Abstract
Minor histocompatibility antigens (mHAs) are known targets of donor T cells after allogeneic hematopoietic stem cell transplantation (HSCT). In contrast, B-cell responses to mHAs have not been extensively characterized and the clinical significance of antibodies to mHAs is unknown. We tested 121 patients who underwent HSCT and 134 healthy donors for immunoglobulin G (IgG) antibodies against 5 mHAs encoded by genes on the Y chromosome (DBY, UTY, ZFY, RPS4Y, and EIF1AY). Antibodies to at least one H-Y protein developed in 52% of male patients with female donors compared with 8.7% of male patients with male donors (P < .0001), and in 41.4% of healthy females compared with 7.8% of healthy males (P < .0001). H-Y antibodies develop 4 to 12 months after transplantation and persist for long periods. The clinical significance of H-Y antibodies was characterized in 75 male patients with hematologic malignancies who received stem cells from female donors (F → M HSCT). The presence of H-Y antibodies correlated with chronic graft-versus-host disease (GVHD) by univariate (odds ratio [OR] = 15.5; P < .0001) and multivariable logistic regression analysis (OR = 56.5; P < .0001). Antibody response to Y-chromosome encoded histocompatibility antigens (H-Y antigens) was also associated with maintenance of disease remission (P < .0001). B cells may provide a new target for immune intervention in chronic GVHD.
Introduction
Most allogeneic hematopoietic stem cell transplantations (HSCTs) use donors who are identical at major histocompatibility alleles (HLA-A, B, DR). In these recipients, minor histocompatibility antigens (mHAs) are presumed to be the primary targets of donor alloimmunity.1,2 To the extent that donor T cells target mHAs expressed by normal recipient tissues, this immune response results in graft-versus-host disease (GVHD) and significant morbidity and mortality following transplantation.3-5 Recent studies have also shown that mHAs can be expressed on hematopoietic tumor cells. When donor T cells target mHAs expressed by leukemia cells, alloimmunity results in graft-versus-leukemia (GVL) and contributes to eradication of malignant cells following transplantation.6-8
Both GVHD and GVL are mediated primarily by mature T cells in the donor stem cell graft. This conclusion is supported by compelling experimental data in animal models as well as by extensive clinical experience.9,10 Thus, depletion of donor T cells from the stem cell graft can prevent severe GVHD but also increases the risk of relapse after HSCT. Moreover, infusion of donor T cells, without other therapy, can induce complete remission in some patients with relapsed leukemia after HSCT.11-13 Although T cells clearly play a central role in alloimmunity, recent studies have shown that mHAs also elicit antibody responses after allogeneic HSCT.14 The present studies were undertaken to investigate whether patients develop B-cell responses to a defined panel of mHAs, and to examine whether these antibody responses contribute to either GVHD or GVL after allogeneic HSCT.
To characterize antibody responses to mHAs, we focused on a limited set of genes located on the Y chromosome. Each of these H-Y genes has an X-chromosome homolog that is 91% to 99% identical at the amino acid level,15 ubiquitously expressed,16 and escapes X inactivation.15,17 Males develop tolerance to these self-antigens, but female T cells are capable of recognizing peptides derived from H-Y proteins following transplantation into male recipients.18 Although mHAs are also encoded by autosomal genes,2 many human mHAs are known to be H-Y-derived peptides presented by either HLA class I18-22 or class II molecules.23-25 We established a sensitive enzyme-linked immunosorbent assay (ELISA) to measure antibody responses to 5 recombinant H-Y proteins and their X homologs. Detailed analysis of 75 male patients who received stem cells from female donors demonstrated that antibody responses to H-Y mHAs are associated with chronic GVHD and maintenance of remission following allogeneic HSCT.
Methods
Samples from patients and healthy donors
All studies were approved by the Dana-Farber/Harvard Cancer Center investigational review board (IRB), and informed consent for sample collection and in vitro studies was obtained from all patients and donors. We obtained 483 plasma samples from 121 male patients who underwent allogeneic HSCT between January 1993 and January 2003. Patients who died less than 6 months after transplantation were not included in this study. Plasma samples were also obtained from 134 healthy adults. Samples were cryopreserved and stored at -70°C until use.
Preparation of recombinant proteins
Five H-Y genes (DBY, UTY, ZFY, RPS4Y, and EIF1AY) and each corresponding X homolog (DBX, UTX, ZFX, RPS4X, and EIF1AX) were reverse transcribed from male peripheral blood mononuclear cells and polymerase chain reaction (PCR) amplified with primers derived from GenBank sequences (Supplementary Data Set 1; see the Supplementary Data Set link at the top of the online article, on the Blood website). Due to the large size of UTY and UTX (4044 bp, 148 kDa), we expressed these genes as 3 overlapping 60-kDa fragments. Plasma samples reactive with any one of the 3 fragments were scored as positive for UTY or UTX respectively. Each H-Y and H-X clone was expressed with a C-terminal V5 epitope tag and 6 histidine residues in E coli and purified by histidine affinity chromatography.14 HIVp24 was expressed and purified in a similar fashion.14
ELISA for antibodies to recombinant H-Y and H-X proteins
Purified H-Y, H-X, and HIVp24 proteins were individually diluted to 5.0 mcg/mL in carbonate binding buffer before coating 96-well ELISA plates (NUNC Scientific, Rochester, NY) with 50 μL (0.25 μg antigen) per well and final antigen concentration was adjusted to provide equal anti-V5 detection. Plasma samples diluted 1:50 were tested for the presence of immunoglobulin G (IgG) antibodies reactive with each of the recombinant proteins.14 The quantity of IgG specific for each protein was measured by absorption at 550 nm to 450 nm (optical density [OD] units). Since all patients and donors were known to be negative for antibody to HIVp24, reactivity with recombinant HIVp24 was used as a negative control and was subtracted from each individual's H-Y or H-X measurement. OD values for p24 were low in all samples (98% < 0.05) and were assumed to reflect the level of nonspecific background binding in the assay. Antibody titers specific for DBY were determined by testing serial dilutions (1:50 to 1:15 000) of selected plasma samples. Results were compared with reactivity of the same diluted samples against 0.25 μg/well Epstein-Barr virus (EBV) EBNA1 (Advanced Biotechnologies, Columbia, MD).
Statistical methods
Several statistical approaches were previously explored to establish cut-off points for DBY and DBX ELISAs.14 To establish cut-off points for the remaining 4 H-Y antigens, the distributions of the ELISA values for healthy male donors were examined and 97.5 percentile values were considered cut-off points. An OD of 0.1 was established as the cut-off value for positive antibody reactivity for all antigens. For 2 × 2 table analysis, a 2-sided Fisher exact test and a 2-sided exact McNemar test were performed. For 2-sample comparison of continuous variables, a 2-sided Wilcoxon ranksum test was performed. A P value of less than .05 was considered significant, and multiple comparisons were not adjusted. Smoothing spline curve estimation technique was used to characterize the time to development of antibody response to DBY and UTY after HSCT. Heatmaps were generated using dChip software.26 The cumulative incidence curves for chronic GVHD and relapse were constructed reflecting time to relapse and chronic GVHD as competing risks.27 Time to chronic GVHD and time to relapse were measured from the date of stem cell infusion. Patients without chronic GVHD or relapse were censored at the time last known alive or at the time of death. There were 2 nonrelapse deaths in 17 patients with no chronic GVHD and no relapse. Potential predictors of acute and chronic GVHD and relapse were tested in a multivariable logistic regression model of H-Y antibody response. Backward elimination of variables was also performed in logistic regression analysis.
Results
H-Y antibodies in male HSCT patients and healthy individuals
Plasma samples from 134 healthy adults and 121 male patients who underwent HSCT, obtained 6 months to 10 years after allogeneic HSCT, were tested for antibodies to 5 H-Y proteins and their H-X homologs. Clinical characteristics of these patients, the stem cell transplants, and the times of sample collection are described in Table 1. As summarized in Table 2, antibodies to at least one H-Y protein were present in 39 of 75 (52%) male HSCT patients with female donors (F → M HSCT). Twenty-three (30.6%) of these patients developed antibodies to more than one H-Y protein. In contrast, only 4 of 46 (8.7%) male patients with male donors (M → M HSCT) and 5 of 64 (7.8%) healthy males had detectable antibodies to any of these recombinant H-Y proteins, and none of these individuals developed antibodies to more than a single H-Y antigen. Each of these comparisons is highly statistically significant (Table 2, legend). In contrast to healthy males, 29 of 70 (41.4%) healthy females were found to have antibodies to at least one H-Y protein (P < .0001).
Characteristics of hematopoietic stem cell transplantation patients
. | Donor → Recipient . | . | |
|---|---|---|---|
. | M → M n = 46, (%) . | F → M n = 75, (%) . | |
| Primary disease | |||
| Acute myeloid leukemia | 9 (20) | 17 (23) | |
| Acute lymphoblastic leukemia | 3 (7) | 8 (11) | |
| Chronic myeloid leukemia | 18 (39) | 19 (25) | |
| Chronic lymphocytic leukemia | 1 (2) | 5 (7) | |
| Myelodysplastic syndrome | 6 (13) | 11 (15) | |
| Non-Hodgkin lymphoma | 4 (9) | 10 (13) | |
| Hodgkin disease | 1 (2) | 2 (3) | |
| Multiple myeloma | 1 (2) | 2 (3) | |
| Other | 3 (7) | 1 (1) | |
| Good prognosis* | |||
| Yes | 23 (50) | 31 (41) | |
| No | 23 (50) | 44 (59) | |
| Patient age | |||
| Median (range), y | 47 (21-70) | 45 (19-66) | |
| Donor age | |||
| Median (range), y | 34 (21-68) | 39 (14-69) | |
| HSC donor | |||
| Unrelated | 20 (44) | 24 (32) | |
| Related | 26 (56) | 51 (68) | |
| HSC source | |||
| Bone marrow | 24 (52) | 44 (59) | |
| Peripheral blood stem cells | 22 (48) | 31 (41) | |
| HSC processing | |||
| Unmanipulated | 31 (67) | 60 (80) | |
| T-cell depleted† | 15 (33) | 15 (20) | |
| Conditioning regimen | |||
| Myeloablative | 32 (70) | 53 (71) | |
| Nonmyeloablative | 14 (30) | 22 (29) | |
| Total samples tested | 115 | 368 | |
| Pretransplantation samples | 10 | 31 | |
| Collected day 0-180 | 32 | 54 | |
| Collected after day 180 | 73 | 283 | |
| Median samples/patient (range) | 2 (1-6) | 4 (1-21) | |
| Days after HSCT that the last sample was collected | |||
| Median (range), days | 505 (180-3881) | 428 (180-3249) | |
. | Donor → Recipient . | . | |
|---|---|---|---|
. | M → M n = 46, (%) . | F → M n = 75, (%) . | |
| Primary disease | |||
| Acute myeloid leukemia | 9 (20) | 17 (23) | |
| Acute lymphoblastic leukemia | 3 (7) | 8 (11) | |
| Chronic myeloid leukemia | 18 (39) | 19 (25) | |
| Chronic lymphocytic leukemia | 1 (2) | 5 (7) | |
| Myelodysplastic syndrome | 6 (13) | 11 (15) | |
| Non-Hodgkin lymphoma | 4 (9) | 10 (13) | |
| Hodgkin disease | 1 (2) | 2 (3) | |
| Multiple myeloma | 1 (2) | 2 (3) | |
| Other | 3 (7) | 1 (1) | |
| Good prognosis* | |||
| Yes | 23 (50) | 31 (41) | |
| No | 23 (50) | 44 (59) | |
| Patient age | |||
| Median (range), y | 47 (21-70) | 45 (19-66) | |
| Donor age | |||
| Median (range), y | 34 (21-68) | 39 (14-69) | |
| HSC donor | |||
| Unrelated | 20 (44) | 24 (32) | |
| Related | 26 (56) | 51 (68) | |
| HSC source | |||
| Bone marrow | 24 (52) | 44 (59) | |
| Peripheral blood stem cells | 22 (48) | 31 (41) | |
| HSC processing | |||
| Unmanipulated | 31 (67) | 60 (80) | |
| T-cell depleted† | 15 (33) | 15 (20) | |
| Conditioning regimen | |||
| Myeloablative | 32 (70) | 53 (71) | |
| Nonmyeloablative | 14 (30) | 22 (29) | |
| Total samples tested | 115 | 368 | |
| Pretransplantation samples | 10 | 31 | |
| Collected day 0-180 | 32 | 54 | |
| Collected after day 180 | 73 | 283 | |
| Median samples/patient (range) | 2 (1-6) | 4 (1-21) | |
| Days after HSCT that the last sample was collected | |||
| Median (range), days | 505 (180-3881) | 428 (180-3249) | |
Good prognosis included acute myeloid leukemia in first complete remission and chronic myeloid leukemia in stable phase
T-cell-depleted transplantations included either CD6 or CD8 depletion
Frequency of antibody response to recombinant H–Y proteins and H–X homologs in male patients who underwent transplantation and in healthy individuals
. | DBY . | UTY . | ZFY . | RPS4Y . | EIF1AY . | ≥1 H–Y . | ≥2 H–Y . | ≥1 H–X . |
|---|---|---|---|---|---|---|---|---|
| F → M HSCT, n = 75 (%) | 35 (46.6) | 18 (24) | 12 (16) | 4 (5.3) | 6 (8) | 39 (52) | 23 (30.6) | 13 (17.3) |
| M → M HSCT, n = 46, (%) | 0 | 0 | 4 (8.7) | 0 | 0 | 4 (8.7) | 0 | 6 (13) |
| Male donors, n = 64 (%) | 1 (1.6) | 0 | 0 | 1 (1.6) | 3 (4.7) | 5 (7.8) | 0 | 5 (7.8) |
| Female donors, n = 70 (%) | 11 (15.7) | 4 (5.7) | 3 (4.3) | 15 (21.4) | 9 (12.9) | 29 (41.4) | 8 (11.4) | 12 (17.1) |
. | DBY . | UTY . | ZFY . | RPS4Y . | EIF1AY . | ≥1 H–Y . | ≥2 H–Y . | ≥1 H–X . |
|---|---|---|---|---|---|---|---|---|
| F → M HSCT, n = 75 (%) | 35 (46.6) | 18 (24) | 12 (16) | 4 (5.3) | 6 (8) | 39 (52) | 23 (30.6) | 13 (17.3) |
| M → M HSCT, n = 46, (%) | 0 | 0 | 4 (8.7) | 0 | 0 | 4 (8.7) | 0 | 6 (13) |
| Male donors, n = 64 (%) | 1 (1.6) | 0 | 0 | 1 (1.6) | 3 (4.7) | 5 (7.8) | 0 | 5 (7.8) |
| Female donors, n = 70 (%) | 11 (15.7) | 4 (5.7) | 3 (4.3) | 15 (21.4) | 9 (12.9) | 29 (41.4) | 8 (11.4) | 12 (17.1) |
Statistical comparisons: DBY, UTY, ≥1 H–Y, ≥2 H–Y antibody frequency between male recipients with female donors (F → M HSCT) and either male recipients with male donors (M → M HSCT) or healthy males; P < .001, 2-sided Fisher exact test. DBY, UTY, ZFY, RPS4Y, and ≥2 H–Y antibody frequency between F → M HSCT and healthy females; P < .008, 2-sided Fisher exact test. DBY, RPS4Y, ≥1 H–Y, and ≥2 H–Y antibody frequency between female donors and male donors; P < .007, 2-sided Fisher exact test. Comparison of ≥1 H–Y with ≥1 H–X in F → M HSCT and healthy females; P < .0001, 2-sided McNemar test.
In F → M HSCT patients, DBY antibodies were detected most frequently (46.6%), UTY and ZFY antibodies were detected less frequently, and few patients developed antibodies to RPS4Y or EIF1AY. The frequencies of responses to individual H-Y proteins were different in healthy females. Antibodies to RPS4Y were detected more frequently, and antibodies to DBY, UTY, and ZFY developed less frequently, in healthy females than in male recipients of stem cells from female donors (P < .008; Table 2).
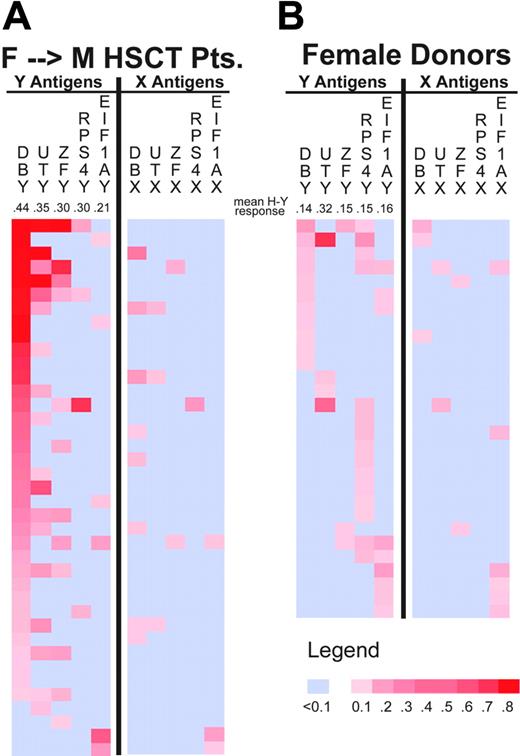
Quantitative anti-H-Y IgG values obtained for samples from 39 F → M HSCT patients who developed one or more H-Y antibodies are depicted in “heatmap” format in Figure 1A. Antibodies were detected against multiple H-Y antigens in 23 patients and anti-DBY was present in every multiresponder. DBY-positive samples with ELISA values measuring 0.5 OD units have high titer antibodies that remain detectable at more than 1:15 000 dilution and exceed IgG antibody titers determined for EBV EBNA1 protein (data not shown).
Quantitation of IgG antibodies specific for H-Y and H-X proteins. This heatmap depicts IgG ELISA results for 39 sex-mismatched patients (A) and 29 healthy females (B) who developed antibody responses to any H-Y or H-X recombinant protein. Individual samples are represented in rows and each H-Y or H-X protein is represented in a separate column. Negative ELISA results are shown in light blue. The magnitude of positive ELISA results are represented by increasing tones of red. Below each H-Y antigen label, the mean ELISA result for the respective seropositive samples is presented.
Quantitation of IgG antibodies specific for H-Y and H-X proteins. This heatmap depicts IgG ELISA results for 39 sex-mismatched patients (A) and 29 healthy females (B) who developed antibody responses to any H-Y or H-X recombinant protein. Individual samples are represented in rows and each H-Y or H-X protein is represented in a separate column. Negative ELISA results are shown in light blue. The magnitude of positive ELISA results are represented by increasing tones of red. Below each H-Y antigen label, the mean ELISA result for the respective seropositive samples is presented.
Quantitative anti-H-Y IgG measurements for 29 healthy females who had detectable antibodies to any H-Y protein are summarized in Figure 1B. In contrast to F → M HSCT patients, only 8 (11.4%) healthy females had antibodies against multiple H-Y antigens. The intensity of reactivity against H-Y proteins was also lower in healthy females. The mean of each positive H-Y antibody response detected in F → M HSCT patients was greater than the response in healthy females (Figure 1). This was confirmed by directly testing all F → M HSCT and healthy female samples by serial dilution for DBY response. While none of the healthy females had DBY responses detectable at 1:500 dilution, 17 of 35 (49%) were detectable at 1:1000, and 8 of these remained detectable at 1:15 000 dilution (data not shown).
Antibodies to any of the 5 H-X proteins were only detected in 7.8% to 17.7% of all tested individuals (Table 2). Figure 1A shows that all F → M H-X responders were also H-Y responders and the magnitude of each H-X response was less than the corresponding H-Y response. There were only 5 of 255 (2%) samples in which H-X reactivity was greater than a positive H-Y antibody response. These results suggest that antibody responses are primarily directed against H-Y proteins.
H-Y antibodies develop 4 to 12 months after HSCT
The temporal development of H-Y antibodies was examined by measuring anti-DBY and anti-UTY IgG in serial samples from 16 F → M HSCT patients. As shown in Figure 2, antibodies to DBY and UTY were not detectable prior to transplantation or in the first 3 months after HSCT. In each of these patients, H-Y antibodies developed 4 to 12 months after transplantation. Of the 39 F → M HSCT patients who developed H-Y antibody, 31 had at least one subsequent sample test positive for H-Y antibody (median elapsed time between positive samples: 281 days; range: 30-1425 days). Once DBY or UTY antibodies developed in any F → M HSCT patient, they remained detectable in every subsequent sample tested.
Detection of DBY and UTY antibodies after stem cell transplantation. Sixteen F → M HSCT patients had 5 or more serial plasma samples tested for DBY and UTY antibodies. All had one or more samples test H-Y antibody-negative before later samples tested positive, and 12 had at least 2 negative samples. Eleven patients developed both DBY and UTY antibodies. Four developed only DBY antibody, and one developed only UTY antibody. Smoothing spline curves are superimposed on the scatterplots for anti-DBY and anti-UTY.28
Detection of DBY and UTY antibodies after stem cell transplantation. Sixteen F → M HSCT patients had 5 or more serial plasma samples tested for DBY and UTY antibodies. All had one or more samples test H-Y antibody-negative before later samples tested positive, and 12 had at least 2 negative samples. Eleven patients developed both DBY and UTY antibodies. Four developed only DBY antibody, and one developed only UTY antibody. Smoothing spline curves are superimposed on the scatterplots for anti-DBY and anti-UTY.28
Patient, donor, and transplant variables are not associated with H-Y antibodies after HSCT
We examined clinical features of the group of 75 F → M HSCT patients to determine whether they were associated with the development of antibodies to H-Y proteins. Univariate analysis showed that patient age, patient diagnosis, patient prognosis, donor age, and degree of HLA compatibility were not associated with the development of H-Y antibodies (Table 3). H-Y antibodies occurred more frequently with HLA-matched related donors, but this trend was not statistically significant. Serologic responses were detected equally in patients receiving bone marrow, mobilized peripheral blood stem cells, T-cell depletion, and nonmyeloablative conditioning regimens.
Univariate analysis of patient, donor, and transplantation characteristics and subsequent development of H–Y antibodies
. | ≥1 H–Y antibody detected . | . | . | ||
|---|---|---|---|---|---|
| Transplantation characteristics . | Yes n = 39 (%) . | No n = 36 (%) . | P . | ||
| Primary disease | .64* | ||||
| Acute myeloid leukemia | 9 | 8 | |||
| Acute lymphoblastic leukemia | 4 | 4 | |||
| Chronic myeloid leukemia | 11 | 8 | |||
| Chronic lymphocytic leukemia | 3 | 2 | |||
| Myelodysplastic syndrome | 5 | 6 | |||
| Non-Hodgkin lymphoma | 4 | 6 | |||
| Hodgkin disease | 2 | 0 | |||
| Multiple myeloma | 1 | 1 | |||
| Other | 0 | 1 | |||
| Good prognosis | >.99 | ||||
| Yes | 16 (41) | 15 (42) | |||
| No | 23 (59) | 21 (58) | |||
| Patient age | .69 | ||||
| Median (range), y | 45 (19-59) | 44 (19-66) | |||
| Donor age | .58 | ||||
| Median (range), y | 39 (16-69) | 39 (14-69) | |||
| HSC donor | .06† | ||||
| HLA-matched related | 29 (74) | 19 (53) | |||
| HLA-matched unrelated | 6 (15) | 13 (36) | |||
| HLA-mismatched related | 1 (3) | 2 (6) | |||
| HLA-mismatched unrelated | 3 (8) | 2 (6) | |||
| HSC source | .82 | ||||
| Bone marrow | 22 (56) | 22 (61) | |||
| Peripheral blood stem cells | 17 (44) | 14 (39) | |||
| Stem cell processing | .39 | ||||
| Unmanipulated | 33 (85) | 27 (75) | |||
| T-cell depleted | 6 (15) | 9 (25) | |||
| Conditioning regimen | .61 | ||||
| Myeloablative | 29 (74) | 24 (67) | |||
| Nonmyeloablative | 10 (26) | 12 (33) | |||
. | ≥1 H–Y antibody detected . | . | . | ||
|---|---|---|---|---|---|
| Transplantation characteristics . | Yes n = 39 (%) . | No n = 36 (%) . | P . | ||
| Primary disease | .64* | ||||
| Acute myeloid leukemia | 9 | 8 | |||
| Acute lymphoblastic leukemia | 4 | 4 | |||
| Chronic myeloid leukemia | 11 | 8 | |||
| Chronic lymphocytic leukemia | 3 | 2 | |||
| Myelodysplastic syndrome | 5 | 6 | |||
| Non-Hodgkin lymphoma | 4 | 6 | |||
| Hodgkin disease | 2 | 0 | |||
| Multiple myeloma | 1 | 1 | |||
| Other | 0 | 1 | |||
| Good prognosis | >.99 | ||||
| Yes | 16 (41) | 15 (42) | |||
| No | 23 (59) | 21 (58) | |||
| Patient age | .69 | ||||
| Median (range), y | 45 (19-59) | 44 (19-66) | |||
| Donor age | .58 | ||||
| Median (range), y | 39 (16-69) | 39 (14-69) | |||
| HSC donor | .06† | ||||
| HLA-matched related | 29 (74) | 19 (53) | |||
| HLA-matched unrelated | 6 (15) | 13 (36) | |||
| HLA-mismatched related | 1 (3) | 2 (6) | |||
| HLA-mismatched unrelated | 3 (8) | 2 (6) | |||
| HSC source | .82 | ||||
| Bone marrow | 22 (56) | 22 (61) | |||
| Peripheral blood stem cells | 17 (44) | 14 (39) | |||
| Stem cell processing | .39 | ||||
| Unmanipulated | 33 (85) | 27 (75) | |||
| T-cell depleted | 6 (15) | 9 (25) | |||
| Conditioning regimen | .61 | ||||
| Myeloablative | 29 (74) | 24 (67) | |||
| Nonmyeloablative | 10 (26) | 12 (33) | |||
Comparison between acute myeloid leukemia/myelodysplastic syndrome/chronic myeloid leukemia (myeloid diseases) versus all other disease categories
Comparison between matched related versus matched unrelated
Correlation of H-Y antibody with clinical outcomes
Overall, grade II-IV acute GVHD occurred in 33% and either limited or extensive chronic GVHD occurred in 60% of the 75 sex-mismatched male patients. In univariate analysis, antibody response to one or more H-Y antigens was not associated with acute GVHD but was significantly associated with the development of chronic GVHD (OR: 15.5; P < .0001; Table 4). The correlation of H-Y antibodies and chronic GVHD was explored for confounding effects through logistic regression analysis taking into account risk factors for chronic GVHD assessed by univariate analysis. This analysis further supported the highly significant correlation between antibody response to H-Y and chronic GVHD after F → M HSCT (adjusted OR: 56.5; P < .0001 for chronic GVHD versus no chronic GVHD with relapse; adjusted OR: 22.2 for chronic GVHD versus no chronic GVHD without relapse, P = .006). Thirteen patients relapsed 5 months to 4 years after undergoing transplantation. None of these patients had developed antibodies to H-Y and disease relapse was significantly associated with both absence of chronic GVHD (P < .0001) and absence of antibody to H-Y antigens (P < .0001).
Logistic regression model to examine H–Y antibodies and transplantation outcomes
. | ≥1 H–Y antibody detected . | . | . | . | |
|---|---|---|---|---|---|
| Clinical outcomes . | Yes n = 39 (%) . | No n = 36 (%) . | Unadjusted odds ratio (95% CI) . | Adjusted odds ratio*(95% CI) . | |
| Acute GVHD II-IV | 10 (26) | 15 (42) | 0.48 (0.18-1.28) | 0.24 (0.05-1.23) | |
| Acute GVHD 0-I | 29 (74) | 21 (58) | P = .15 | P = .11 | |
| Chronic GVHD | 34 (87) | 11 (31) | 15.5 (4.8-50.1) | 56.5 (7.5-425) | |
| No cGVHD | 5 (13) | 25 (69) | P < .0001 | P < .0001 | |
| Relapse | 0 | 13 (36) | 0.02 (0.001-0.39) | — | |
| No relapse | 39 (100) | 23 (64) | P < .0001 | ||
| cGVHD and no relapse | 34 (87) | 11 (31) | 7.4 (2.1-25.8)† | 22.2 (2.4-206)† | |
| No cGVHD and no relapse | 5 (13) | 12 (33) | P = .001‡ | P = .006‡ | |
| Relapse and no cGVHD | 0 (0) | 13 (36) | 0.08 (0.004-1.7)† | — | |
. | ≥1 H–Y antibody detected . | . | . | . | |
|---|---|---|---|---|---|
| Clinical outcomes . | Yes n = 39 (%) . | No n = 36 (%) . | Unadjusted odds ratio (95% CI) . | Adjusted odds ratio*(95% CI) . | |
| Acute GVHD II-IV | 10 (26) | 15 (42) | 0.48 (0.18-1.28) | 0.24 (0.05-1.23) | |
| Acute GVHD 0-I | 29 (74) | 21 (58) | P = .15 | P = .11 | |
| Chronic GVHD | 34 (87) | 11 (31) | 15.5 (4.8-50.1) | 56.5 (7.5-425) | |
| No cGVHD | 5 (13) | 25 (69) | P < .0001 | P < .0001 | |
| Relapse | 0 | 13 (36) | 0.02 (0.001-0.39) | — | |
| No relapse | 39 (100) | 23 (64) | P < .0001 | ||
| cGVHD and no relapse | 34 (87) | 11 (31) | 7.4 (2.1-25.8)† | 22.2 (2.4-206)† | |
| No cGVHD and no relapse | 5 (13) | 12 (33) | P = .001‡ | P = .006‡ | |
| Relapse and no cGVHD | 0 (0) | 13 (36) | 0.08 (0.004-1.7)† | — | |
— indicates odds ratio is not determinable.
Logistic regression model variables included patient age, donor age, related versus unrelated donors, bone marrow versus peripheral blood stem cell source, myeloablative versus nonablative conditioning regimens, T-cell depletion, disease at conditioning, good prognosis at conditioning, and acute GVHD
“No cGVHD and no relapse” is the referent group
cGVHD and no relapse versus no cGVHD and no relapse
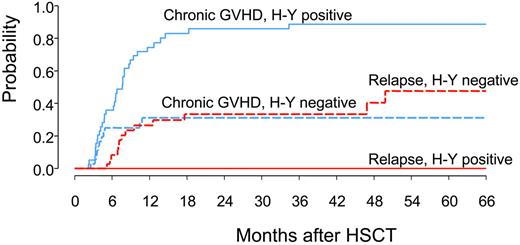
The impact of antibodies to H-Y proteins on the cumulative incidence of chronic GVHD and disease relapse is shown in Figure 3. Disease relapse and chronic GVHD were considered competing risks in this analysis. In the presence of antibodies to at least one H-Y protein, the cumulative incidence of chronic GVHD reached 89% at 5 years after transplantation, compared with only 31% in the absence of H-Y antibodies (P < .0001). In the absence of antibodies to H-Y, the cumulative incidence of relapse reached 48% at 5 years after transplantation, compared with 0% in the presence of H-Y antibodies (P < .0001).
The cumulative incidence of chronic GVHD and relapse as a function of H-Y antibody response. Cumulative incidence of chronic GVHD (cGVHD) and disease relapse were determined as competing risks for H-Y antibody-positive and -negative patients. Those patients who did not experience chronic GVHD or relapse were censored at the time of last follow-up or death.
The cumulative incidence of chronic GVHD and relapse as a function of H-Y antibody response. Cumulative incidence of chronic GVHD (cGVHD) and disease relapse were determined as competing risks for H-Y antibody-positive and -negative patients. Those patients who did not experience chronic GVHD or relapse were censored at the time of last follow-up or death.
Discussion
It is well established that major histocompatibility complex antigens can elicit antibody responses after exposure to HLA-disparate cells, but it has only recently been demonstrated that minor histocompatibility antigens can also elicit B-cell responses in vivo.14 HLA antibodies are known to contribute to solid organ graft rejection29 and transfusion-related acute lung injury (TRALI),30,31 and thus clearly have clinical significance in well-defined circumstances. In contrast, the contribution of mHA antibodies to immune-mediated disease has not previously been examined and the clinical significance of mHA antibodies is unknown. In this context, the initial aim of our study was to establish the specificity and frequency of antibody response to a panel of well-defined mHAs following allogeneic HSCT and in healthy individuals. Five putative mHAs encoded by genes on the Y chromosome were selected because several such proteins are known to contain peptide epitopes that can be recognized by female T cells.21-25 Moreover, genetically disparate recipient/donor pairs can be readily identified and transplantation of female stem cells into male donors is known to be associated with an increased incidence of GVHD following transplantation.32-35 This increased incidence of GVHD is presumably due to donor immune responses directed against Y-encoded antigens and clonal T-cell responses to at least some of these H-Y proteins have previously been well documented.
In our analysis of 121 patients and 134 healthy volunteers, male patients with female donors and healthy females were found to have a significantly greater frequency of antibody response than male patients with male donors and healthy males (P < .0001). The frequency of antibody responses to individual H-Y proteins varied, but antibodies were detected to each H-Y protein, including ZFY and EIF1AY, which have not previously been identified as targets of T-cell immunity. Since the Y chromosome includes several other H-Y genes15 it is likely that additional H-Y proteins not tested in our assays also elicit immune responses. Only a small number of H-Y peptide T-cell epitopes have been identified thus far, but the high frequency of antibody response in patients and healthy donors suggests that many additional T-cell epitopes have not yet been identified. Moreover, the persistence of high-titer H-Y antibody likely results from coordinated T- and B-cell responses to these antigens. This has been confirmed in recent studies documenting the persistence of both T- and B-cell responses to multiple distinct DBY peptide epitopes in a patient with chronic GVHD.25
While the overall frequency of H-Y antibodies was similar in F → M HSCT patients (52%) and healthy females (41.4%), the frequency and titer of antibodies to individual proteins was different in these 2 groups. Ninety percent of F → M HSCT patients who developed H-Y antibodies had antibodies to DBY and 59% of responding patients had high-titer antibodies to multiple H-Y proteins. In healthy females, H-Y antibodies were not directed against a single immunodominant antigen and fewer individuals had antibodies to multiple H-Y proteins. Nevertheless, the prevalence of H-Y antibodies in healthy females suggests that H-Y proteins are also highly immunogenic in this group. Previous studies have documented the persistence of H-Y-specific T cells for many years after normal pregnancy.36,37 Our studies suggest that exposure to male cells (presumably through pregnancy or blood transfusion) also results in the development of antibody responses to H-Y proteins. The detection of H-Y antibodies by ELISA may therefore represent a rapid and efficient method of identifying those females who have developed immunity to H-Y proteins. Further studies will now be directed to determine whether the presence of pre-existing H-Y antibodies in female stem cell donors affects the clinical outcome of stem cell transplantation in male recipients. If the presence of H-Y antibodies is associated with increased risk of GVHD in male recipients, sensitive assays such as those described in this report may facilitate the screening and selection of potential stem cell donors.
The second objective of our study was to determine whether H-Y antibodies were associated with clinical outcomes after stem cell transplantation. As summarized in Table 3, none of the pretransplantation variables we examined influenced the development of H-Y antibodies after transplantation. Notably, H-Y antibodies were not detectable in the early posttransplantation period and were not associated with acute GVHD. In contrast, H-Y antibodies developed 4 to 12 months after transplantation and appeared to be temporally associated with the onset of chronic GVHD. This association was clearly evident in univariate analysis and was confirmed in multivariable logistic regression modeling which demonstrated a highly significant association between the presence of H-Y antibodies and chronic GVHD. Although both acute and chronic GVHD are presumed to be mediated primarily by donor T cells, these observations suggest that B-cell immunity may also contribute to the pathogenesis of chronic GVHD. Our studies do not suggest any role for B-cell immunity in acute GVHD.
The clinical manifestations of chronic GVHD share many features with systemic autoimmune diseases such as scleroderma and systemic lupus erythematosus (SLE). These diseases are characterized by the production of autoantibodies to a variety of intracellular antigens.38 In some tissues, deposition of immune complexes plays an important role in tissue destruction. However, many manifestations of disease cannot be attributed to either immune complex deposition or reactivity with cell surface molecules.39 Animal models have provided some evidence that autoantibodies can also play a direct role in disease pathogenesis,40 and this has led to the evaluation of B-cell-directed therapy with rituximab for a variety of autoimmune diseases.41 Early results showed promising results, and a recent prospective randomized trial has confirmed that rituximab is highly effective in the treatment of rheumatoid arthritis.42 In patients with chronic GVHD, antibodies to ubiquitously expressed mHAs may be analogous to autoantibodies in conventional autoimmune diseases. These observations suggest that B-cell-directed therapy should also be tested in patients with chronic GVHD. Indeed, a recent report documented clinical improvement in 4 of 8 patients with chronic GVHD after a single infusion of rituximab.43 Further studies in larger numbers of patients will be necessary to evaluate the therapeutic role of rituximab in this disease. Chronic GVHD continues to be a major cause of morbidity and mortality affecting 50% to 70% of HSCT patients and current therapies remain inadequate.44 If B-cell-directed therapy is effective, these studies may also help define the mechanisms whereby antibodies to mHAs contribute to the pathogenesis of this disease.
It is noteworthy that our studies also found a highly significant association between H-Y antibodies and maintenance of disease remission. Overall, disease relapse occurred in 17% of male patients with female donors. However, none of these relapses occurred in patients who had developed H-Y antibodies. Larger prospective studies will be necessary to confirm the association between H-Y antibodies and disease remission and to define the extent to which this association is independent of chronic GVHD. Unlike conventional autoimmune diseases where autoantibodies do not play any beneficial role, these observations suggest that H-Y antibodies may contribute to the prevention of relapse following transplantation. If this beneficial role is confirmed, inhibition of these responses with agents such as rituximab may also increase the risk of relapse. B-cell-directed therapy following transplantation should therefore be closely monitored to ensure that overall transplantation outcomes are not compromised.
Prepublished online as Blood First Edition Paper, December 21, 2004; DOI 10.1182/blood-2004-09-3660.
Supported by National Institutes of Health grants AI29530, HL70149, and the Ted and Eileen Pasquarello Tissue Bank and Research Fund; the Clinical Investigator Training Program (D.M.), Harvard/MIT Health Sciences and Technology, Beth Israel Deaconess Medical Center, in collaboration with Pfizer Inc and K08 HL69132. R.J.S. is a Scholar in Clinical Research of the Leukemia and Lymphoma Society.
Presented in abstract form at the 45th annual meeting of the American Society of Hematology, San Diego, CA, December 8, 2003 (abstract no. 519).
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Assistance with patient sample procurement and processing was provided by Leah Gordon, Evan McEwing, Marcia Chesterfield, Armond Paul, Heather Daley, and Christine Canning.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal