Abstract
In most cell types constitutive and ligand-induced apoptosis is a caspase-dependent process. In neutrophils, however, the broad-spectrum caspase inhibitor z-VAD-fmk enhances tumor necrosis factor-α (TNFα)-induced cell death, and this has been interpreted as evidence for caspase-dependent and -independent cell death pathways. Our aim was to determine the specificity of the effect of z-VAD-fmk in neutrophils and define the potential mechanism of action. While confirming that z-VAD-fmk (> 100 μM) enhances TNFα-induced neutrophil apoptosis, lower concentrations (1-30 μM) completely blocked TNFα-stimulated apoptosis. Boc-D-fmk, a similar broad-spectrum caspase inhibitor, and z-IETD-fmk, a selective caspase-8 inhibitor, caused a concentration-dependent inhibition of only TNFα-stimulated apoptosis. Moreover, the caspase-9 inhibitor, Ac-LEHD-cmk, had no effect on TNFα-induced apoptosis, and z-VAD-fmk and Boc-D-fmk inhibited TNFα-stimulated reactive oxygen species (ROS) generation. These data suggest that TNFα-induced apoptosis in neutrophils is fully caspase dependent and uses a mitochondrial-independent pathway and that the proapoptotic effects of z-VAD-fmk are compound specific and ROS independent.
Introduction
Tumor necrosis factor-α (TNFα)-stimulated killing of human neutrophils remains an ill-defined event. This process may, however, play an essential role in the resolution of granulocytic inflammation since TNFα is abundantly expressed in acute inflammation and one of a small number of biologic agents shown capable of stimulating neutrophil apoptosis.1,2
Recent studies have suggested that TNFα can induce cell death in neutrophils via distinct caspase-dependent and -independent pathways. This conclusion was based on the finding that high concentrations of z-VAD-fmk, a widely used broad-spectrum caspase inhibitor, enhance rather than inhibit TNFα-induced apoptosis.3,4 The cell death induced by the combination of z-VAD-fmk and TNFα lacked the usual nuclear features of apoptosis and was dependent on mitochondrial reactive oxygen species (ROS) generation.4 While the importance of caspase activation in constitutive and TNFα-stimulated apoptosis remains controversial, this result challenges the findings of a number of previous studies.1 Moreover, despite evidence suggesting that caspases are activated when neutrophils undergo apoptosis,5 we and others have been unable to demonstrate inhibition of constitutive apoptosis with either z-VAD-fmk or selective caspase-8 and -9 inhibitors z-IETD-fmk and Ac-LEHD-cmk.6,7 These data cannot be explained by a lack of cell permeability, as these compounds inhibit the relevant caspase activity in intact cells and abolish Fas-L and gliotoxin-induced neutrophil apoptosis.1,4
This study was designed to establish the involvement of caspases in TNFα-stimulated neutrophil apoptosis and to assess the specificity of z-VAD-fmk to enhance TNFα-mediated killing.
Methods
Neutrophil isolation and culture
Neutrophils were purified from healthy donors and cultured in Dulbecco modified Eagle medium containing 10% autologous serum.8,9 Cells were pre-incubated with the broad-spectrum caspase inhibitors z-VAD-fmk (0.03-300 μM) or Boc-D-fmk (1-1000 μM), the caspase-8 and -9 inhibitors z-IETD-fmk (0.01-10 μM), z-LEHD-fmk (0.01-10 μM) (Merck Biosciences, Nottingham, United Kingdom), or vehicle for 30 minutes before addition of TNFα (200 U/mL) (R&D, Abingdon, United Kingdom) or phosphate-buffered saline (PBS) for the times indicated. To investigate the role of ROS in z-VAD-fmk-augmented apoptosis, cells were pre-incubated with the ROS scavenger diphenyleneiodonium chloride (DPI) (10 μM) (Sigma, Poole, United Kingdom). Approval was obtained from the Cambridge research ethics committee for these studies. Informed consent was provided according to the Declaration of Helsinki.
Assessment of apoptosis
TNFRI/RII surface expression
Neutrophils were cultured as detailed in “Neutrophil isolation and culture”, centrifuged (500g, 3 minutes at 4°C), washed, and incubated on ice for 30 minutes with mAb for TNFRI (MAB225, 1:40) or TNFRII (MAB226, 1:40) (R&D). The cells were washed with ice-cold PBS, followed by a fluorescein isothiocyanate (FITC)-conjugated goat antimouse F(ab')2 secondary (1:100 in PBS on ice for 30 minutes). The cells were then washed, resuspended in ice-cold PBS, and TNFRI/TNFRII surface expression quantified by fluorescence-activated cell-sorter scanner (FACs) analysis.10
Cellular ROS generation
Neutrophils (11.1 × 106 cells/mL) were cultured for 30 minutes in the presence or absence of 0.03-300 μM z-VAD-fmk or Boc-D-fmk before addition of TNFα (200 U/mL). Cells (100 μL) were added to a microtiter plate followed by an equal volume of lucigenin (0.25 mM for extracellular ROS release) or luminol (1 mM for intra- and extracellular ROS release). ROS generation was assessed kinetically using a chemiluminescence microtiter plate luminometer (Dynex Technologies, Billingshurst, Sussex, United Kingdom).
Statistical analysis
Data are presented as mean ± SEM and analyzed using a Student t test or ANOVA. Values of P less than .05 were considered significant.
Results and discussion
Effects of z-VAD-fmk on TNFα-mediated neutrophil apoptosis
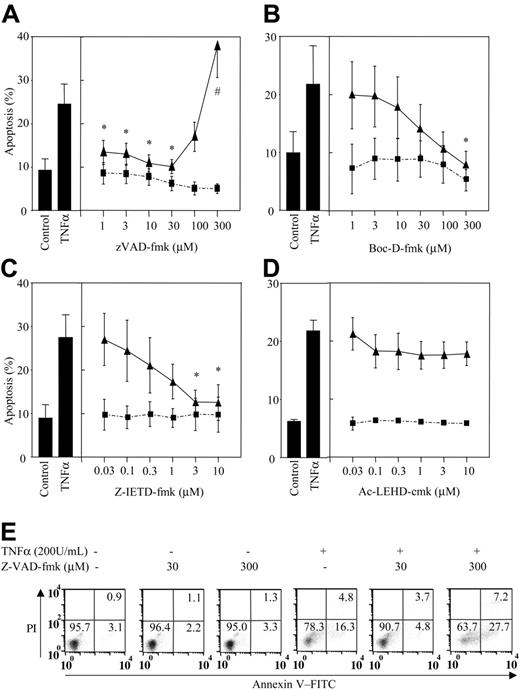
z-VAD-fmk caused a biphasic effect on TNFα-stimulated neutrophil apoptosis. Hence, while z-VAD-fmk at or above 100 μM enhanced the extent of apoptosis with TNFα, this compound caused full inhibition at lower concentrations (Figure 1A) (IC50 0.7 μM). Moreover, the effect of the higher concentrations of z-VAD-fmk on TNFα-mediated neutrophil killing appeared to be compound specific since Boc-D-fmk, an alternative broad-spectrum caspase inhibitor, and z-IETD-fmk, a caspase-8 inhibitor, caused full inhibition only of the proapoptotic effect of TNFα (Figure 1B-C) with IC50 values of 39 μM and 0.46 μM, respectively. These data reestablish caspases as essential in TNFα-induced apoptosis in neutrophils and suggest that the ability of z-VAD-fmk to enhance TNFα-induced killing is inhibitor specific and observed only at supraphysiological concentrations. This conclusion is supported by the distinct apoptotic/necrotic cell morphology observed in TNFα and z-VAD-fmk-treated cells.3
High concentrations of z-VAD-fmk augment TNFα-stimulated neutrophil apoptosis. Human neutrophils were incubated in the presence of (A) z-VAD-fmk, (B) Boc-D-fmk, (C) z-IETD-fmk (caspase 8 inhibitor), or (D) Ac-LEHD-cmk (caspase 9 inhibitor) at the concentrations shown or vehicle control for 30 minutes prior to the addition of 200 U/mL TNFα (▴) or PBS (▪). The cells were then incubated for 6 hours and percent apoptosis determined by annexin V-FITC staining. (E) A representative experiment showing z-VAD-fmk inhibition of TNFα-induced apoptosis at low inhibitor concentrations (30 μM) and the augmentation of cell death at the higher concentrations (300 μM). *P < .05 (significant inhibition of TNFα-stimulated apoptosis), #P < .05 (significant augmentation of TNFα-stimulated apoptosis). All values are mean ± SEM of n = 3-5 separate experiments, each performed in triplicate. Numbers in quadrants indicate percentage of cells.
High concentrations of z-VAD-fmk augment TNFα-stimulated neutrophil apoptosis. Human neutrophils were incubated in the presence of (A) z-VAD-fmk, (B) Boc-D-fmk, (C) z-IETD-fmk (caspase 8 inhibitor), or (D) Ac-LEHD-cmk (caspase 9 inhibitor) at the concentrations shown or vehicle control for 30 minutes prior to the addition of 200 U/mL TNFα (▴) or PBS (▪). The cells were then incubated for 6 hours and percent apoptosis determined by annexin V-FITC staining. (E) A representative experiment showing z-VAD-fmk inhibition of TNFα-induced apoptosis at low inhibitor concentrations (30 μM) and the augmentation of cell death at the higher concentrations (300 μM). *P < .05 (significant inhibition of TNFα-stimulated apoptosis), #P < .05 (significant augmentation of TNFα-stimulated apoptosis). All values are mean ± SEM of n = 3-5 separate experiments, each performed in triplicate. Numbers in quadrants indicate percentage of cells.
Effects of z-VAD-fmk on TNFRI/II expression and TNFα-mediated NF-κB stimulation
We and others have shown that inhibition of NF-κB causes a major potentiation of the proapoptotic effect of TNFα.1,10 Moreover, the killing effect of TNFα appears dependent on the dual activation of TNFRI (a recognized caspase-7 target11 ) and TNFRII, which are rapidly shed or internalized following TNFα stimulation.12,13 We postulated that the enhanced killing effect of TNFα might reflect retention of functional TNFRI/II on the neutrophil surface or inhibition of NF-κB. TNFα caused a 95% ± 2% and 97% ± 1% loss in TNFRI and TNFRII surface loss by 6 hours, respectively. Neither z-VAD-fmk nor Boc-D-fmk (3-300 μM) affected TNFRI or TNFRII expression in vehicle (3% ± 1% and 0.5% ± 0.5% at 300 μM, respectively) or TNFα-treated cells (98% ± 2% and 92.4% ± 4% at 300 μM, respectively). Likewise, using interleukin-8 (IL-8) secretion as a readout of TNFα-stimulated NF-κB activation,14-16 z-VAD-fmk, Boc-D-fmk, and z-IETD-fmk all failed to modify the release of IL-8 (data not shown), a finding that concurs with Liu and coworkers.3
z-VAD-fmk does not enhance TNFα-induced ROS production
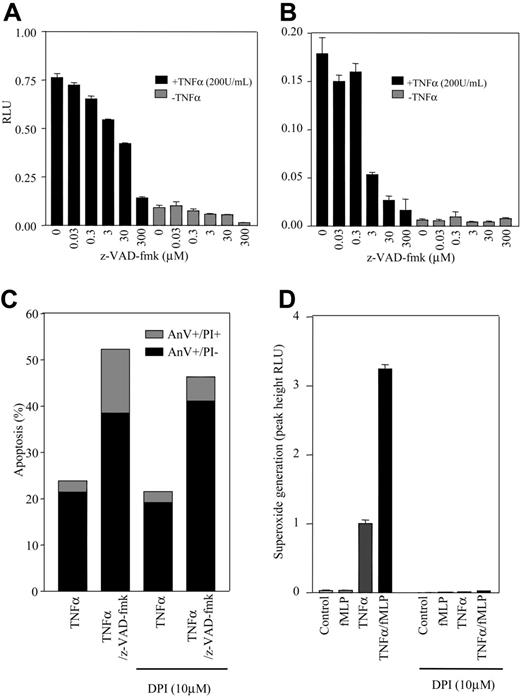
Recent studies have suggested that while z-VAD-fmk can still enhance TNFα-stimulated apoptosis in patients lacking a functional NADPH oxidase, this effect is not seen in mitochondrial-deficient neutrophil cytoplasts.3 Moreover, the augmentation of apoptosis by z-VAD-fmk could be inhibited by ROS scavengers and mitochondrial inhibitors, suggesting that the cytotoxic effect of z-VAD-fmk is ROS dependent. To explore this, we performed detailed kinetic analysis of the effects of z-VAD-fmk on TNFα-stimulated ROS generation using lucigenin and luminol. As shown in Figure 2A-B, z-VAD-fmk caused a concentration-dependent and near-complete inhibition of steady-state TNFα-stimulated ROS production. Near identical data were obtained using Boc-D-fmk (data not shown). The inclusion of an ROS scavenger, DPI (1 μM), which caused a 99% decrease of TNFα/fMLP (formyl methionyl leucyl phenylalanine, a known neutrophil agonist), stimulated ROS generation (Figure 2D) and did not prevent the augmentation of cell death observed with z-VAD-fmk (Figure 2C). However, DPI did decrease the percentage of AnV+/PI+ cells, suggesting that the onset of secondary necrosis may be ROS sensitive. The involvement of a mitochondrial-dependent pathway in TNFα and TNFα/z-VAD-fmk-induced neutrophil apoptosis is further undermined by the inability of the highly effective caspase-9 inhibitor Ac-LEHD-cmk to inhibit TNFα or TNFα/z-VAD-fmk-induced cell death (Figure 1D).
z-VAD-fmk inhibits TNFα-stimulated intra- and extracellular ROS accumulation. Neutrophils were cultured for 30 minutes in the presence or absence of 0.03-300 μM z-VAD-fmk before the addition of TNFα (200 U/mL) or PBS for a further 30 minutes. (A) Lucigenin (0.25 mM) or (B) luminol was then added and steady-state ROS generation assessed using a luminometer. For reference, these values are approximately 100 times lower than the values obtained under TNFα-primed, 100 nM fMLP-stimulated conditions. (C) The inclusion of a ROS scavenger, DPI (10 μM), did not effect z-VAD-fmk (300 μM) augmentation of TNFα-stimulated cell death, (D) but was sufficient to inhibit TNFα-primed (30 minutes)/fMLP stimulated (48 seconds) ROS generation. *P < .05 (significant inhibition of TNFα-stimulated ROS generation analyzed by ANOVA). Data represent the mean ± SEM of n = 3 separate experiments each performed in triplicate. AnV, annexin V; RLU, relative light units.
z-VAD-fmk inhibits TNFα-stimulated intra- and extracellular ROS accumulation. Neutrophils were cultured for 30 minutes in the presence or absence of 0.03-300 μM z-VAD-fmk before the addition of TNFα (200 U/mL) or PBS for a further 30 minutes. (A) Lucigenin (0.25 mM) or (B) luminol was then added and steady-state ROS generation assessed using a luminometer. For reference, these values are approximately 100 times lower than the values obtained under TNFα-primed, 100 nM fMLP-stimulated conditions. (C) The inclusion of a ROS scavenger, DPI (10 μM), did not effect z-VAD-fmk (300 μM) augmentation of TNFα-stimulated cell death, (D) but was sufficient to inhibit TNFα-primed (30 minutes)/fMLP stimulated (48 seconds) ROS generation. *P < .05 (significant inhibition of TNFα-stimulated ROS generation analyzed by ANOVA). Data represent the mean ± SEM of n = 3 separate experiments each performed in triplicate. AnV, annexin V; RLU, relative light units.
In summary, these data indicate that TNFα-induced apoptosis in human neutrophils is fully inhibited by a range of caspase inhibitors including z-VAD-fmk and do not support the view that TNFα uses a caspase-independent pathway. The augmentation of the TNFα-induced killing effect previously reported with z-VAD-fmk may represent a nonphysiological cytotoxic effect of this agent.
Prepublished online as Blood First Edition Paper, November 30, 2004; DOI 10.1182/blood-2004-07-2870.
Supported by the Wellcome Trust, MRC (Medical Research Council), Isaac Newton Trust and British Lung Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal