | 7 | 14 | 25 | 37 | 30 | 61 | 386 | ||||||||
. | . | Cytogenetic-molecular groups, no. . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytogenetic group . | Normal . | t(9;22)/BCR-ABL . | t(4;11)/AF4-MLL . | t(1;19)/E2A-PB XI . | del(6q) . | Hyperdiploid . | Miscellaneous . | del(9p)/p15-p16 . | Not evaluable . | Total . | ||||||||
| Normal | 86 | 4 | 3 | 2 | 0 | 0 | 1* | 18 | 0 | 114 | ||||||||
| t(9;22) | 0 | 64† | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 64 | ||||||||
| t(4;11) | 0 | 0 | 18‡ | 0 | 0 | 0 | 0 | 0 | 0 | 18 | ||||||||
| t(1;19) | 0 | 0 | 0 | 3‡ | 0 | 0 | 0 | 0 | 0 | 3 | ||||||||
| del(6q) | 0 | 1 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 15 | ||||||||
| Hyperdiploid | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 25 | ||||||||
| Miscellaneous | 0 | 2 | 2 | 1 | 0 | 0 | 36 | 0 | 0 | 41 | ||||||||
| del(9p) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | ||||||||
| Not evaluable | 0 | 25 | 1 | 1 | 0 | 0 | 0 | 8 | 61 | 96 | ||||||||
| Not done | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 8 | ||||||||
| Total | 86 | 102 | 24 | 7 | 14 | 25 | 37 | 30 | 61 | 386 | ||||||||
TEL-AML1
One case with positive fluorescence in situ hybridization and negative RT-PCR
One case missing molecular analysis
Pretreatment characteristics of 325 genetically defined patients by cytogenetic-molecular groups
. | . | Cytogenetic-molecular groups . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Total . | Normal . | t(9;22)/BCR-ABL . | t(4;11)/AF4-MLL . | t(1;19)/E2A-PBX1 . | del(6q) . | del(9p)/p15-p16 . | Pseudodiploid . | Hyperdiploid . | |||||||
| Total | 325 | 86 | 102 | 24 | 7 | 14 | 30 | 37 | 25 | |||||||
| Sex, no. (%) | ||||||||||||||||
| Male | 194 (60) | 51 (59) | 54 (53) | 13 (54) | 3 (43) | 11 (78) | 24 (80) | 22 (59) | 16 (61) | |||||||
| Female | 131 (40) | 35 (41) | 48 (47) | 11 (46) | 4 (57) | 3 (22) | 6 (20) | 15 (41) | 9 (39) | |||||||
| Median age, y (range) | 31 (14-60) | 26 (15-58) | 42 (16-60) | 39 (15-59) | 22 (15-58) | 27 (18-57) | 29 (14-59) | 23 (15-59) | 22 (15-53) | |||||||
| Age, no. (%) | ||||||||||||||||
| Younger than 30 y | 148 (46) | 51 (59) | 22 (22) | 7 (29) | 6 (86) | 8 (57) | 15 (50) | 25 (68) | 14 (56) | |||||||
| 31 to 50 y | 124 (38) | 29 (34) | 51 (50) | 14 (58) | 0 (0) | 5 (36) | 10 (33) | 7 (19) | 8 (32) | |||||||
| 51 to 60 y | 53 (16) | 6 (7) | 29 (28) | 3 (13) | 1 (14) | 1 (7) | 5 (17) | 5 (13) | 3 (12) | |||||||
| WBC counts, no. (%) | ||||||||||||||||
| 10 × 109/L or less | 132 (41) | 49 (57) | 36 (35) | 3 (12.5) | 3 (43) | 4 (29) | 10 (33) | 14 (38) | 13 (52) | |||||||
| 10 × 109/L to 30 × 109/L | 64 (20) | 13 (15) | 17 (17) | 5 (21) | 3 (43) | 4 (29) | 7 (23) | 9 (24) | 6 (24) | |||||||
| 30 × 109/L to 50 × 109/L | 32 (10) | 8 (9) | 12 (12) | 3 (12.5) | 0 | 2 (14) | 3 (10) | 2 (5) | 2 (8) | |||||||
| 50 × 109/L to 100 × 109/L | 43 (13) | 6 (7) | 19 (19) | 5 (21) | 0 | 1 (7) | 4 (14) | 7 (19) | 1 (4) | |||||||
| 100 × 109/L or more | 53 (16) | 10 (12) | 17 (17) | 8 (33) | 1 (14) | 3 (21) | 6 (20) | 5 (14) | 3 (12) | |||||||
| Phenotype, no. (%) | ||||||||||||||||
| B | 266 (82) | 66 (77) | 101 (99) | 24 (100) | 7 (100) | 6 (43) | 19 (63) | 25 (68) | 18 (72) | |||||||
| T | 59 (18) | 20 (23) | 1 (1) | 0 | 0 | 8 (57) | 11 (37) | 12 (32) | 7 (28) | |||||||
| CD10 status, no. (%) | ||||||||||||||||
| Positive | 206 (66) | 52 (64) | 92 (93) | 0 (0) | 7 (100) | 6 (46) | 19 (63) | 20 (57) | 10 (42) | |||||||
| Negative | 107 (34) | 29 (36) | 7 (7) | 24 (100) | 0 (0) | 7 (54) | 11 (37) | 15 (43) | 14 (58) | |||||||
| Not done | 12 | 5 | 3 | 0 | 0 | 1 | 0 | 2 | 1 | |||||||
| CD13 status, no. (%) | ||||||||||||||||
| Positive | 77 (26) | 23 (29) | 33 (35) | 0 (0) | 0 (0) | 2 (15) | 1 (4) | 10 (29) | 8 (38) | |||||||
| Negative | 222 (74) | 55 (71) | 62 (65) | 24 (100) | 6 (100) | 11 (85) | 26 (96) | 25 (71) | 13 (62) | |||||||
| Not done | 26 | 8 | 7 | 0 | 1 | 1 | 3 | 2 | 4 | |||||||
| CD33 status, no. (%) | ||||||||||||||||
| Positive | 67 (22) | 19 (24) | 33 (35) | 0 (0) | 0 (0) | 0 (0) | 2 (7) | 7 (20) | 6 (26) | |||||||
| Negative | 235 (78) | 59 (76) | 62 (65) | 23 (100) | 7 (100) | 13 (100) | 26 (93) | 28 (80) | 17 (74) | |||||||
| Not done | 23 | 8 | 7 | 1 | 0 | 1 | 2 | 2 | 2 | |||||||
| CD34 status, no. (%) | ||||||||||||||||
| Positive | 223 (72) | 57 (71) | 92 (95) | 10 (41) | 0 (0) | 8 (57) | 14 (48) | 24 (76) | 18 (75) | |||||||
| Negative | 86 (28) | 23 (29) | 5 (5) | 14 (59) | 7 (100) | 6 (43) | 15 (52) | 10 (24) | 6 (25) | |||||||
| Not done | 16 | 6 | 5 | 0 | 0 | 0 | 1 | 3 | 1 | |||||||
| MDR, no. (%) | ||||||||||||||||
| Yes | 57 (23) | 15 (24) | 16 (21) | 2 (11) | 1 (14) | 1 (9) | 7 (26) | 8 (27) | 7 (37) | |||||||
| No | 193 (87) | 47 (76) | 59 (79) | 17 (89) | 6 (86) | 10 (91) | 20 (74) | 22 (73) | 12 (63) | |||||||
| Not done | 75 | 24 | 27 | 5 | 0 | 3 | 3 | 7 | 6 | |||||||
. | . | Cytogenetic-molecular groups . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Total . | Normal . | t(9;22)/BCR-ABL . | t(4;11)/AF4-MLL . | t(1;19)/E2A-PBX1 . | del(6q) . | del(9p)/p15-p16 . | Pseudodiploid . | Hyperdiploid . | |||||||
| Total | 325 | 86 | 102 | 24 | 7 | 14 | 30 | 37 | 25 | |||||||
| Sex, no. (%) | ||||||||||||||||
| Male | 194 (60) | 51 (59) | 54 (53) | 13 (54) | 3 (43) | 11 (78) | 24 (80) | 22 (59) | 16 (61) | |||||||
| Female | 131 (40) | 35 (41) | 48 (47) | 11 (46) | 4 (57) | 3 (22) | 6 (20) | 15 (41) | 9 (39) | |||||||
| Median age, y (range) | 31 (14-60) | 26 (15-58) | 42 (16-60) | 39 (15-59) | 22 (15-58) | 27 (18-57) | 29 (14-59) | 23 (15-59) | 22 (15-53) | |||||||
| Age, no. (%) | ||||||||||||||||
| Younger than 30 y | 148 (46) | 51 (59) | 22 (22) | 7 (29) | 6 (86) | 8 (57) | 15 (50) | 25 (68) | 14 (56) | |||||||
| 31 to 50 y | 124 (38) | 29 (34) | 51 (50) | 14 (58) | 0 (0) | 5 (36) | 10 (33) | 7 (19) | 8 (32) | |||||||
| 51 to 60 y | 53 (16) | 6 (7) | 29 (28) | 3 (13) | 1 (14) | 1 (7) | 5 (17) | 5 (13) | 3 (12) | |||||||
| WBC counts, no. (%) | ||||||||||||||||
| 10 × 109/L or less | 132 (41) | 49 (57) | 36 (35) | 3 (12.5) | 3 (43) | 4 (29) | 10 (33) | 14 (38) | 13 (52) | |||||||
| 10 × 109/L to 30 × 109/L | 64 (20) | 13 (15) | 17 (17) | 5 (21) | 3 (43) | 4 (29) | 7 (23) | 9 (24) | 6 (24) | |||||||
| 30 × 109/L to 50 × 109/L | 32 (10) | 8 (9) | 12 (12) | 3 (12.5) | 0 | 2 (14) | 3 (10) | 2 (5) | 2 (8) | |||||||
| 50 × 109/L to 100 × 109/L | 43 (13) | 6 (7) | 19 (19) | 5 (21) | 0 | 1 (7) | 4 (14) | 7 (19) | 1 (4) | |||||||
| 100 × 109/L or more | 53 (16) | 10 (12) | 17 (17) | 8 (33) | 1 (14) | 3 (21) | 6 (20) | 5 (14) | 3 (12) | |||||||
| Phenotype, no. (%) | ||||||||||||||||
| B | 266 (82) | 66 (77) | 101 (99) | 24 (100) | 7 (100) | 6 (43) | 19 (63) | 25 (68) | 18 (72) | |||||||
| T | 59 (18) | 20 (23) | 1 (1) | 0 | 0 | 8 (57) | 11 (37) | 12 (32) | 7 (28) | |||||||
| CD10 status, no. (%) | ||||||||||||||||
| Positive | 206 (66) | 52 (64) | 92 (93) | 0 (0) | 7 (100) | 6 (46) | 19 (63) | 20 (57) | 10 (42) | |||||||
| Negative | 107 (34) | 29 (36) | 7 (7) | 24 (100) | 0 (0) | 7 (54) | 11 (37) | 15 (43) | 14 (58) | |||||||
| Not done | 12 | 5 | 3 | 0 | 0 | 1 | 0 | 2 | 1 | |||||||
| CD13 status, no. (%) | ||||||||||||||||
| Positive | 77 (26) | 23 (29) | 33 (35) | 0 (0) | 0 (0) | 2 (15) | 1 (4) | 10 (29) | 8 (38) | |||||||
| Negative | 222 (74) | 55 (71) | 62 (65) | 24 (100) | 6 (100) | 11 (85) | 26 (96) | 25 (71) | 13 (62) | |||||||
| Not done | 26 | 8 | 7 | 0 | 1 | 1 | 3 | 2 | 4 | |||||||
| CD33 status, no. (%) | ||||||||||||||||
| Positive | 67 (22) | 19 (24) | 33 (35) | 0 (0) | 0 (0) | 0 (0) | 2 (7) | 7 (20) | 6 (26) | |||||||
| Negative | 235 (78) | 59 (76) | 62 (65) | 23 (100) | 7 (100) | 13 (100) | 26 (93) | 28 (80) | 17 (74) | |||||||
| Not done | 23 | 8 | 7 | 1 | 0 | 1 | 2 | 2 | 2 | |||||||
| CD34 status, no. (%) | ||||||||||||||||
| Positive | 223 (72) | 57 (71) | 92 (95) | 10 (41) | 0 (0) | 8 (57) | 14 (48) | 24 (76) | 18 (75) | |||||||
| Negative | 86 (28) | 23 (29) | 5 (5) | 14 (59) | 7 (100) | 6 (43) | 15 (52) | 10 (24) | 6 (25) | |||||||
| Not done | 16 | 6 | 5 | 0 | 0 | 0 | 1 | 3 | 1 | |||||||
| MDR, no. (%) | ||||||||||||||||
| Yes | 57 (23) | 15 (24) | 16 (21) | 2 (11) | 1 (14) | 1 (9) | 7 (26) | 8 (27) | 7 (37) | |||||||
| No | 193 (87) | 47 (76) | 59 (79) | 17 (89) | 6 (86) | 10 (91) | 20 (74) | 22 (73) | 12 (63) | |||||||
| Not done | 75 | 24 | 27 | 5 | 0 | 3 | 3 | 7 | 6 | |||||||
Treatment results
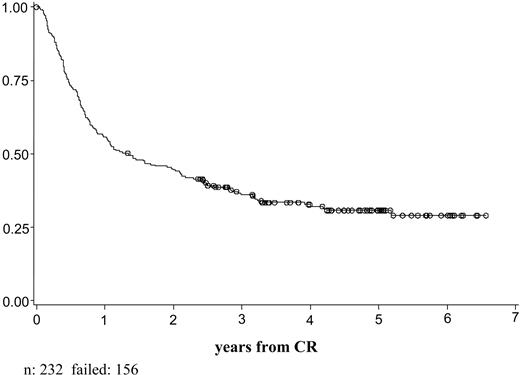
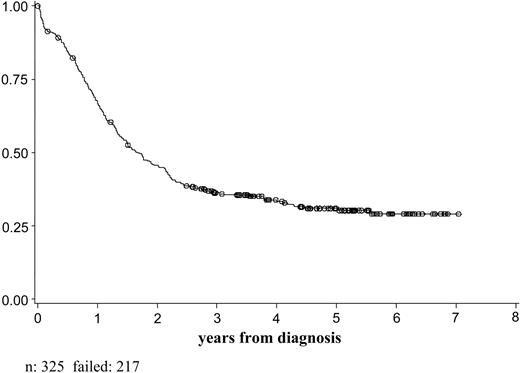
Of the 325 patients registered and eligible for the study who could be considered for the integrated cytogenetic-molecular classification, 317 started the induction treatment and 294 were evaluable for response (Table 5). Of these, 232 patients (79%) obtained CR. The overall DFS was 31% at 5 years (95% CI, 24.4%-37.0%; Figure 1). The OS at 5 years was 31% (95% CI, 25.6%-36.3%; Figure 2). The overall clinical outcome of the patient population considered for the cytogenetic-molecular classification did not differ from that of the other patients enrolled in the trial (CR rate, 80%; DFS and OS at 5 years, 33% [95% CI, 27.7%-37.9%], and 33% [95% CI, 28.3%-37.3%], respectively).
Flow chart of the patients included in the GIMEMA 0496 protocol
. | Clinical trial . | Central handling . | Evaluable integrated cytogenetic-molecular classification . |
|---|---|---|---|
| No. patients eligible for the study | 498 | 414 | 325 |
| No. patients who started induction | 490 | 408 | 317 |
| No. patients evaluable for response | 455 | 380 | 294 |
| No. RC (%) | 363 (80) | 303 (80) | 232 (79) |
. | Clinical trial . | Central handling . | Evaluable integrated cytogenetic-molecular classification . |
|---|---|---|---|
| No. patients eligible for the study | 498 | 414 | 325 |
| No. patients who started induction | 490 | 408 | 317 |
| No. patients evaluable for response | 455 | 380 | 294 |
| No. RC (%) | 363 (80) | 303 (80) | 232 (79) |
DFS of 232 patients obtained CR and considered in the integrated cytogenetic-molecular classification.
DFS of 232 patients obtained CR and considered in the integrated cytogenetic-molecular classification.
OS of 325 patients considered for the integrated cytogenetic-molecular classification.
OS of 325 patients considered for the integrated cytogenetic-molecular classification.
MDR1 expression
The MDR protein expression was assessed by MRK16 binding. MDR1 expression was detected in 57 (23%) of the 250 analyzed cases.
Prognostic value of the integrated molecular-cytogenetic classification in ALL patients at presentation
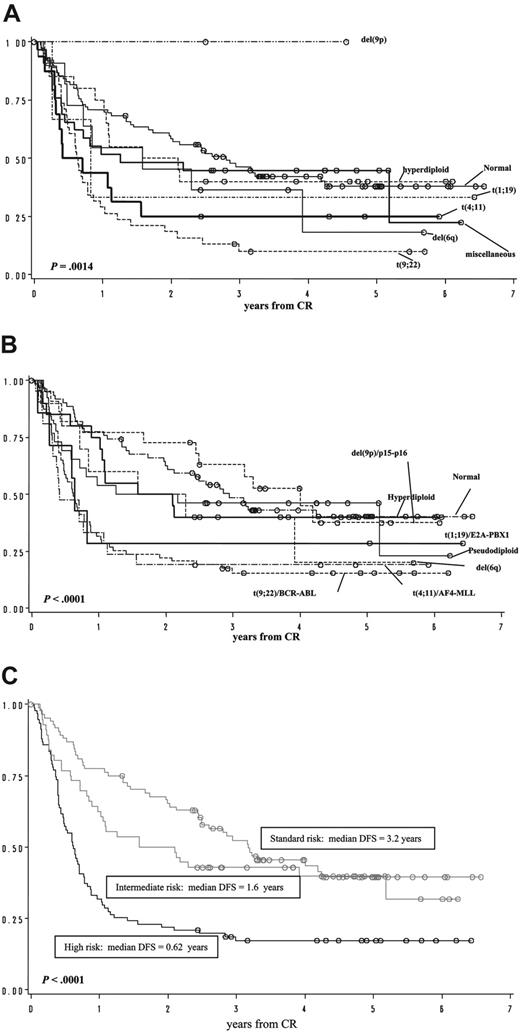
Excluding patients not evaluable for response to induction, 294 of 325 cases could be assessed for their clinical outcome. No significant differences among the 8 cytogenetic-molecular subgroups were observed in terms of CR rate (P = .238). In contrast, on the basis of the median DFS, 3 prognostic groups could be distinguished by the presence of specific cytogenetic-molecular abnormalities. Cases with normal karyotype and isolated 9p/p15-p16 deletions are suggestive of having a relatively better outcome (median DFS, > 3 years) and were considered as a “standard-risk” group, whereas patients with t(9;22), t(4;11), and t(1;19) showed an adverse prognosis (median DFS, 7 months) and were comprised in an “adverse-risk” category. Patients with del(6q), miscellaneous abnormalities, and hyperdiploidies exhibited an intermediate outcome (median DFS, 19 months) and were included in the “intermediate-risk” group. In univariate analysis, with respect to the DFS probability at 2 years, patient stratification on the basis of cytogenetic-molecular features was significantly associated with outcome (P < .001; Figure 3). In a multivariate analysis model including age, WBC count, MDR, and immunophenotype, patients in the “high-risk” group had a statistically significant lower probability to be alive without disease recurrence after 2 years from remission, compared to the “intermediate-risk” (hazard ratio [HR] 2.00; P = .02), and patients in the “standard-risk” showed a significantly higher probability of being alive in remission compared to those in the intermediate-risk group (HR 0.55; P = .04; Table 6). After a more prolonged follow-up, the differences in outcome between the favorable and intermediate groups tend to erode, becoming comparable at 5 years (Figure 3 and Tables 7, 8, 9).
DFS by univariate analysis. DFS according to (A) cytogenetic subgroups, (B) cytogenetic-molecular subgroups, and (C) cytogenetic-molecular risk groups.
DFS by univariate analysis. DFS according to (A) cytogenetic subgroups, (B) cytogenetic-molecular subgroups, and (C) cytogenetic-molecular risk groups.
Multivariate analysis for DFS at 2 years
Variable . | P value . | Hazard ratio . | 95% hazard ratio confidence limits . | . | |
|---|---|---|---|---|---|
| WBCs, × 109/L* | 0.0479 | 1.002 | 1.000 | 1.003 | |
| Age, y† | 0.2061 | 1.010 | 0.994 | 1.026 | |
| Phenotype, B or T | 0.2924 | 1.385 | 0.755 | 2.533 | |
| MDR, negative or positive | 0.7815 | 0.928 | 0.546 | 1.575 | |
| Cytogenetic-molecular risk group | |||||
| Intermediate vs standard | 0.0428 | 0.552 | 0.311 | 0.981 | |
| Intermediate vs high | 0.0231 | 2.006 | 1.100 | 3.659 | |
Variable . | P value . | Hazard ratio . | 95% hazard ratio confidence limits . | . | |
|---|---|---|---|---|---|
| WBCs, × 109/L* | 0.0479 | 1.002 | 1.000 | 1.003 | |
| Age, y† | 0.2061 | 1.010 | 0.994 | 1.026 | |
| Phenotype, B or T | 0.2924 | 1.385 | 0.755 | 2.533 | |
| MDR, negative or positive | 0.7815 | 0.928 | 0.546 | 1.575 | |
| Cytogenetic-molecular risk group | |||||
| Intermediate vs standard | 0.0428 | 0.552 | 0.311 | 0.981 | |
| Intermediate vs high | 0.0231 | 2.006 | 1.100 | 3.659 | |
Range, 0.5-848 × 109/L
Range, 16-60 years
Univariate analysis for DSF according to cytogenetic subgroups
. | +, y . | Median, % . | 95% CI, y . | No. patients . | No. events . |
|---|---|---|---|---|---|
| del(9p) | Not reached* | - | - | 2 | 0 |
| Normal | 2.8 | 49 | 38.2-60.2 | 83 | 48 |
| Hyperdiploid | 1.6 | 50 | 28.1-71.9 | 20 | 12 |
| del(6q) | 1.6 | 45 | 16.0-74.9 | 11 | 8 |
| Miscellaneous | 1.3 | 48 | 30.1-66.5 | 29 | 17 |
| t(1;19) | 0.8 | 33 | 0-86.7 | 3 | 2 |
| t(9;22) | 0.6 | 50 | 34.1-65.9 | 38 | 34 |
| t(4;11) | 0.4 | 50 | 25.5-74.5 | 16 | 12 |
. | +, y . | Median, % . | 95% CI, y . | No. patients . | No. events . |
|---|---|---|---|---|---|
| del(9p) | Not reached* | - | - | 2 | 0 |
| Normal | 2.8 | 49 | 38.2-60.2 | 83 | 48 |
| Hyperdiploid | 1.6 | 50 | 28.1-71.9 | 20 | 12 |
| del(6q) | 1.6 | 45 | 16.0-74.9 | 11 | 8 |
| Miscellaneous | 1.3 | 48 | 30.1-66.5 | 29 | 17 |
| t(1;19) | 0.8 | 33 | 0-86.7 | 3 | 2 |
| t(9;22) | 0.6 | 50 | 34.1-65.9 | 38 | 34 |
| t(4;11) | 0.4 | 50 | 25.5-74.5 | 16 | 12 |
del(9p) 100% at 4 years
Univariate analysis for DSF according to cytogenetic-molecular subgroups
. | +, y . | Median, % . | 95% CI, y . | No. patients . | No. events . |
|---|---|---|---|---|---|
| del(9p)/p15-p16 | 4.0 | 45 | 22.0-68.0 | 22 | 12 |
| Normal | 2.9 | 50 | 37.7-63.1 | 63 | 35 |
| Hyperdiploid | 1.6 | 50 | 28.1-71.9 | 20 | 12 |
| del(6q) | 1.6 | 50 | 19.0-81.0 | 10 | 7 |
| Pseudodiploid | 1.3 | 50 | 30.8-69.2 | 26 | 15 |
| t(1;19)/E2A-PBX1 | 0.6 | 43 | 6.2-79.5 | 7 | 5 |
| t(9;22)/BCR-ABL | 0.6 | 49 | 36.9-61.6 | 63 | 53 |
| t(4;11)/AF4-MLL | 0.4 | 48 | 26.3-69.0 | 21 | 17 |
. | +, y . | Median, % . | 95% CI, y . | No. patients . | No. events . |
|---|---|---|---|---|---|
| del(9p)/p15-p16 | 4.0 | 45 | 22.0-68.0 | 22 | 12 |
| Normal | 2.9 | 50 | 37.7-63.1 | 63 | 35 |
| Hyperdiploid | 1.6 | 50 | 28.1-71.9 | 20 | 12 |
| del(6q) | 1.6 | 50 | 19.0-81.0 | 10 | 7 |
| Pseudodiploid | 1.3 | 50 | 30.8-69.2 | 26 | 15 |
| t(1;19)/E2A-PBX1 | 0.6 | 43 | 6.2-79.5 | 7 | 5 |
| t(9;22)/BCR-ABL | 0.6 | 49 | 36.9-61.6 | 63 | 53 |
| t(4;11)/AF4-MLL | 0.4 | 48 | 26.3-69.0 | 21 | 17 |
Univariate analysis for DSF according to cytogenetic-molecular risk groups
. | +, y . | 95% CI, y . | No. patients . | No. events . |
|---|---|---|---|---|
| Standard | 3.2 | 38.6-60.6 | 85 | 47 |
| Intermediate | 1.6 | 36.9-63.1 | 56 | 34 |
| High | 0.62 | 39.2-59.7 | 91 | 75 |
. | +, y . | 95% CI, y . | No. patients . | No. events . |
|---|---|---|---|---|
| Standard | 3.2 | 38.6-60.6 | 85 | 47 |
| Intermediate | 1.6 | 36.9-63.1 | 56 | 34 |
| High | 0.62 | 39.2-59.7 | 91 | 75 |
Discussion
Despite the efforts to identify at the onset of the disease subgroups of adult patients with ALL with a different prognostic likelihood, the predictive value of the risk models so far proposed is still limited. Considering that cytogenetic and molecular features are among the most important factors for predicting outcome, we hereby propose a risk classification system based on an integrated cytogenetic-molecular characterization of a large series of patients with adult ALL enrolled in the GIMEMA ALL 0496 trial and studied prospectively at presentation. This systematic approach first allowed us to decrease the number of cases with failed cytogenetics (from 25% to 16%) or normal karyotype (from 29.5% to 22%) through the detection of molecular lesions not identified by conventional cytogenetics and vice versa, and second, to depict 8 different cytogenetic-molecular subgroups as much as possible homogeneous for defined genetic abnormalities, such as BCR-ABL or t(9;22), MLL-AF4 or t(4;11), p15 and/or p16 deletion or del(9p), and so forth (Table 5). Within the normal karyotype group, 28 of 114 patients had molecular anomalies missed by conventional cytogenetics. The most frequently detected were p15 and p16 deletions (18 of 28 cases), t(9;22) (4 of 28 cases), and t(4;11) (3 of 28 cases). This confirms previous observations that most of the p15 and p16 deletions are not detected by conventional cytogenetics.24,25 In the group with failed cytogenetic analysis, 35 of 96 cases were found to harbor molecular abnormalities consisting of BCR-ABL (25 of 35), AF4-MLL (1 of 35), and E2A-PBX1 (1 of 35) rearrangements, and p15 or p16 deletions (8 of 35). Considering the patients with normal or not evaluable cytogenetics, most molecular abnormalities are restricted to the failed cytogenetic group (77.4% versus 22.6%; P = < .001).
When the DFS probability at 2 years is considered as an early prognostic end point, the cytogenetic-molecular subgroups delineated in the present study allow prediction of a different clinical outcome likelihood. Cases with no abnormalities or with 9p/p15 and/or p16 deletions have a significantly higher DFS probability, as shown in Figure 3. The prognostic significance of a normal karyotype has been seldom estimated in adult ALL and varies in different series. The results of our study are consistent with the outcome of a series of 443 adult patients reported by the Groupe Français de Cytogénétique Hématologique (GFCH),3 which found the normal karyotype group to be associated with a better prognosis (median event-free survival [EFS], 24 months; EFS 44%) than the abnormal karyotype group (P < .001) in B-lineage ALL. This finding was not confirmed by the Medical Research Council (MRC) UKALL XA trial,4 which showed that DFS for patients with a diploid karyotype was comparable with the poor-outcome categories. This result, however, could be due to the presence of poor-risk genetic lesions missed by conventional cytogenetics, because in this study molecular investigations were not performed. The frequency of normal karyotypes has been reported to range between 16% and 34% for adult ALL patients; clinical features associated with this subgroup include a median age ranging between 30 and 44 years, a median WBC count between 10 and 30 × 109/L, and a T-cell phenotype in 18% to 38% of cases.1-5,26 In the present series, the subgroup with normal karyotype represented 27% of cases and showed more frequently than the abnormal cytogenetic-molecular subgroups a young age (27 years; range, 14-58 years), a low WBC count (6.8 × 109/L; range, 0.6-700 × 109/L), whereas the prevalence of T-cell phenotype was similar to that of the other groups (23%).
In addition to normal karyotype, the standard-risk group included cases carrying abnormalities of the short arm of chromosome 9, including p15 and p16 deletions. These abnormalities have been found in approximately 10% of childhood ALL with a higher percentage in T-ALL and in 5% to 16% of adults.27-31 Frequently, 9p deletions have been associated with other abnormalities, in most cases the t(9;22) translocation, and are considered a secondary change.5,24,30 In our series, 9p, p15 and/or p16 deletions were found in 52 (25%) of 211 analyzed cases emerging as the most frequent molecular abnormalities after BCR-ABL. In 30 cases, 19 with B- and 11 with T-cell phenotype, this group of anomalies was present as an isolated change, suggesting that it may be considered as a primary change in ALL. Comparing this group with the 22 cases carrying 9p abnormalities in addition to other changes consisting of a t(9;22) (11 cases), other structural changes included in the pseudodiploid subgroup (7 cases), a del(6q) (2 cases), a t(1;19) (1 case), and a t(4;11) (1 case), patients with isolated 9p/p15 and/or p16 deletion showed a similar median age (27 versus 29 years), but a lower median WBC count (21 versus 46 × 109/L). In a pediatric series treated with a Children's Cancer Group risk-adjusted protocol, Heerema et al27 reported that deletions of 9p were associated with an adverse prognosis in B-lineage ALL and, in the homozygous form, also in T-ALL. This finding has been confirmed by Carter et al,32 who reported that homozygous and hemizygous deletions of p16 are independent predictors of relapse. Disagreement still exists about the correlation with prognostic and immunophenotypic features of 9p abnormalities in adult ALL. Fizzotti et al33 found a significant relationship between p16 deletion and a subset of ALL with features of aggressive disease (high leukemic cell mass and high WBC counts), but no differences by immunophenotype. Indeed, the GFCH reported no impact on prognosis and no significant correlation with B- or T-lineage ALL.3 Similar findings were observed by Faderl et al,24 who reported no difference in the outcome of patients with homozygous p15 and p16 gene deletions with respect to patients with no deletions or loss of only one allele. The correlation of 9p abnormalities with an adverse prognosis may depend on their frequent association with additional karyotypic changes, often consisting in poor-risk aberrations such as t(9;22), t(4;11), or complex abnormalities. Conversely, our study suggests that the p15 and/or p16 deletions, when present as an isolated change, are not associated with a high risk of relapse at short term.
The intermediate prognostic category included patients with del(6q), cases with diploid karyotype, and a miscellaneous group of other structural changes not encompassed by the other 2 risk groups, which were too infrequent to be assigned a prognostic significance in their own right, as well as cases with only numerical acquisitions. Partial deletions of the long arm of chromosome 6 have mainly been reported in pediatric T-ALL, with a frequency ranging between 6% and 10% of patients, and an association with a favorable prognosis. In adults, too few cases have been reported so far to allow definitive clinical and biologic correlations. Our study highlights the preferential correlation of this anomaly with a T-cell phenotype, especially when it is present as isolated change, as reported elsewhere.11 Moreover, the result of our analysis makes previous not unfavorable prognostic suggestions somewhat questionable, on the basis of the median DFS of 19 months recorded in the del(6q) subgroup. The hyperdiploid group, which includes 9 cases (32%) with so-called “high” hyperdiploidy (> 51 chromosomes), accounts for 8% of successfully investigated cases, in line with what so far is described in adult ALL. More than 50% of the hyperdiploid cases described in the literature were of B-cell phenotype, were younger than 30 years, and a normal WBC count.3,4 The clinical outcome of patients with hyperdiploidy varies in different series, being more favorable in children than in adults, where a poor outcome has been repeatedly reported.3,5,26 Similarly, in the present study a median DFS of 19 months has been recorded in the hyperdiploid group, despite the absence of unfavorable structural changes, indicating that adults may not enjoy the good prognosis reported in children. Adverse risk features, which may correlate with an unfavorable outcome, such as WBC count more than 30 × 109/L and age older than 30 years,34,35 were observed in this group in 24% and 44% of cases, respectively.
The t(9;22), t(4;11), and t(1;19) translocations defined a cytogenetic-molecular group with unfavorable outcome. With respect to the first 2 abnormalities, our data are consistent with what is reported by several groups in terms of poor outcome.3-5 The association with a poor prognosis of the t(1;19) translocation, which in this series was found in 4% of cases, is still debated. In pediatric ALL, where this aberration is more common (8%), improved outcome has been obtained with a more intensified treatment.36 In adults, the GFCH3 described 11 cases, of which 9 failed therapy within 12 months from diagnosis; conversely, the MRC UKALL XA4 did not confirm the adverse outcome, reporting a 3-year DFS of 54% in 10 patients, 4 of them undergoing bone marrow transplantation. In the present study, t(1;19) characterizes a subset of relatively young patients with CD10+, often CyIg+, B-lineage ALL who do not express CD34 and myeloid associated antigens. Despite the high CR rate, this anomaly was associated with early treatment failure in 5/7 patients.37
Our study emphasizes the feasibility of a combined cytogenetic-molecular classification of adult patients with ALL in the context of a multicenter clinical trial. At 2 years, the stratification in 3 risk groups derived from this approach emerges, in univariate and multivariate analysis, as a variable significantly associated with DFS probability. Although at 5 years the favorable and intermediate groups had an overall comparable outcome, this model highlights a category of patients with at least 2 years of relatively favorable outcome during which, on the basis of the presence of minimal residual disease, the impact of consolidative treatment strategies including newer drugs should be evaluated.
In conclusion, our data indicate that a combined broad cytogenetic-molecular classification of adults with ALL at presentation provides a framework that clearly distinguishes groups of patients with a different likelihood of relapse and that, in the context of multicenter protocols, this integrated approach is essential for the design of a risk-adapted therapeutic algorithm. The challenge of future trials will be to further extend the number of cytogenetic-molecular profiled cases by using novel techniques, such as spectral karyotyping and comparative genomic hybridization, as well as to complement the genetic screening with other parameters in order, ultimately, to permit a more refined and targeted clinical management.
Appendix
The following institutions and investigators participated in this study: Ospedale SG Moscati, Avellino, Ettore Volpe; Istituto di Ematologia L. A. Seràgnoli, Bologna, Michele Baccarani; Ospedale S Giovanni Bosco, Napoli, Eustachio Miraglia; Ospedale S. Francesco, Attilio Gabbas; Ospedale S. Carlo, Potenza, Francesco Ricciuti; Università Cattolica, Roma, Giuseppe Leone; Ospedale Cardarelli, Napoli, Vincenzo Mettivier; Ospedale S Giovanni Battista, Torino, Mario Boccadoro; Ospedale SS Antonio e Biagio, Alessandria, Alessandro Levis; Ospedale S Maria Goretti, Latina, Angelo De Blasio; Università di Palermo, Pietro Citarrella; Ospedale S Antonio Abate, Gallarate, Ruggero Mozzana; Arcispedale S Maria Nuova, Reggio Emilia, Luigi Gugliotta; Ospedale S Eugenio, Roma, Sergio Amadori; Università di Sassari, Maurizio Longinotti; Ospedale S Martino, Genova, Gino Santini; Dipartimento di Medicina Interna, Università di Genova, Riccardo Ghio; Ospedale A Di Summa Brindisi, Giovanni Quarta; Ospedale A Sclavo, Siena, Francesco Lauria; Ospedale Maggiore, Lodi, Giulio Nalli; Ospedale E Morelli, Sondalo, Renzo Epis; CRO Aviano, Umberto Tirelli; Università di Palermo, Vincenzo Abbadessa; Ospedale S Salvatore, Pesaro, Giuseppe Visani; Ospedale S Croce e Carle, Cuneo, Andrea Gallamini; Ospedale SS Annunziata, Taranto, Patrizio Mazza; Ospedale Maggiore della Carità, Novara, Giancarlo Avanzi.
Prepublished online as Blood First Edition Paper, January 13, 2005; DOI 10.1182/blood-2004-07-2922.
Supported by the Associazione Italiana contro le leucemie–Sezione di Roma (Romail); the Consiglio Nazionale delle Ricerche (CNR), Ministero dell'istruzione, dell'Università e della Ricerca (MIUR), and Associazione Italiana Ricerca sul Cancro (AIRC; C.M.); Progetto Finalizzato Oncologia, Fondazione Internazionale di Ricerca in Medicina (FIRMS); and the Istituto Superiore di Sanità (R.F.).
A complete list of the members of the Gruppo Italiano Malattie EMatologiche dell' Adulto (GIMEMA) appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Francesca Paoloni for biostatistical analysis and Sandra De Simone for data management.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal