Abstract
Monocyte cytokines (ie, monokines) induce natural killer (NK) cells to produce interferon-γ (IFN-γ), which is critical for monocyte clearance of infectious pathogens and tumor surveillance. Human CD56bright NK cells produce far more IFN-γ in response to monokines than do CD56dim NK cells. The kinases and phosphatases involved in regulating IFN-γ production by monokine-activated NK cells are not clearly identified. SHIP1 is a 5′ inositol phosphatase that dephosphorylates the phosphatidylinositol-3 kinase (PI-3K) product PI3,4,5P3. Here, we show that constitutive expression of SHIP1 is distinctly lower in CD56bright NK cells compared with CD56dim NK cells, suggesting it could be an important negative regulator of IFN-γ production in monokine-activated NK cells. Indeed, overexpression of SHIP1 in CD56bright NK cells followed by monokine activation substantially lowered IFN-γ production. This effect was not seen when NK cells were infected with a SHIP1 mutant containing an inactive catalytic domain. Finally, NK cells in SHIP1–/– mice produced more IFN-γ in response to monokines in vivo than did NK cells from wild-type mice. Collectively, these results demonstrate that SHIP1 negatively regulates monokine-induced NK cell IFN-γ production in vitro and in vivo and provide the first molecular explanation for an important functional distinction observed between CD56bright and CD56dim human NK subsets.
Introduction
Natural killer (NK) cells are CD56+CD16+CD3– large granular lymphocytes that constitute approximately 10% of peripheral blood mononuclear cells.1 NK cells are critical components of the innate immune system by virtue of their ability to produce important immunoregulatory cytokines, such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF).1,2 NK cells also mediate cytotoxicity against a variety of target cells that lack matching major histocompatibility complex (MHC) ligands. In peripheral blood 2 distinct subsets of human NK cells have been identified that differ in their phenotype and function.3 Most NK cells in blood are CD56dim and express high levels of CD16, whereas the minority are CD56bright and CD16dim/neg. Functionally, immunoregulatory CD56bright NK cells produce abundant cytokines in response to monokine costimulation, while CD56dim NK cells are inefficient cytokine producers yet efficient effectors of natural and antibody-dependent target cell lysis.4,5 The molecular basis for the functional distinction of cytokine secretion between NK subsets is unknown.
CD56bright NK cells are powerful producers of IFN-γ, an immunoregulatory cytokine that plays a critical role in the clearance of infectious pathogens and in tumor immune surveillance.6-8 Macrophages infected with intracellular pathogens or stimulated by bacterial products are able to induce CD56bright NK cell production of IFN-γ via the secretion of proinflammatory monokines such as interleukin-12 (IL-12), IL-15, and IL-18. NK cells constitutively express functional receptor complexes for these monokines.9 Whereas the stimulation of human NK cells with a single monokine does not induce significant IFN-γ secretion, combinations of monokines such as IL-12 and IL-18 synergistically induce high amounts of IFN-γ.4,9 The biology of NK cells with regard to their monokine receptors and production of immunoregulatory cytokines has not been well studied, and many questions remain unanswered. In particular, the kinases and phosphatases that are responsible for the induction and regulation of IFN-γ by the different monokines in NK cells are not well defined. The receptors for IL-12, IL-15, and IL-18 do not share receptor chains,10,11 and none of the known receptor subunits possess intrinsic tyrosine kinase activity. Stimulation with IL-12, IL-15, and IL-18 has been associated with the induction of phosphorylation and/or activation of the src tyrosine kinase lck and the serine/threonine mitogen-activated protein kinase (MAPK)12-16 and activates different signal transducers and activators of transcription (STATs).15,17,18 A characteristic of the signaling events of IL-18 is the phosphorylation of the serine/threonine kinase IL-1-receptor–associated kinase (IRAK) and the sequential activation of nuclear factor–κB (NF-κB).11,19
SHIP1 is a hematopoietic cell–specific SH2 containing 5′ inositol phosphatase and a negative regulator of cell proliferation, survival, and cell activation.20 SHIP1 mediates the degradation of the phosphatidylinositol-3 kinase (PI-3K) product phosphatidylinositol (3,4,5) triphosphate (PI3,4,5P3) into phosphatidylinositol (3,4) bisphosphate (PI3,4P2), linking its activity to the PI-3K pathway. Whereas the FcγRIIB inhibitory signaling in B cells is dependent on SHIP1, the killer cell immunoglobulin-like receptor (KIR) inhibitory signaling in NK cells requires the tyrosine phosphatase SHP1 but not SHIP1.21-23 There is, however, considerable evidence demonstrating a role for SHIP1 as a negative regulator for FcγR activation signaling and some cytokine receptor signaling.24-26 SHIP1 is constitutively expressed in human NK cells, and activation of CD16 induces the transient translocation of SHIP1 to raft domains where it interacts with the receptor complex and negatively modulates antibody-dependent cellular cytotoxicity (ADCC).27,28 In mouse NK cells SHIP1 influences the development of the NK cell receptor repertoire, and its absence can alter the outcome in bone marrow transplantation29 ; however, whether SHIP1 has a role in regulating IFN-γ gene expression in NK cells following monokine stimulation has not been addressed.
Here we provide what we believe to be the first molecular evidence that explains the functional distinction in cytokine production between the CD56bright and CD56dim NK subsets. We show that SHIP1 mRNA and protein levels are constitutively lower in CD56bright NK cells compared with CD56dim NK cells, that SHIP1 is down-modulated by activating monokines, and that SHIP1 negatively regulates NK cell IFN-γ production induced by monokines.
Materials and methods
Cells lines and NK cell preparations
The human IL-2–dependent NK cell line NK92 (gift from Dr H. Klingemann, Rush Cancer Center, Chicago, IL) was maintained in culture in RPMI 1640 medium (Invitrogen, Carlsbad, CA) containing Glutamax supplemented with 20% heat-inactivated fetal bovine serum (FBS) (Invitrogen) and 150 IU/mL recombinant human IL-2 (rhIL-2) (Hoffman-LaRoche, Nutley, NJ).
All work with human cells was approved by The Ohio State University Institutional Review Board. Human NK cells were isolated from peripheral blood leukopacks of healthy individuals (American Red Cross, Columbus, OH) by incubation for 30 minutes with RosetteSep NK cell antibody cocktail (StemCell Technologies, Vancouver, BC), followed by Ficoll-Hypaque density gradient centrifugation and by plastic adherence overnight to remove the monocyte population. The fresh NK cell preparations were more than 85% CD56+, as determined by direct immunofluorescence using an anti-CD56 phycoerythrin (PE)–conjugated monoclonal antibody (mAb) (Immunotech, Marseille, France). CD56bright and CD56dim NK cell subsets were purified based on CD56 cell surface density by fluorescence-activated cell sorting (FACS) upon staining with the anti-CD56–PE.4 NK cells from Ship1–/– and Ship1+/+ mice were purified by DX5/NK1.1 positive selection from single-cell suspensions of splenocytes (Miltenyi Biotec, Auburn, CA). The amphotropic-packaging cell line Phoenix (gift from Dr G. P. Nolan, Stanford University, CA) was maintained in culture in Dulbecco modified Eagle medium (DMEM) (Invitrogen)/10% FBS medium and grown for 16 to 18 hours to 80% confluence prior to transfection by calcium phosphate–DNA precipitation (Profection system; Promega, Madison, WI).
Retroviral infection of NK92 cell line and primary human NK cells
PINCO–wild-type (PINCO-wt) SHIP1 retroviral vector and the mutant pTRE-D675A SHIP1 vector were kindly provided by Dr Martin Sattler (Dana-Farber Cancer Institute, Harvard University, Boston, MA) and Dr Larry R. Rohrschneider (Fred Hutchinson Cancer Research Center, Seattle, WA).30-32 To generate PINCO-D675A SHIP1 vector, the cDNA fragment encoding the D675A SHIP1 mutant was obtained after EcoRI digestion of pTRE-D675A SHIP1 plasmid and were cloned into the EcoRI-digested PINCO retroviral vector. Correct orientation of the cDNA in the plasmid was detected by restriction enzyme and sequence analyses. For infection, the NK92 cell line and primary NK cells were pretreated for 2 or 3 days and infected in the presence of 900 IU/mL IL-2 or in the presence of the combination of 300 IU/mL IL-2 and 100 ng/mL KL (Amgen, Seattle, WA) to enrich for CD56brignt NK cells. Retroviral infections were performed following previously published standards.33 Briefly, infectious supernatants from PINCO, PINCO-wt SHIP1, and PINCO-D675A SHIP1–transfected Phoenix cells were collected 48 hours after transfection and used for 3 cycles of infection. Upon infection, NK92 cells were sorted (FACS Vantage; BD Biosciences, San Jose, CA) for green fluorescent protein (GFP) expression, and primary NK cells were sorted for GFP and CD56 expression upon staining with an anti-CD56 allophycocyanin (APC)–conjugated monoclonal Ab (Immunotech). GFP+CD56+ primary NK cells were used immediately after sorting. Expression of the wt or D675A SHIP1 fusion proteins was confirmed in NK92 cells by Western blot using an anti-SHIP1 Ab (UBI, Lake Placid, NY).
Mice
C57BL/2 Ship1–/– and Ship1+/+ mice, kindly provided by G. Krystal (The Terry Fox Laboratory, Vancouver, BC, Canada), were 5- to 7-week-old littermates.34 All animal work was approved by The Ohio State University Animal Care and Use Committee, and mice were treated in accordance with the institutional guidelines for animal care.
Cell culture conditions
Prior to monokine stimulation, NK92 cells were cultured in IL-2–free medium containing 10% FBS for 24 hours or in medium containing 0.1% bovine serum albumin (BSA) for 4 hours. Cells were next incubated in medium plus 10% FBS at 37°C (0.5 × 106 to 1.0 × 106/mL) for the indicated times with the addition of different monokine(s). Monokines included recombinant human IL-12 (rhIL-12) and recombinant murine IL-12 (rmuIL-12) kindly provided by Genetics Institute (Cambridge, MA), rhuIL-18 and rmuIL-18 (BASF Bioresearch, Worcester, MA), and rhIL-15 and rmuIL-15 (Amgen). Where noted, the PI-3K inhibitor LY294002 (Calbiochem, San Diego, CA) was added at the indicated concentrations for 45 minutes at 37°C before monokine stimulation. The inhibitor was present throughout stimulation.
Western blot analysis
Cells were harvested, washed once with ice-cold phosphate-buffered saline (PBS), and lysed (108 cells per milliliter of lysis buffer: 1% Nonidet P-40 [NP-40], 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] pH 7.5, 0.15 to 0.5 M NaCl, 10% glycerol supplemented with protease and phosphatase inhibitors, 1 mM phenylmethylsulfonylfluoride [PMSF], 1 mM Na3VO4, 50 mM NaF, 10 mM β-glycerol-phosphate, 1 mM EDTA [ethylenediaminetetraacetic acid], and a protease inhibitor cocktail tablet from Roche Applied Science, Indianapolis, IN), as described.33 Alternatively, cells were directly lysed in Laemmli buffer (2 × 105 cells per 20 μL). Western blotting was performed according to previously published protocols,35 and Ab-reactive proteins were detected with horseradish peroxidase–labeled sheep anti–rabbit, –mouse, and/or –goat immunoglobulin (Ig) sera and enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL). Proteins were analyzed by 4% to 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (BIO-RAD Laboratories, Hercules, CA) using reducing conditions. Monoclonal and polyclonal antibodies used were polyclonal rabbit sera anti-SHIP1 and anti-SHP1 from UBI; monoclonal anti-SHIP1 Ab from Santa Cruz Biotechnology (Santa Cruz, CA); monoclonal anti–growth factor receptor-bound protein-2 (anti-GRB2) from Transduction Laboratories (San Diego, CA); and the antiactin goat polyclonal Ab from Sigma (St Louis, MO).
Detection of IFN-γ production by intracellular flow cytometry
Following 18 hours of coculture with IL-12 and IL-18, PINCO-infected and PINCO-SHIP1–infected NK92 cells were cultured for 4 hours with brefeldin A, harvested, stained with anti-CD56 APC mAb, and fixed and permeabilized using Cytofix/Cytoperm reagent (BD Biosciences). Cells were then stained with an antihuman IFN-γ-APC mAb or isotype control–APC mAb (BD Biosciences). A similar protocol was used for the detection of murine IFN-γ from splenocytes harvested from Ship1–/– and Ship1+/+ mice 24 hours after intraperitoneal injection of IL-12 and IL-18. Murine cells were stained with DX5-PE and NK1.1-PE (pan-NK) mAbs and antimouse IFN-γ–fluorescein isothiocyanate (FITC) (BD Pharmigen, San Diego, CA) or isotype control–FITC mAbs. Cells were assessed on a FACSCalibur cytometer (BD Biosciences), and analyses were performed using the WinMDI (J. Trotter, Scripts Institute, La Jolla, CA) software program.
IFN-γ ELISA assays
For human IFN-γ ELISA was performed as described4 using commercially available mAb pairs (Endogen, Woburn, MA). Cell-free supernatants were collected after 18 hours of incubation at 37°C. The detection limit was 10 to 30 pg/mL. Results are shown as mean of triplicate wells ± SEM. Cell-free supernatants were harvested after 24 hours and analyzed in duplicate for murine IFN-γ enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN).
RT-PCR and real-time RT-PCR
Total mRNA was extracted using TRIZOL (Invitrogen) or RNeasy Mini kits (Qiagen, Valencia, CA), and cDNA was generated according to the manufacturer's recommendations (Invitrogen). Reverse transcriptase–polymerase chain reaction (RT-PCR) amplification of SHIP1 mRNA was carried using a 5′ primer (5′-GCCTACACCAAGCAGAAAGC-3′), a 3′ primer (5′-GGACCGTTCTTGGAGACAAA-3′), Amplitaq Gold (Applied Biosystems, Foster City, CA), and the following conditions: 94°C for 5 minutes, followed by 20 (NK92) and 25 to 30 (primary NK cells) cycles of 94°C (45 seconds), 55°C (45 seconds), and 72°C (45 seconds). The 18S rRNA levels were monitored as control for equal loading using a 5′ primer (5′-CGGCTACCACATCCAAGGAA-3′), a 3′ primer (5′-GCTGGAATTACCGCGGCT), and the following conditions of PCR: 18 to 20 cycles of 94°C (45 seconds), 55°C (45 seconds), and 72°C (45 seconds).
Real-time RT-PCR reactions for human IFN-γ transcripts were performed as a multiplex reaction with primer/probe set specific for the IFN-γ mRNA (forward, 5′-GAAAAGCTGACTAATTATTCGGTAACTG-3′; reverse, 5′-GTTCAGCCATCACTTGGATGAG-3′; probe, 5′FAM-CTTGAATGTCCAACGCAAAGCAATACATGA-3′TAMRA) and an internal 18S rRNA control. Complementary DNA from phytohemagglutinin (PHA)–activated human lymphocytes served as positive controls for cytokine transcripts, and water (no template) was used as a negative control. Reactions were performed using an ABI prism 7700 sequence detector (Taqman; Applied Biosystems), and data were analyzed with the Sequence Detector version 1.6 software to establish the PCR cycle at which the fluorescence exceeded a set threshold, CT, for each sample. Data were analyzed according to the comparative CT method, as previously described,9 using the internal control (18S) transcript levels to normalize differences in sample loading and preparation. Results represent the n-fold difference of transcript levels in a particular sample compared with calibrator cDNA (cDNA samples of unstimulated PINCO-infected or untreated NK92 cells). Results are expressed as the mean ± SEM of triplicate reaction wells.
Statistics
Data were compared using Student 2-tailed t test. P less than .05 was considered statistically significant.
Results
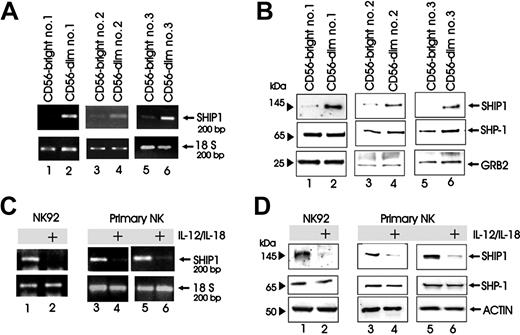
We first postulated that SHIP1 could prove to be a negative regulator for NK cytokine production following monokine costimulation. Based on this we asked if resting human CD56bright NK cells that have a significantly enhanced capacity to invoke a cytokine response to monokine costimulation compared with CD56dim NK cells3 would constitutively express less SHIP1. Figure 1A-B shows that in 3 consecutive healthy donors, resting CD56bright NK cells constitutively express less SHIP1 mRNA and protein when compared with CD56dim NK cells from the same donors. Equivalent levels of the tyrosine phosphatase SHP1 protein were noted in both populations (Figure 1B).
SHIP1 expression in NK cells. (A-B) CD56bright (lanes 1, 3, and 5) and CD56dim (lanes 2, 4, and 6) primary human NK cells were FACS purified from 3 separate donors. Cell pellets were collected and analyzed for SHIP1 transcript by RT-PCR with 18S rRNA used for nomalization (A) or directly lysed in Laemmli buffer and analyzed for protein by Western blot (B). (B) Western blot was performed using anti-SHIP1 (top panels), anti-SHP1 (middle panels), and anti-GRB2 (bottom panels) Abs sequentially on the same filter. (C-D) NK92 and enriched primary human NK cells were costimulated with monokines IL-12 (10 ng/mL) and IL-18 (100 ng/mL) for 24 hours (panel C, lanes 1-6; panel D, lanes 1-4) or 48 hours (panel D, lanes 5 and 6), after which cell pellets were collected and analyzed for SHIP1 transcript by RT-PCR (C) and for protein by Western blot (D). (D) Western blot was performed with anti-SHIP1 (top panels), anti-SHP1 (middle panels), and antiactin (bottom panels) Abs sequentially in the same filter.
SHIP1 expression in NK cells. (A-B) CD56bright (lanes 1, 3, and 5) and CD56dim (lanes 2, 4, and 6) primary human NK cells were FACS purified from 3 separate donors. Cell pellets were collected and analyzed for SHIP1 transcript by RT-PCR with 18S rRNA used for nomalization (A) or directly lysed in Laemmli buffer and analyzed for protein by Western blot (B). (B) Western blot was performed using anti-SHIP1 (top panels), anti-SHP1 (middle panels), and anti-GRB2 (bottom panels) Abs sequentially on the same filter. (C-D) NK92 and enriched primary human NK cells were costimulated with monokines IL-12 (10 ng/mL) and IL-18 (100 ng/mL) for 24 hours (panel C, lanes 1-6; panel D, lanes 1-4) or 48 hours (panel D, lanes 5 and 6), after which cell pellets were collected and analyzed for SHIP1 transcript by RT-PCR (C) and for protein by Western blot (D). (D) Western blot was performed with anti-SHIP1 (top panels), anti-SHP1 (middle panels), and antiactin (bottom panels) Abs sequentially in the same filter.
We next asked if induction of an NK cytokine response by costimulation with 2 proinflammatory monokines (IL-12 and IL-18) would also modulate SHIP1 expression. As shown for both transcript (Figure 1C) and protein (Figure 1D) in the NK92 cell line and in primary human NK cells, 24- and 48-hour costimulation with these monokines resulted in a decrease in NK cell SHIP1 without any change in the levels of the tyrosine phosphatase SHP1 protein.
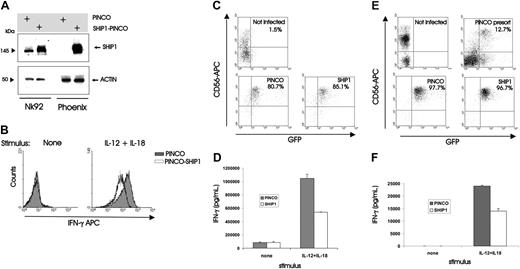
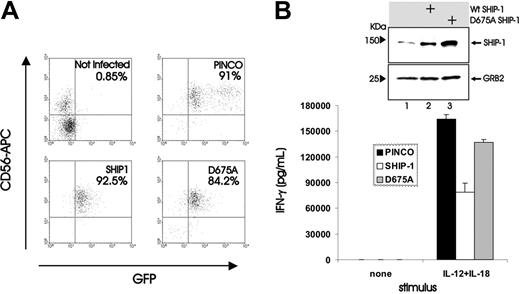
To test our original hypothesis that SHIP1 could serve as a negative regulator of NK cytokine production following monokine costimulation, we next overexpressed SHIP1 in the NK92 cell line by a described method that uses the GFP-expressing Epstein-Barr virus (EBV)/retroviral PINCO hybrid vector.28,31 After confirming the protein expression of SHIP1 in the GFP+ fraction of cells (Figure 2A), we showed that NK92 cells overexpressing SHIP1 produced nearly 60% less IFN-γ than NK92 cells infected with the vector-GFP control upon costimulation with IL-12 plus IL-18 (mean fluorescence intensity [MFI] of 170 versus 71.1, n = 2; Figure 2B), consistent with results by both ELISA and real-time RT-PCR (see Figure 3). We next performed the identical experiment using primary human NK cells (Figure 2C-D) and measured IFN-γ production in supernatants of cultures before and after costimulation with IL-12 plus IL-18. Once again, a significantly lower amount of IFN-γ was detected from NK cells infected with SHIP1 versus the GFP+ vector-only control (P < .001, n = 7; Figure 2D). Most IFN-γ production following monokine costimulation comes from the less abundant CD56bright NK cell subset3 that expresses less SHIP1 compared with the CD56dim NK cell subset (Figure 1). To test whether the lower constitutive expression of SHIP1 allows for more abundant IFN-γ production by the CD56bright NK subset, we overexpressed SHIP1 in primary CD56bright NK cells and demonstrated significantly lower IFN-γ production compared with mock-transfected CD56bright NK cells (P = .003, n = 3; Figure 2F). Thus, the lower constitutive expression of SHIP1 in CD56bright NK cells is at least in part responsible for the relatively high IFN-γ production following monokine costimulation compared with CD56dim NK cells.
Overexpression of SHIP1 blunts monokine-activated IFN-γ production in NK92 and primary human NK cells. (A-B) NK92 cells were retrovirally infected using the PINCO-GFP vector or PINCO vector encoding both SHIP1 and GFP cDNAs. (A) Infected cells were FACS sorted for GFP, and lysates were prepared. Protein lysates were next blotted with anti-SHIP1 and antiactin Abs. Lysates from the transfected Phoenix packaging cell line served as a positive control. (B) NK92 cells that were either infected with PINCO-GFP or with PINCO-SHIP1-GFP were each sorted for GFP+ cells and then incubated overnight with IL-12 (10 ng/mL) plus IL-18 (100 ng/mL), after which cells were assessed for IFN-γ protein production using intracellular flow cytometry. Results are representative of 2 experiments. (C-D) Enriched primary human NK cells were infected with PINCO-GFP or PINCO-SHIP1-GFP vectors. (C) Infected cells were FACS sorted for CD56 and GFP. (D) Sorted CD56+GFP+ NK cells were costimulated for 18 hours with IL-12 and IL-18, and supernatants were then harvested and quantified for IFN-γ production by ELISA. This experiment is representative of 7 performed with similar results. (E-F) Enriched primary CD56bright NK cells were infected with PINCO-GFP or PINCO-SHIP1-GFP vectors. (E) Infected cells were FACS sorted for CD56bright and GFP. (F) Sorted CD56bright GFP+ NK cells were costimulated for 18 hours with IL-12 and IL-18, and supernatants were then harvested and quantified for IFN-γ production by ELISA. This experiment is representative of 3 performed with similar results. Error bars indicate ± SEM (see “Materials and Methods”).
Overexpression of SHIP1 blunts monokine-activated IFN-γ production in NK92 and primary human NK cells. (A-B) NK92 cells were retrovirally infected using the PINCO-GFP vector or PINCO vector encoding both SHIP1 and GFP cDNAs. (A) Infected cells were FACS sorted for GFP, and lysates were prepared. Protein lysates were next blotted with anti-SHIP1 and antiactin Abs. Lysates from the transfected Phoenix packaging cell line served as a positive control. (B) NK92 cells that were either infected with PINCO-GFP or with PINCO-SHIP1-GFP were each sorted for GFP+ cells and then incubated overnight with IL-12 (10 ng/mL) plus IL-18 (100 ng/mL), after which cells were assessed for IFN-γ protein production using intracellular flow cytometry. Results are representative of 2 experiments. (C-D) Enriched primary human NK cells were infected with PINCO-GFP or PINCO-SHIP1-GFP vectors. (C) Infected cells were FACS sorted for CD56 and GFP. (D) Sorted CD56+GFP+ NK cells were costimulated for 18 hours with IL-12 and IL-18, and supernatants were then harvested and quantified for IFN-γ production by ELISA. This experiment is representative of 7 performed with similar results. (E-F) Enriched primary CD56bright NK cells were infected with PINCO-GFP or PINCO-SHIP1-GFP vectors. (E) Infected cells were FACS sorted for CD56bright and GFP. (F) Sorted CD56bright GFP+ NK cells were costimulated for 18 hours with IL-12 and IL-18, and supernatants were then harvested and quantified for IFN-γ production by ELISA. This experiment is representative of 3 performed with similar results. Error bars indicate ± SEM (see “Materials and Methods”).
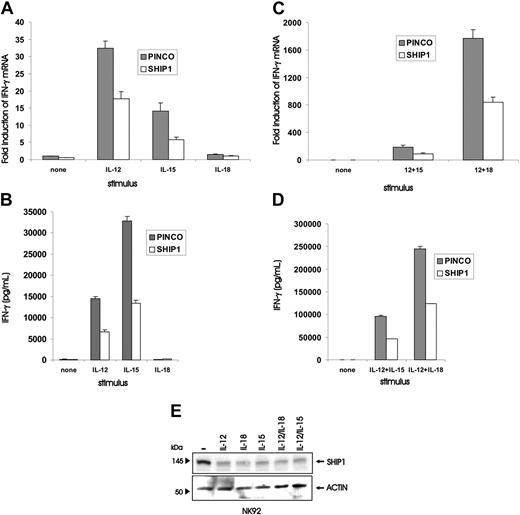
Quantification of IFN-γ gene and protein expression in NK92 cells overexpressing SHIP1, or quantification of SHIP1 protein in NK92 cells, each following stimulation or costimulation by monokines. PINCO and PINCO-SHIP1–infected NK92 cells were stimulated for 18 hours with IL-12 (10 ng/mL), IL-18 (20 ng/mL), or IL-15 (100 ng/mL) and quantified for (A) induction of IFN-γ transcript by real-time RT-PCR or (B) secretion of IFN-γ protein by ELISA. Likewise, PINCO and PINCO-SHIP1–infected NK92 cells were stimulated for 18 hours with combinations of IL-12 and IL-15 or IL-12 and IL-18 and were quantified for (C) induction of IFN-γ transcript by real-time RT-PCR or (D) secretion of IFN-γ protein by ELISA. (E) Western blot analysis was performed on lysates of NK92 cells treated individually for 48 hours with IL-12, IL-15, or IL-12 and IL-18, or treated for 48 hours with IL-12 and IL-15 or IL-18, and then blotted with either anti-SHIP1 or antiactin Abs. This experiment is representative of at least 3 performed with similar results.
Quantification of IFN-γ gene and protein expression in NK92 cells overexpressing SHIP1, or quantification of SHIP1 protein in NK92 cells, each following stimulation or costimulation by monokines. PINCO and PINCO-SHIP1–infected NK92 cells were stimulated for 18 hours with IL-12 (10 ng/mL), IL-18 (20 ng/mL), or IL-15 (100 ng/mL) and quantified for (A) induction of IFN-γ transcript by real-time RT-PCR or (B) secretion of IFN-γ protein by ELISA. Likewise, PINCO and PINCO-SHIP1–infected NK92 cells were stimulated for 18 hours with combinations of IL-12 and IL-15 or IL-12 and IL-18 and were quantified for (C) induction of IFN-γ transcript by real-time RT-PCR or (D) secretion of IFN-γ protein by ELISA. (E) Western blot analysis was performed on lysates of NK92 cells treated individually for 48 hours with IL-12, IL-15, or IL-12 and IL-18, or treated for 48 hours with IL-12 and IL-15 or IL-18, and then blotted with either anti-SHIP1 or antiactin Abs. This experiment is representative of at least 3 performed with similar results.
We next asked if the negative regulatory effects of SHIP1 overexpression were limited to NK cells costimulated with IL-12 plus IL-18 or whether this effect was applicable to other costimulatory signals or even individual monokines. NK92 cells were first rested in the absence of cytokines and then stimulated with either IL-12, IL-15, or IL-18 alone. Cells infected with the PINCO control vector responded to IL-12 alone and IL-15 alone with an induction of IFN-γ transcript and protein, while NK92 cells infected with PINCO-SHIP1 showed a significant decrease in IFN-γ gene and protein expression under the same conditions (P ≤ .004, n = 5 and 4, respectively; Figure 3A-B). IL-18 alone produced no IFN-γ. The combination of IL-12 plus IL-15 induced substantially more IFN-γ transcript and protein than either cytokine alone in NK92 cells infected with PINCO, and this expression was significantly blunted in NK92 cells infected with PINCO-SHIP1 (P < .01, n = 4; Figure 3C-D). Finally, to determine if the down-modulation of SHIP1 itself was limited to the synergistic costimulation of IL-12 and IL-18, NK92 cells were activated with single monokines or the different combinations noted above and then probed for SHIP1 protein expression. Each individual cytokine, including IL-18, resulted in down-modulation of SHIP1 that was comparable to the down-modulation seen with monokine costimulation (Figure 3E).
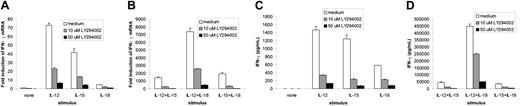
SHIP1 dephosphorylates the PI-3K product PI3,4,5P3 into PI3,4P2. To confirm that PI-3K has a role in regulating IFN-γ production following monokine costimulation in NK cells, we used a specific PI-3K inhibitor LY294002. This inhibits PI-3K by competing with adenosine triphosphate (ATP) for specific binding sites on PI-3K.36 We assayed both NK92 (not shown) and primary NK cells for IFN-γ transcript and protein production following stimulation with individual monokines or costimulation by IL-12 plus IL-15 or IL-18 and did so in the presence of increasing concentrations of LY294002. Indeed, LY294002 inhibits monokine-activated NK cell IFN-γ gene and protein expression in a dose-dependent fashion (Figure 4A-D). Complete inhibition of IFN-γ was observed using 50 μM LY294002, a dose that inhibits the IL-15–induced phosphorylation of the downstream PI-3K target AKT, without affecting STAT-5 phosphorylation or NK cell viability (not shown).
Effect of the PI-3K inhibitor LY294002 on IFN-γ production by monokine-activated primary human NK cells. Enriched primary NK cells were first incubated (45 minutes, 37°C) in medium containing either dimethyl sulfoxide (DMSO) vehicle control or the indicated concentration of LY294002, followed by an additional 18 hours of incubation in the presence of IL-12 (10 ng/mL), IL-18 (100 ng/mL), and/or IL-15 (100 ng/mL). Cell pellets were then collected for quantification of IFN-γ transcript by real-time RT-PCR (A-B), and supernatants were collected and quantified for IFN-γ protein production by ELISA (C-D). This experiment is representative of 4 experiments performed with similar results. Error bars indicate ± SEM.
Effect of the PI-3K inhibitor LY294002 on IFN-γ production by monokine-activated primary human NK cells. Enriched primary NK cells were first incubated (45 minutes, 37°C) in medium containing either dimethyl sulfoxide (DMSO) vehicle control or the indicated concentration of LY294002, followed by an additional 18 hours of incubation in the presence of IL-12 (10 ng/mL), IL-18 (100 ng/mL), and/or IL-15 (100 ng/mL). Cell pellets were then collected for quantification of IFN-γ transcript by real-time RT-PCR (A-B), and supernatants were collected and quantified for IFN-γ protein production by ELISA (C-D). This experiment is representative of 4 experiments performed with similar results. Error bars indicate ± SEM.
We next assessed which domain of SHIP1 was critical for its inhibitory effects on NK cell IFN-γ gene expression. An alanine to aspartic acid amino acid substitution in position 675 completely abrogates the inositol 5′ phosphatase activity of SHIP1.37 We therefore infected both NK92 and primary NK cells with a PINCO-GFP–expressing retrovirus carrying a wild-type SHIP1 or catalytic mutant SHIP1 D675A cDNA (Figure 5A). In contrast to cells infected with the WT SHIP1 construct, both primary (Figure 5B) and NK92 cells (not shown) infected with the SHIP1 D675A construct failed to show any significant diminution in IFN-γ production upon monokine costimulation with IL-12 and IL-18, demonstrating that the SHIP1 phosphatase activity was required to exert its negative regulatory effect on monokine-stimulated NK cell IFN-γ gene expression.
Effect of catalytic mutant SHIP1 (D675A) overexpression on IFN-γ production induced by monokine costimulation of NK cells. (A) Enriched primary NK cells were retrovirally infected using insertless PINCO, the PINCO vector encoding wild-type SHIP1, or the PINCO vector encoding the catalytic mutant D675A SHIP1. Infected cells were FACS sorted for CD56 and GFP, plated in medium, and costimulated with IL-12 plus IL-18 for 18 hours. (B) Supernatants were then collected and assayed for IFN-γ by ELISA. An immunoblot shows levels of SHIP1 and GRB2 in cell lysates from NK92 cells infected with insertless PINCO (lane 1), the PINCO vector encoding wild-type SHIP1 (lane 2), or the PINCO vector encoding the catalytic mutant D675A SHIP1 (lane 3). This experiment showed no significant decrease in IFN-γ production between primary NK cells infected with insertless PINCO and NK cells infected with the PINCO D675A SHIP1 vector and is representative of 3 experiments performed with similar results. Error bars indicate ± SEM.
Effect of catalytic mutant SHIP1 (D675A) overexpression on IFN-γ production induced by monokine costimulation of NK cells. (A) Enriched primary NK cells were retrovirally infected using insertless PINCO, the PINCO vector encoding wild-type SHIP1, or the PINCO vector encoding the catalytic mutant D675A SHIP1. Infected cells were FACS sorted for CD56 and GFP, plated in medium, and costimulated with IL-12 plus IL-18 for 18 hours. (B) Supernatants were then collected and assayed for IFN-γ by ELISA. An immunoblot shows levels of SHIP1 and GRB2 in cell lysates from NK92 cells infected with insertless PINCO (lane 1), the PINCO vector encoding wild-type SHIP1 (lane 2), or the PINCO vector encoding the catalytic mutant D675A SHIP1 (lane 3). This experiment showed no significant decrease in IFN-γ production between primary NK cells infected with insertless PINCO and NK cells infected with the PINCO D675A SHIP1 vector and is representative of 3 experiments performed with similar results. Error bars indicate ± SEM.
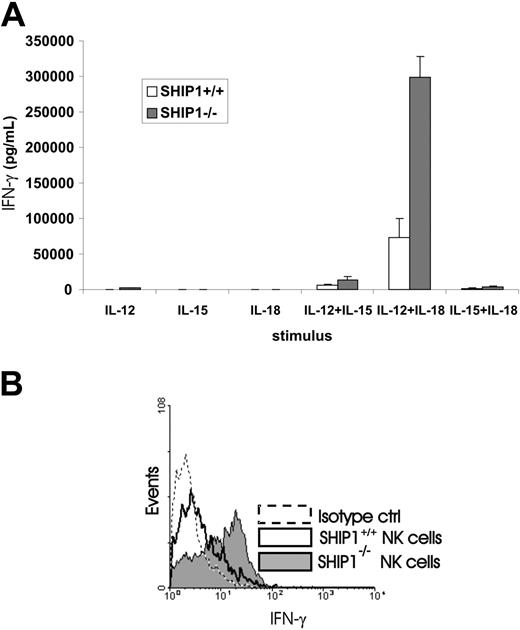
The overexpression of SHIP1 in primary human NK cells supported the notion that this phosphatase could serve as a negative regulator for IFN-γ production in monokine-activated NK cells. The substantially lower constitutive expression of SHIP1 in CD56bright compared with CD56dim NK cells suggested that this differential expression could account for the clear difference of monokine-activated IFN-γ production within the 2 subsets as previously described.3 In further support of this, we compared NK cell IFN-γ production in WT mice with that seen in Ship1–/– mice following monokine costimulation. Using purified NK cells from each genetic group, we found that Ship1–/– NK cells costimulated with IL-12 plus IL-15 or IL-18 secreted significantly higher levels of IFN-γ in vitro compared with NK cells from WT mice (P ≤ .007, n = 5; Figure 6A).
Assessment of NK cell IFN-γ production in WT and Ship1–/– mice following monokine stimulation. (A) NK cells were isolated from spleens of WT and Ship1–/– mice and then stimulated for 24 hours in vitro with individual monokines IL-12 (10 ng/mL), IL-18 (20 ng/mL), or IL-15 (10 ng/mL) or with a combination of IL-12 plus IL-15, IL-12 plus IL-18, or IL-15 plus IL-18. Supernatants were then collected and assayed for IFN-γ by ELISA. Data summarize results from 5 mice. (B) Wild-type and Ship1–/– mice received an intraperitoneal injection of IL-12 (1 μg) plus IL-18 (0.5 μg). After 24 hours spleens were harvested and splenocytes were cultured for 4 hours in brefeldin A and permeabilized and stained with DX5-PE and NK1.1-PE mAbs (pan-NK) and anti-muIFN-γ–FITC mAb. The histogram shows flow analysis for IFN-γ staining in DX5+ NK1.1+ NK cells from WT and Ship1–/– mice. Results are representative of 5 experiments. Error bars indicate ± SEM.
Assessment of NK cell IFN-γ production in WT and Ship1–/– mice following monokine stimulation. (A) NK cells were isolated from spleens of WT and Ship1–/– mice and then stimulated for 24 hours in vitro with individual monokines IL-12 (10 ng/mL), IL-18 (20 ng/mL), or IL-15 (10 ng/mL) or with a combination of IL-12 plus IL-15, IL-12 plus IL-18, or IL-15 plus IL-18. Supernatants were then collected and assayed for IFN-γ by ELISA. Data summarize results from 5 mice. (B) Wild-type and Ship1–/– mice received an intraperitoneal injection of IL-12 (1 μg) plus IL-18 (0.5 μg). After 24 hours spleens were harvested and splenocytes were cultured for 4 hours in brefeldin A and permeabilized and stained with DX5-PE and NK1.1-PE mAbs (pan-NK) and anti-muIFN-γ–FITC mAb. The histogram shows flow analysis for IFN-γ staining in DX5+ NK1.1+ NK cells from WT and Ship1–/– mice. Results are representative of 5 experiments. Error bars indicate ± SEM.
Likewise, we assessed this response to monokine costimulation in vivo. Twenty-four hours after intraperitoneal injection of IL-12 and IL-18 into both WT and Ship1–/– mice, we killed the mice and performed intracellular staining for IFN-γ on DX5+ and NK1.1+ splenocytes. The results are presented in Figure 6B and reveal that gated DX5+ NK1.1+ NK cells from Ship1–/– mice produce significantly more IFN-γ than NK cells from WT mice (22.6 ± 1.75 MFI versus 8.5 ± 1.81 MFI, P < .001, n = 5). Additionally, the percentage of NK cells producing IFN-γ is 3-fold higher in Ship1–/– than in WT mice (59.67% ± 2.33% versus 17.72% ± 1.96%, P < .001, n = 5).
Discussion
IFN-γ is critical for successful defense against obligate intracellular pathogens,38 and supportive evidence is mounting for a critical role in defending against malignant transformation.39 NK cells provide the first, immediate source of IFN-γ during the immune response, while the adaptive arm is developing its response to infection or malignant transformation.3 We previously reported that freshly isolated human CD56bright NK cells consistently produce significantly more IFN-γ following monokine stimulation than do CD56dim NK cells. Further, the CD56bright NK cell subset is the primary source of NK cell–derived IFN-γ when cells are stimulated with recombinant monokines or when the endogenous source of monokines is provided by lipopolysaccharide (LPS)–activated macrophages.4 Among the activating monokines, the combination of IL-12 and IL-18 represents the optimal stimulus for the production of IFN-γ by CD56bright NK cells.9 The molecular basis for the noted differences in cytokine production by CD56bright and CD56dim NK cell subsets has not been elucidated and was explored in the current study.
SHIP1 is a 5′ inositol phosphatase whose expression is restricted to the hematopoietic lineage where, depending on the cell type, it acts as a negative regulator of cell activation, proliferation, survival, degranulation, or ADCC.20 Moreover, although SHIP1 can be subjected to posttranslational control, its activity is often modulated by simply altering the level of its protein.40,41 For example, transforming growth factor-β (TGF-β) up-regulates SHIP1 protein in plasmocytoma cells, thereby contributing to the induction of apoptosis.42 Additionally, LPS-induced up-regulation of SHIP1 protein in macrophages and mast cells is necessary for tolerance of endotoxin.43 Here, we provide the first molecular evidence that can account for differences in cytokine (IFN-γ) production previously noted by monokine-stimulated human NK cell subsets. We report that constitutive gene expression of SHIP1 is substantially lower in the human CD56bright NK cell subset compared with the CD56dim NK cell subset and, through studies that increase or eliminate intracellular SHIP1, we define it as a novel negative regulator of mouse and human NK cell IFN-γ production following monokine stimulation. Further we show that monokine stimulation of NK cells decreases SHIP1 expression.
Phenotypically, CD56brightCD16neg/low and CD56dimCD16high NK cells differ in the expression of NK receptors, cytokine and chemokine receptors, and adhesion molecules. Whether CD56bright and CD56dim NK cells represent different stages of NK differentiation, totally distinct subsets derived from separate precursors, or simply different stages of activation has been discussed.3,44 Based on our data showing that CD56bright NK cells have less constitutive expression of SHIP1 than CD56dim NK cells and that monokine stimulation down-regulates SHIP1, one could also hypothesize that the CD56bright NK cell may simply represent an NK cell that has been exposed to monokine stimulation in vivo. The clear predominance of CD56bright NK cells over CD56dim NK cells in the parafollicular T-cell–rich regions of human lymph nodes might provide the environment whereby macrophages, dendritic cells (DCs), or T cells in close proximity could produce cytokines for local IFN-γ production, thereby down-regulating SHIP1 expression in the CD56bright NK cell.45,46 Indeed, Loza and Perussia44 have shown that IL-12 can down-modulate CD16 in NK cells, which is expressed at lower surface density or not at all on CD56bright NK cells compared with CD56dim NK cells. On the other hand, evidence now suggests that human CD56bright NK cells may differentiate from hematopoietic precursors within lymph nodes.47 In the latter scenario, it could be that the release of CD56bright NK cells from the lymph node (LN) into the peripheral circulation results in less monokine activation, which in turn up-regulates SHIP1 while CD56bright NK cells might differentiate to the more cytolytic CD56dim NK cell that predominates in the circulation. While comparable subsets of functionally distinct NK cells are not yet apparent in the mouse, this study establishes a comparable role for Ship1 in the mouse. Further, the observation that a 3-fold greater fraction of NK cells from Ship–/– mice was found to produce IFN-γ when compared with Ship+/+ mice suggests that a fraction of NK cells from WT mice must constitutively express sufficient Ship1 to normally repress IFN-γ gene expression following monokine costimulation. It is therefore possible that variable levels of Ship1 in WT mice contribute to functionally distinct NK subsets in mice, similar to CD56dim and CD56bright NK subsets seen in humans. It will be interesting to determine if murine NK cells with constitutively higher Ship1 expression have enhanced natural cytotoxicity as is seen in the human CD56dim NK cell subset.3
We noted that modulation in SHIP1 transcript and modulation in protein were congruent and therefore lend support for some component of transcriptional down-regulation by proinflammatory monokine stimulation. However, the involvement of epigenetic, transcriptional, or posttranscriptional mechanisms remains to be investigated. Interestingly, the antiinflammatory monokine TGF-β/activin induces transcriptional up-regulation of SHIP1. Specifically, Valderrama-Carvajal et al42 demonstrated that SMAD2, SMAD3, and SMAD4 are required for the TGF-β/activin–induced increase in SHIP1 gene transcription in 293 cells and in murine embryonic fibroblasts. These data, combined with our data demonstrating that proinflammatory monokines can down-regulate SHIP1 transcript and protein, suggest that in CD56bright and CD56dim NK cells the expression of SHIP1 is likely regulated at the transcriptional level and involves an interplay between elements of proinflammatory and anti-inflammatory signaling pathways.
We provide evidence that the SHIP1-induced inhibition of IFN-γ production is likely mediated by the ability of SHIP1 to break down the PI-3K products PI3,4,5P3. By overexpressing the catalytically inactive SHIP1 mutant we show that SHIP1 regulation of IFN-γ gene expression in NK cells depends on SHIP1's phosphatase activity. Accordingly, the PI-3K inhibitor LY294002 blocked IFN-γ mRNA induction and protein expression induced by monokine-stimulated NK cells. These data are consistent with a model in which the PI3,4,5P3 second messager regulates IFN-γ production in NK cells.
How SHIP1 regulates the amount of IFN-γ induced by monokines in NK cells is not fully understood. An analysis of possible transcriptional factors and kinase pathways involved is now under investigation. A possible common transcriptional factor regulated by SHIP1 following monokine stimulation of NK cells that is known to be involved in IFN-γ production is NF-κB. In fact, there is an NF-κB binding site within in the IFN-γ promoter,48 and SHIP1 is a potent inhibitor of the NF-κB pathway.26,49 A kinase that is a direct target of SHIP1 phosphatase activity and could regulate NK cell–mediated IFN-γ production is the serine/threonine kinase PKB (protein kinase B),50 also known as AKT. In fact, increases in levels of PI3,4,5P3 result in activation of PKB via its phosphorylation at Thr308 by the PI3,4,5P3 binding pleckstrin homology domain (PH)–containing serine/threonine kinase pyruvate dehydrogenase kinase 1 (PDK1).51 Interestingly, it has been observed in T cells that the expression of a constitutively active form of PKB results in an increase in the CD3-mediated production of IFN-γ.52 This strongly suggests that SHIP1 may also regulate IFN-γ production in T cells. Indeed, it has recently been reported that T cells from mice heterozygous for both Pten and SHIP exhibit a modest increase in IFN-γ production after CD3 stimulation.53 Other possible SHIP1 intermediary regulators are the MAPK, extracellular signal-regulated kinase (ERK), and p38 kinases whose activities not only induce IFN-γ gene expression in NK cells54,55 but are also down-modulated by SHIP1.49 Accordingly we observed that ERK activity is higher in monokine-stimulated Ship1–/– NK cells compared with WT NK cells (data not shown).
Collectively, our data show that SHIP1 is a negative regulator of IFN-γ production in NK cells and its expression defines the functional distinction between CD56bright and CD56dim NK cell production of IFN-γ. We also show that monokines that induce NK cell production of IFN-γ down-regulate SHIP1 expression. The identification of the activating and inhibitory molecules that regulate CD56bright and CD56dim NK cell function is important for the development of molecularly targeted therapies that in turn can be used to manipulate these innate immune effector cells against infection and cancer in vivo.56
Prepublished online as Blood First Edition Paper, December 16, 2004; DOI 10.1182/blood-2004-10-4072.
Supported by National Cancer Institute grants CA95426 and CA68458.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Danilo Perrotti, Susan Whitman, and Aharon Freud for useful discussions, Tamara Vukosavlievic for help in the analysis of real-time PCR experiments, Amy K. Ferketich for help in statistical analysis, and Tiffany Hughes and Trent Marburger for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal