Abstract
Delivery of biologically active peptides into cells may help elucidate intracellular signal transduction pathways, identify additional in vivo functions, and develop new therapeutics. Although p21 was first identified as a major regulator of cell cycle progression, it is now clear that p21 subserves multiple functions. The amino terminus of p21 interacts with cyclins and cyclin-dependent kinases, while the carboxyl terminus interacts with proliferating cell nuclear antigen (PCNA), growth arrest and DNA damage–inducible gene 45 (GADD45), calmodulin, SET, and CCAAT/enhancer binding protein-α (C/EBP-α). A chimeric peptide, p21-IRS, consisting of the carboxyl terminal domain of p21 conjugated to a pentapeptide (RYIRS) rapidly enters lymphoid cells and activates apoptosis. In the present study, we investigate the molecular events involved in p21-activated apoptosis. Comparison of p21-IRS with other known proapoptotic agents demonstrates that p21-IRS activates a novel apoptotic pathway: mitochondria are central to the process, but caspases and a decrease in Δψm are not involved. Targeting the p21 peptide to specific cell populations may allow development of novel therapies to eliminate aberrant cells in human diseases.
Introduction
Apoptosis, or programmed cell death, is a complex process involving both pro- and antiapoptotic signals. Two major signaling pathways resulting in apoptosis have been described: the death receptor pathway, exemplified by Fas or tumor necrosis factor (TNF) receptor–mediated caspase-8 activation, and the stress- or mitochondria-mediated caspase-9 activation pathway.1 Mitochondria are central integrators of apoptotic signals, and disruption of the outer mitochondrial membrane is often the point of no return in cell death.
p21 was originally identified as a cyclin-dependent kinase (CDK) inhibitor, 2-4 and was subsequently shown to play an important role in regulating cell growth and differentiation. The N-terminal domain of p21 shares homology with the N-termini of p27 and p57, both of which are necessary and sufficient to inhibit CDK activity. The C-terminus of p21 interacts with many important proteins, including proliferating cell nuclear antigen (PCNA), growth arrest and DNA damage–inducible gene 45 (GADD45), calmodulin, SET, and CCAAT/enhancer binding protein-α (C/EBP-α)5 p21 can inhibit or induce apoptosis, depending on its subcellular locations and other conditions.6,7 Localization of p21 in the nucleus is proapoptotic, 5,7 while p21 mutants lacking the N-terminus signal peptide remain in the cytosol and protect cells from apoptosis.8 Three major mechanisms have been proposed to explain the antiapoptotic role of p21: (1) p21-dependent cell cycle arrest, permitting DNA repair9 ; (2) p21 binding to and inactivation of cyclin A/Cdk2 complexes; and (3) p21 stabilization of the inhibitor of apoptosis protein C (c-IAP) and direct interaction with procaspase-3 to block the activation of caspase-3 and subsequent apoptosis.8 The mechanism by which p21 promotes apoptosis is still elusive, but may involve interactions with other proteins such as GADD45.5,7 Mutoh et al10 reported that a peptide corresponding to residues 139-164 of p21 fused to the internalization peptide from Antennapedia (AP) inhibited growth of human lymphoma cells by inducing necrosis. We reported previously that a synthetic peptide corresponding to residues 139-160 of p21 and fused to the IRS tag (RYIRS; designated p21-IRS) caused apoptosis.11 In this study, we elucidate the mechanisms underlying p21-IRS–induced apoptosis.
Materials and methods
Materials
Reagents used include annexin V–fluorescein isothiocyanate (FITC; BD Pharmingen, San Diego, CA); 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylben-zimidazolylcarbocyanine iodide (JC-1), tetramethylrhodamine/AM (TMRM), 5-(and-6)-chloromethyl-2′, 7′-dichlorodihydrofluorescein diacetate (carboxyl-DCFDA/AM), rhod-2, fluo-3, and ATP Determination Kit (Molecular Probes, Eugene, OR); caspase inhibitor I (Z-VAD), A23187, and apamin (CalBiochem, San Diego, CA); propidium iodine (PI), staurosporine, tetraethylammonium (TEA), amiloride, econazole, nicardipine, iberiotoxin, N-acetyl-L-cysteine (LNAC), myxothiazol, stigmatellin, rotenone and antimycin A (Sigma, St Louis, MO); Manganese (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP) (OXIS International, Portland, OR); QX222 (Tocris, Ellisville, MO); DNeasy Tissue Kit (Qiagen, San Diego, CA); anti–cytochrome C antibody (BD PharMingen); anti-AIF antibody (Santa Cruz Biotechnology, Santa Cruz, CA); anti-actin antibody (Oncogene, Boston, MA); anti-Fas antibody, CH-11 (Medical and Biological Lab, Watertown, MA); APO-1/Fas monoclonal antibody, SM1/23 (Bender MedSystems, San Bruno, CA); and polyclonal anti–caspase-9 antibody (Cell Signaling Technology, Beverly, MA).
p21-IRS is composed of the C-terminus of p21 (residues 139-160, GRKRRQTSMTDFYHSKRRLIFS) followed by an IRS tag (RYIRS). It was synthesized and purified by United Biochemical Research (Seattle, WA) and its composition and purity were confirmed by mass spectrometry. The peptide was dissolved at 10 mM in dimethyl sulfoxide (DMSO) and stored at room temperature for several months.
Cell culture
The human leukemia cell line U937 was cultured in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 2 mM glutamine, 200 μg/mL penicillin, and 100 μg/mL streptomycin sulfate. Cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2 unless specifically described in some experiments.
Annexin V and PI staining
A quantity of 400 μL of a suspension of U937 cells (1 × 106 cells/mL) was treated with 20 μM p21-IRS for 3 hours, and washed twice with phosphate-buffered saline (PBS). Cell pellets were suspended in 150 μL binding buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 140 mM NaCl, and 2.5 mM CaCl2, pH 7.4), and stained with 5 μL annexin V–FITC in the dark at room temperature for 15 minutes. Prior to fluorescence-activated cell sorting (FACS) analysis (FACScan; BD Biosciences), 250 μL binding buffer and 5 μL PI (250 μg/mL) were added. Cells staining with annexin V alone or with annexin V plus PI were considered apoptotic. The percentage of apoptotic cells were determined using the formula: % apoptotic cells = (% apoptotic cells treated with p21-IRS – % apoptotic cells in medium)/(100 – % apoptotic cells in medium) × 100.
Internuclear DNA fragmentation
U937 cells (5 × 106) were treated with p21-IRS (20 μM) for 3 hours, the cell pellets were washed once with PBS, and genomic DNA was prepared using a DNeasy Tissue Kit according to the manufacturer's instructions. Total genomic DNA (4.5 μg) was loaded onto a 1.5% agarose gel, and internuclear DNA fragmentation was visualized after ethidium bromide staining.
Isolation of mitochondrial and cytosolic proteins
U937 cells treated with 20 μM p21-IRS for 3 hours were harvested and washed once with PBS. Cell pellets were suspended in ice-cold buffer A (250 mM sucrose, 20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA [ethylenediaminetetraacetic acid] and 1 mM EGTA [ethylene glycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid]) supplemented with fresh protease inhibitors (1 μM phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL leupeptin, and 10 μg/mL aprotinin) and 1 mM dithiothreitol (DTT) and incubated on ice for 20 minutes. Cell suspensions were drawn up and down 30 to 40 times through a 30 1/2 gauge needle at 4°C avoiding generation of bubbles, and centrifuged at 855 g for 5 minutes at 4°C to remove cytoplasmic membranes and unbroken cells. The supernatant was transferred to a new tube and spun at 16 060 g for 30 minutes at 4°C to bring down mitochondria. The supernatant was transferred to a fresh tube, and the mitochondrial fraction was saved for Western blotting. The supernatant was further centrifuged at 167 789 g at 4°C for one hour to obtain the cytosolic fraction.
Mitochondrial membrane potential
Changes in mitochondrial membrane potential (Δψm) were measured using 2 different fluorescent dyes, TMRM and JC-1. TMRM accumulates more within mitochondria having higher Δψm.14,15 A quantity of 200 μL of a suspension of U937 cells (1 × 106 cells/mL) pretreated with 20 μM p21-IRS for 3 hours was incubated with 1 μLof 50 μM TMRM in the dark at room temperature for 15 minutes, and fluorescence was measured by FACS.
JC-1 binds more specifically to mitochondrial membranes.16 JC-1 exists as a monomer at low concentrations with emission at 530 nm and aggregates at higher concentrations with emission at 590 nm. A quantity of 200 μL(1 × 106 cells/mL) of U937 treated with p21-IRS was washed once with PBS, and suspended in 200 μL PBS with 5 μg/mL JC-1 in the dark at room temperature for 10 minutes. Cells were washed twice with PBS and resuspended in 200 μL PBS before FACS analysis.
Reactive oxygen species
Carboxyl-DCFA/AM is a nonpolar compound that can easily diffuse into and be trapped inside cells. In the presence of reactive oxygen species (ROSs), it is further oxidized to the fluorescent 2,7-dichlorofluorescein which can be detected by FACS. To detect intracellular ROSs, U937 cells were incubated with 5 μM carboxyl-DCFA/AM at 37°C for 30 minutes and then washed once with PBS. Cells were then treated with 20 μM p21-IRS and analyzed immediately by FACS.
Cytosolic and mitochondrial Ca2+
Fluo-3 was used to measure cytosolic calcium concentration. Briefly, 1 × 106 U937 cells/mL were loaded with 5 μM fluo-3/AM, 0.02% pluronic F-127, and 0.25 mM sulfinpyrazone for one hour at room temperature, washed twice with RPMI 1640 to remove nonhydrolyzed fluo-3/AM, and suspended in RPMI 1640 (without fetal bovine serum [FBS]) at 1 × 106 cells/mL. Either separately or in combination, 20 μM p21-IRS and 5 mM EGTA were added to the cell suspension, and fluo-3/AM fluorescence was monitored kinetically using a fluorometer (Spectra MAX GEMINI; Molecular Devices, Sunnyvale, CA) with excitation set at 488 nm and emission at 522 nm. To determine the calcium concentration in mitochondria, 1 × 106 cells/mL were loaded with 8 μM rhod-1/AM and 0.02% pluronic F-127 at 4°C for 15 minutes, washed once, suspended in RPMI 1640 (without FBS) at 1 × 106 cells/mL, and incubated at room temperature for another 30 minutes. Then, 20 μM p21-IRS, 5 mM EGTA, or 10 μM A23187 was added, and the fluorescence was recorded kinetically using a fluorometer with excitation at 568 nm and emission at 605 nm.
Intracellular ATP
U937 cells (1 × 106 cells/mL) were treated with 20 μM p21-IRS for 3 hours, washed twice with PBS, and suspended in distilled water at 1 × 106 cells/mL. Cells were boiled for 2 minutes and then immediately cooled on ice. Cell debris was removed by centrifugation at 9503 g for 2 minutes at 4°C, and the supernatant was transferred to a new tube and kept on ice. Intracellular adenosine triphosphate (ATP) was measured using the ATP Determination Kit (Molecular Probes, Eugene, OR) based on the manufacturer's instructions.
Generation of ρ° cells
U937 cells were treated with 50 ng/mL ethidium bromide for 3 months in RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, 200 μg/mL penicillin, 100 μg/mL streptomycin sulfate, 100 μg/mL pyruvate, and 50 μg/mL uridine.17 mtDNA encodes proteins involved in mitochondrial respiratory chain complexes I, III, IV, and V, and dysfunction of these complexes resulting from the loss of mtDNA can be rescued by addition of pyruvate and uridine. Depletion of mtDNA from U937 ρ° cells was confirmed with actimycin A, an inhibitor of complex III, that causes apoptosis in wild-type but not U937 ρ° cells.
Generation of reticulocytes and hemolysis assay
Japanese white rabbits, weighing 1.9 kg to 2.0 kg, were made anemic by subcutaneous injection of 2.5% phenylhydrazine-HCl solution (Sigma) according to the following schedule: 1.0 mL on day 1, 0.8 mL on day 2, 0.6 mL on day 3, 0.8 mL on day 4, and 1 mL on day 5.18 Control rabbits were injected with the same volumes of sterile distilled water. On the sixth or seventh day, 5 mL blood was taken from the ear vein of the rabbits and collected in the tubes containing heparin. Cells were stained with Brilliant Cresyl Blue19 (Sigma) each day to determine the ratio of reticulocytes to red blood cells (RBCs). RBCs and reticulocytes were separated from plasma and buffy coat by centrifugation at 1000g for 5 minutes, washed 3 times with RPMI 1640 without phenol red, and suspended in RPMI 1640. For hemolysis assay, rabbit RBCs or reticulocytes (2 × 106 cells/mL in RPMI 1640) were treated with the indicated concentrations of p21-IRS at 37°C for 4 hours, and the released lactate dehydrogenase (LDH) were analyzed using an LDH kit (Roche, Palo Alto, CA).
Results
Apoptosis induced by p21-IRS
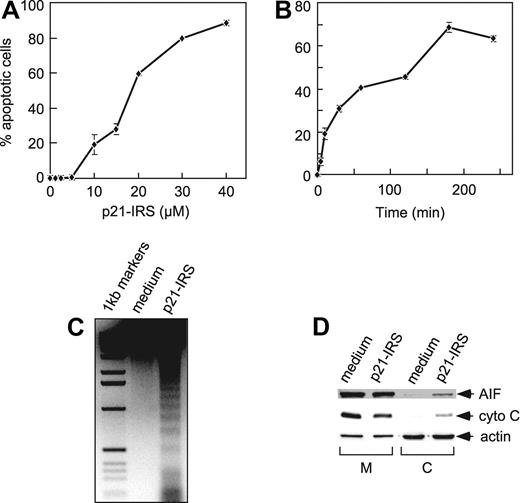
We previously reported that p21-IRS induced apoptosis in T lymphocytes.11 Subsequently, we observed that p21-IRS caused apoptosis in a number of different cell types, including B cells, T cells, monocytes, and macrophages of human or mouse origin (not shown). We selected the p53-deficient human monocyte–like line U937 for the studies reported here. p21-IRS induced apoptosis of U937 cells in a dose-dependent manner (Figure 1A), and the induction of apoptosis was rapid: 5 minutes after addition of 20 μM p21-IRS, 8% of the cells were apoptotic (Figure 1B). At 60 minutes, 42% of the cells were apoptotic, and by 3 hours more than 70% were apoptotic.
p21-IRS–induced apoptosis in U937 cells. U937 cells were incubated with indicated amounts of p21-IRS for 3 hours (A) or with 20 μM p21-IRS for the indicated times (B). Each point was the average of 3 independent experiments. (C) Internucleosomal DNA fragmentation. (D) Release of AIF and cytochrome C from mitochondria in cells treated with p21-IRS. M indicates mitochondria; C, cytosol; cyto C, cytochrome C.
p21-IRS–induced apoptosis in U937 cells. U937 cells were incubated with indicated amounts of p21-IRS for 3 hours (A) or with 20 μM p21-IRS for the indicated times (B). Each point was the average of 3 independent experiments. (C) Internucleosomal DNA fragmentation. (D) Release of AIF and cytochrome C from mitochondria in cells treated with p21-IRS. M indicates mitochondria; C, cytosol; cyto C, cytochrome C.
A hallmark of apoptosis is internucleosomal DNA degradation. Genomic DNA extracted from U937 cells 3 hours after incubation with 20 μM p21-IRS showed obvious DNA fragmentation, whereas the DNA from the untreated cells remained intact (Figure 1C).
p21-IRS damages mitochondria but does not involve caspases or affect mitochondrial potential
Release of cytochrome C and apoptosis-inducing factor (AIF) from mitochondria was also investigated. U937 were treated with 20 μM p21-IRS for 3 hours, and the mitochondrial and cytosolic fractions were analyzed by Western blot. Both cytochrome C and AIF were partially released from mitochondria to cytosol in the cells treated with p21-IRS but not in cells treated with medium alone (Figure 1D).
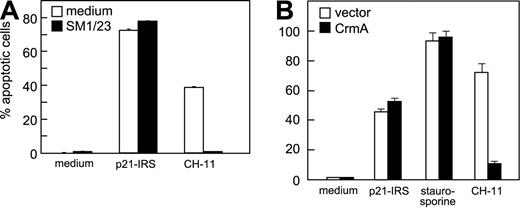
To evaluate whether Fas was involved in p21-IRS–induced apoptosis, U937 cells were pretreated with SM1/23, a monoclonal antibody (mAb) that blocks Fas/FasL-mediated apoptosis. SM1/23 mAb completely abrogated CH-11 (anti-Fas mAb)–induced apoptosis, but did not affect p21-IRS–induced apoptosis (Figure 2A). To test the involvement of caspase-8, Jurkat cells overexpressing CrmA, an inhibitor of activation of caspase-8, were treated with p21-IRS, staurosporine, or CH11 mAb. CH11-induced apoptosis was inhibited by overexpression of Crm A, whereas that induced by staurosporine or p21-IRS was not (Figure 2B).
Fas and caspase-8 are not involved in p21-IRS–induced apoptosis. (A) U937 cells were incubated with SM1/23, a mAb that disrupts Fas-mediated apoptosis, prior to the addition of p21-IRS or CH-11. (B) Jurkat cells overexpressing CrmA or transfected with the expression vector were treated with p21-IRS, staurosporine, or CH-11 for 3 hours, 4 hours, or overnight, respectively, and apoptosis determined.
Fas and caspase-8 are not involved in p21-IRS–induced apoptosis. (A) U937 cells were incubated with SM1/23, a mAb that disrupts Fas-mediated apoptosis, prior to the addition of p21-IRS or CH-11. (B) Jurkat cells overexpressing CrmA or transfected with the expression vector were treated with p21-IRS, staurosporine, or CH-11 for 3 hours, 4 hours, or overnight, respectively, and apoptosis determined.
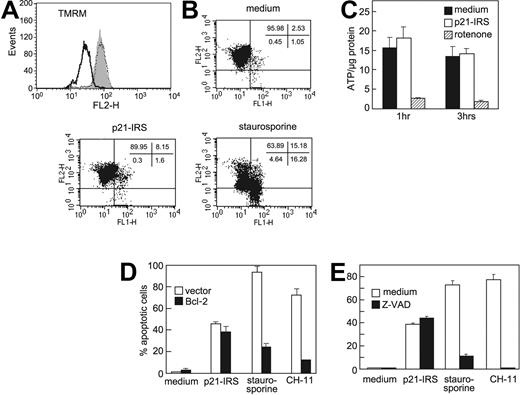
Many agents that cause apoptosis mediate an early loss of mitochondrial potential (Δψm).20-22 To address whether p21-IRS affects Δψm, U937 cells were treated with p21-IRS or staurosporine and then stained with TMRM. Cells treated with staurosporine exhibited a dramatic shift in fluorescence compared with control cells, indicating depolarization of mitochondrial membrane potential (Figure 3A). However, no shift in fluorescence was observed in p21-IRS–treated cells, indicating that p21-IRS does not affect Δψm. These findings were confirmed by using JC-1, a dye that is more specific for mitochondrial membrane potential (Figure 3B).
p21-IRS does not disrupt the mitochondrial inner membrane. U937 cells were incubated with medium, p21-IRS, or staurosporine, and Δψm was determined. (A) TMRM fluorescence of cells treated with medium (gray fill), staurosporine (black line), or p21-IRS (dotted line). (B) JC-1 fluorescence. The percentage of cells in each quadrant is shown. (C) p21-IRS does not affect intracellular ATP. U937 cells were treated with p21-IRS or rotenone for 1 hour or 3 hours and intracellular ATP determined. The results shown were the average of 3 replicas. (D) Overexpression of Bcl-2 could not rescue cells from p21-IRS–induced cell death. Jurkat cells overexpressing Bcl-2 were incubated with p21-IRS, staurosporine, or CH-11 for 3 hours, 4 hours, or overnight, respectively, and the percentage of apoptotic cells determined. Results are the average of 3 experiments. (E) The caspase inhibitor Z-VAD does not affect p21-IRS–induced apoptosis. Cells were preincubated with Z-VAD prior to the indicated treatment.
p21-IRS does not disrupt the mitochondrial inner membrane. U937 cells were incubated with medium, p21-IRS, or staurosporine, and Δψm was determined. (A) TMRM fluorescence of cells treated with medium (gray fill), staurosporine (black line), or p21-IRS (dotted line). (B) JC-1 fluorescence. The percentage of cells in each quadrant is shown. (C) p21-IRS does not affect intracellular ATP. U937 cells were treated with p21-IRS or rotenone for 1 hour or 3 hours and intracellular ATP determined. The results shown were the average of 3 replicas. (D) Overexpression of Bcl-2 could not rescue cells from p21-IRS–induced cell death. Jurkat cells overexpressing Bcl-2 were incubated with p21-IRS, staurosporine, or CH-11 for 3 hours, 4 hours, or overnight, respectively, and the percentage of apoptotic cells determined. Results are the average of 3 experiments. (E) The caspase inhibitor Z-VAD does not affect p21-IRS–induced apoptosis. Cells were preincubated with Z-VAD prior to the indicated treatment.
Mitochondria are the major source of cellular ATP, and mitochondrial integrity is required for the production of ATP. We therefore asked whether p21-IRS affects intracellular ATP levels. U937 cells treated with the electron transport chain inhibitor rotenone for 1 or 3 hours showed a sharp decline in intracellular ATP (Figure 3C), whereas cells treated with p21-IRS were indistinguishable from cells incubated in medium alone (Figure 3C). Thus, p21-IRS does not affect intracellular ATP.
Bcl-2 is thought to preserve mitochondrial membrane integrity and agents that induce apoptosis often are ineffective in cells overexpressing Bcl-2. Therefore, we explored whether p21-IRS could cause apoptosis in Jurkat cells stably transfected with Bcl-2. These cells were protected from cell death induced by staurosporine and CH-11, but were not protected from p21-IRS–induced apoptosis (Figure 3D).
Caspases, a family of aspartate-specific cysteine proteases, often play a major role in many physiologic and pathologic apoptotic processes. Upstream caspases, such as caspase-8, are activated by engagement of death receptors exemplified by Fas, whereas downstream caspases such as caspase-9 are often activated by mitochondrial damage. Our observation that p21-IRS–treated U937 cells release cytochrome C and AIF suggested that downstream caspases might be involved in p21-IRS–induced apoptosis. To investigate this, U937 cells were treated with staurosporine or p21-IRS, and cell extracts were assessed for the inactive precursor and mature cleaved forms of caspase-3 and caspase-9. No cleavage or either procaspase-3 and procaspase-9 was observed in cells treated with either medium or p21-IRS. In contrast, both forms were nearly totally cleaved in cells incubated with staurosporine (data not shown). These observations indicated that cleavage of procaspase-3 and procaspase-9 are not required for p21-IRS–induced apoptosis.
In order to assess involvement of other caspases, U937 cells were pretreated with Z-VAD, a broad-spectrum caspase inhibitor. Cells treated with Z-VAD or medium were equally susceptible to p21-IRS, whereas Z-VAD blocked apoptosis induced by either staurosporine or CH-11 (Figure 3E).
Role of intracellular ions in p21-IRS–mediated apoptosis
Homeostasis is important for proper cell function and is achieved by a balance of osmotic pressure across the plasma membrane. Most cells maintain their osmotic balance through the Na+/K+ ATPase pump, which sustains high intracellular K+ and low intracellular Na+.K+ efflux is an early event in apoptosis.23,24 Ions also act as cofactors for many enzymes. Thus, exploring the changes in intracellular ion content could lead to a greater understanding of the mechanisms of apoptosis induced by p21-IRS.
U937 cells pretreated with the Na+ channel blockers, QX222 or amiloride, were protected from p21-IRS–induced apoptosis (Table 1), suggesting that the Na+ channel is involved and that changes in intracellular Na+ are partially responsible for activating the death program. U937 cells pretreated with the K+ channel blockers TEA or apamin were also largely protected from apoptosis induced by p21-IRS (Table 1), indicating that K+ plays a critical role in programmed cell death. Cells incubated in a high K+ buffer (150 mM K+) were highly resistant to p21-IRS–induced apoptosis (Table 1).
Effects of ion channel and electron transport complex inhibitors on p21-IRS–induced apoptosis
. | Inhibitor (dose) . | Percent inhibition of apoptosis ± SD . |
|---|---|---|
| Na+ channel | QX222 (15 mM) | 37.0 ± 2.2 |
| Amiloride (1.5 mM) | 46.0 ± 1.4 | |
| Ca2+ channel | Econazole (20 μM) | 44.0 ± 2.3 |
| Nicardipine (20 μM) | 71.0 ± 6.2 | |
| Ni2+ (0.5 mM) | 30.0 ± 1.1 | |
| EDTA (10 mM) | 68.0 ± 0.5 | |
| EGTA (5 mM) | 55.0 ± 6.6 | |
| K+ channel | TEA (50 mM) | 73.6 ± 1.1 |
| Apamin (50 μM) | 84.0 ± 8.2 | |
| High K+ buffer | 83.0 ± 3.8 | |
| Electron transport complex | ||
| Complex I | Rotenone (20 μM) | 49.0 ± 1.6 |
| Complex II | TTFA (500 μM) | 52.0 ± 3.6 |
| Complex III | Actimycin A (30 μM) | 6.5 ± 5.1 |
| Myxothiazol (10 μM) | 0 | |
| Stigmatellin (10 μM) | 0 | |
| Complex IV | KCN (7.5 mM) | 53.0 ± 3.5 |
. | Inhibitor (dose) . | Percent inhibition of apoptosis ± SD . |
|---|---|---|
| Na+ channel | QX222 (15 mM) | 37.0 ± 2.2 |
| Amiloride (1.5 mM) | 46.0 ± 1.4 | |
| Ca2+ channel | Econazole (20 μM) | 44.0 ± 2.3 |
| Nicardipine (20 μM) | 71.0 ± 6.2 | |
| Ni2+ (0.5 mM) | 30.0 ± 1.1 | |
| EDTA (10 mM) | 68.0 ± 0.5 | |
| EGTA (5 mM) | 55.0 ± 6.6 | |
| K+ channel | TEA (50 mM) | 73.6 ± 1.1 |
| Apamin (50 μM) | 84.0 ± 8.2 | |
| High K+ buffer | 83.0 ± 3.8 | |
| Electron transport complex | ||
| Complex I | Rotenone (20 μM) | 49.0 ± 1.6 |
| Complex II | TTFA (500 μM) | 52.0 ± 3.6 |
| Complex III | Actimycin A (30 μM) | 6.5 ± 5.1 |
| Myxothiazol (10 μM) | 0 | |
| Stigmatellin (10 μM) | 0 | |
| Complex IV | KCN (7.5 mM) | 53.0 ± 3.5 |
Ca2+ is another ion implicated in apoptosis. Elevated cytosolic Ca2+ has been shown to activate nucleases that lead to the degradation of nuclear DNA. Ca2+ is also a prominent modulator of mitochondrial permeability transition. To examine the role of Ca2+ in p21-IRS–induced apoptosis, U937 cells were pretreated with 3 types of Ca2+ channel blockers: econazole (voltage-independent); nicardipine (voltage-dependent); or Ni2+ (inorganic chemical). All 3 inhibitors protected cells from p21-IRS–induced apoptosis to some degree (Table 1). Incubation of U937 cells in medium supplemented with EGTA or EDTA significantly reduced p21-IRS–induced apoptosis (Table 1), indicating that influx of Ca2+ from external sources was involved. To exclude the possibility that ion channel blockers prevented uptake of p21-IRS, immunohistochemistry was performed using anti-IRS antibodies as previously described.11 p21-IRS uptake was identical in cells treated with medium or ion channel blockers (not shown).
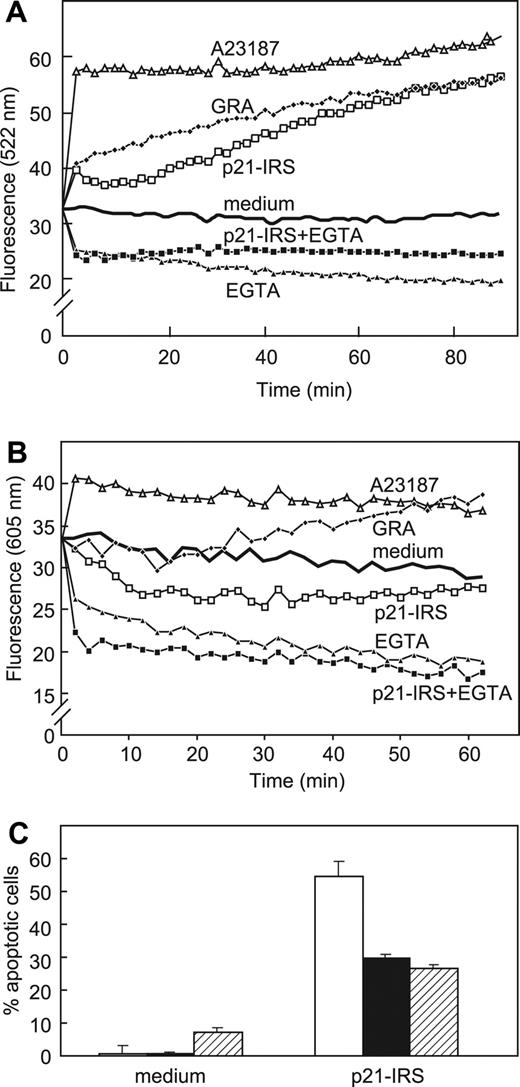
Cytosolic and mitochondrial Ca2+ levels were measured directly using fluo-3 and rhod-2, respectively (Figure 4). Cytosolic Ca2+ ([Ca2+]i) increased almost immediately after addition of p21-IRS or granulysin, a lytic molecule made by activated human T cells.25 However, U937 cells preincubated in medium supplemented with EGTA did not exhibit an increase in [Ca2+]i after addition of p21-IRS, suggesting that influx of extracellular Ca2+ is the major source of elevated Ca2+ in cytosol (Figure 4A). As we previously reported, granulysin causes an increase in mitochondrial Ca2+.26 In contrast, p21-IRS led to the release of Ca2+ from mitochondria (Figure 4B).
p21-IRS increases cytosolic Ca2+ but decreases mitochondrial Ca2+. U937 cells were incubated with fluorescent dyes fluo-3 (A) or rhod-2 (B), prior to the addition of the indicated compounds. (C) Iberiotoxin, a specific Ca2+-dependent K+ channel blocker, partially blocks p21-IRS–induced apoptosis. Cells were preincubated with medium (open bar), 10 μM iberiotoxin (black bar), or 15 μM iberiotoxin (striped bar) prior to the addition of p21-IRS.
p21-IRS increases cytosolic Ca2+ but decreases mitochondrial Ca2+. U937 cells were incubated with fluorescent dyes fluo-3 (A) or rhod-2 (B), prior to the addition of the indicated compounds. (C) Iberiotoxin, a specific Ca2+-dependent K+ channel blocker, partially blocks p21-IRS–induced apoptosis. Cells were preincubated with medium (open bar), 10 μM iberiotoxin (black bar), or 15 μM iberiotoxin (striped bar) prior to the addition of p21-IRS.
Iberiotoxin, a specific Ca2+-dependent K+ channel blocker, was used to clarify the relationship between the increase in [Ca2+]i and K+ efflux in p21-IRS–induced apoptosis. Cells pretreated with iberiotoxin were less susceptible to p21-IRS (Figure 4C), suggesting that change of [Ca2+]i precedes K+ efflux, and that the K+ efflux is dependent on the increase in [Ca2+]i.
Reactive oxygen species are elevated in p21-IRS–treated cells
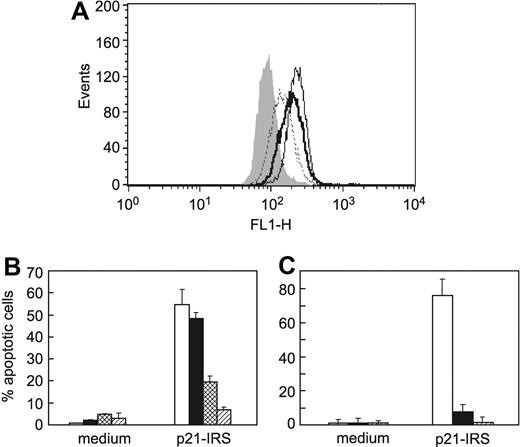
Reactive oxygen species (ROSs) are associated with many kinds of apoptosis, 27 as well as diseases such as stroke, ischemia, and neurodegenerative disorders.28-32 To evaluate the role of ROSs in p21-IRS–induced apoptosis, carboxyl-DCFDA/AM was employed to detect the production of ROSs. Carboxyl-DCFDA/AM fluorescence increases in cells treated with staurosporine or p21-IRS (Figure 5A), indicating an increase in ROSs. To confirm that ROSs contribute to apoptosis, U937 cells were treated with either LNAC, the glutathione precursor that is widely used as an intracellular free radical scavenger, or MnTBAP, a cell permeable super oxide dismutase mimetic, prior to the addition of p21-IRS. Both LNAC and MnTBAP almost completely blocked p21-IRS–induced apoptosis (Figure 5B-C). These findings indicate that ROSs play a critical role in p21-IRS–induced apoptosis, and that abrogation of the increase in ROSs rescues cells from the death program.
Generation of ROSs led to apoptosis in U937 cells. (A) p21-IRS–treated U937 cells produce ROSs. ROS production in U937 cells incubated with medium (gray fill); p21-IRS for 1 hour (dotted line); p21-IRS for 3 hours (solid line); or staurosporine for 4 hours (bold line). (B) The ROS scavenger LNAC rescues cells from p21-IRS–induced apoptosis. U937 cells were preincubated with medium (open bar), or with LNAC at 10 mM (black bar), 15 mM (hatched bar), or 20 mM (stippled bar) before the addition of p21-IRS. (C) The ROS scavenger MnTBAP rescues cells from p21-IRS–induced apoptosis. U937 cells were preincubated with medium (open bar) or with MnTBAP at 50 μM (black bar) or 200 μM (striped bar) before the addition of p21-IRS.
Generation of ROSs led to apoptosis in U937 cells. (A) p21-IRS–treated U937 cells produce ROSs. ROS production in U937 cells incubated with medium (gray fill); p21-IRS for 1 hour (dotted line); p21-IRS for 3 hours (solid line); or staurosporine for 4 hours (bold line). (B) The ROS scavenger LNAC rescues cells from p21-IRS–induced apoptosis. U937 cells were preincubated with medium (open bar), or with LNAC at 10 mM (black bar), 15 mM (hatched bar), or 20 mM (stippled bar) before the addition of p21-IRS. (C) The ROS scavenger MnTBAP rescues cells from p21-IRS–induced apoptosis. U937 cells were preincubated with medium (open bar) or with MnTBAP at 50 μM (black bar) or 200 μM (striped bar) before the addition of p21-IRS.
To pinpoint the site(s) of ROS generation on the electron transport chain, U937 cells were pretreated with rotenone (complex I inhibitor); TTFA (complex II inhibitor); actimycin A (complex III center i inhibitor); myxothiazol or stigmatellin (complex III center o inhibitors); or KCN (complex IV inhibitor) before exposure to p21-IRS. Rotenone, TTFA, and KCN partially protected cells from p21-IRS–mediated apoptosis, whereas no effect was observed in cells pretreated with actimycin A, myxothiazol, or stigmatellin (Table 1), suggesting that complexes I, II, and IV are the sites of the ROS production.
Ca2+ influx and ROS production can be dependent33-35 or occur in parallel.36 Since the increase in [Ca2+]i occurred very rapidly in U937 after incubation with p21-IRS (Figure 4A), we investigated when ROSs were generated. After incubated with DCFDA-CM for 30 minutes at room temperature, cells were treated with p21-IRS, and production of ROSs was monitored kinetically. No change in ROSs was detected during the first 30 minutes (not shown), indicating that ROS production occurs much later than the rise in [Ca2+]i. To address whether ROS generation is dependent on [Ca2+]i, the Ca2+ channel blocker nicardipine was incubated with U937 before the addition of p21-IRS. p21-IRS caused a similar increase in ROSs in cells pretreated with nicardipine as in those pretreated with medium (not shown), further demonstrating the calcium independence of the increase in ROSs. Thus, the p21-IRS–induced increase in [Ca2+]i occurs quite early, whereas ROS production occurs relatively late; ROS generation is not dependent on Ca2+ influx, but disruption of an increase in either [Ca2+]i or ROSs can block p21-IRS–induced apoptosis.
Cells lacking functional mitochondria are resistant to p21-IRS
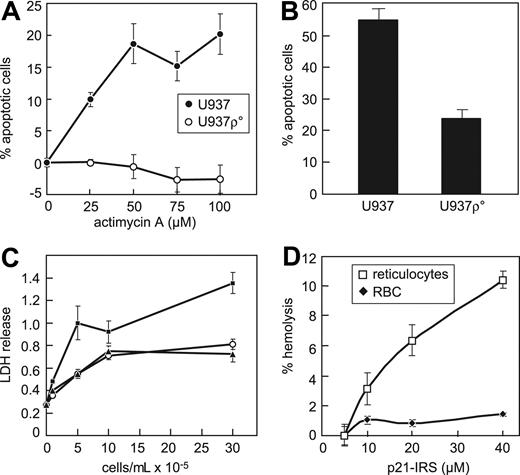
Mitochondria are sites of ROS generation and serve as reservoirs for intracellular Ca2+, suggesting that they are essential components in the p21-IRS–induced apoptosis. To address whether intact respiration in mitochondria is required for p21-IRS–induced apoptosis, U937 ρ° cells deficient in mitochondrial DNA were generated. U937 ρ° cells were unaffected by antimycin A (Figure 6A), and were more resistant to p21-IRS–induced apoptosis: after a 2.5-hour incubation with p21-IRS, 55% of wild-type cells were apoptotic, but only 25% of U937 ρ° cells underwent apoptosis (Figure 6B).
Mitochondria are required for p21-IRS–induced apoptosis. (A) U937 cells, but not U937 ρ° cells, are susceptible to actimycin A. (B) U937 ρ° cells are largely resistant to p21-IRS–induced apoptosis. (C) p21-IRS does not hemolyze red blood cells. Medium (○); p21-IRS (▴); 1% Triton X (▪). (D) p21-IRS lyses rabbit reticulocytes but not red blood cells.
Mitochondria are required for p21-IRS–induced apoptosis. (A) U937 cells, but not U937 ρ° cells, are susceptible to actimycin A. (B) U937 ρ° cells are largely resistant to p21-IRS–induced apoptosis. (C) p21-IRS does not hemolyze red blood cells. Medium (○); p21-IRS (▴); 1% Triton X (▪). (D) p21-IRS lyses rabbit reticulocytes but not red blood cells.
Because red blood cells lack mitochondria and other organelles, we were interested to learn whether they were susceptible to p21-IRS. p21-IRS did not cause hemolysis of human red blood cells, even when incubated at a high concentration (40 μM) for up to 4 hours (Figure 6C). Reticulocytes have ribosomes and mitochondria, but no nuclei. Immature reticulocytes were more susceptible to p21-IRS–induced release of LDH than were erythrocytes (Figure 6D), indicating that mitochondria and/or other organelles contribute to p21-IRS–induced apoptosis.
Discussion
p21 is a major regulator of cell cycle progression. The amino terminus of p21 interacts with cyclins and cyclin-dependent kinases, while the carboxyl terminus interacts with PCNA. Chimeric peptides consisting of the carboxyl terminal domain of p21 conjugated to cell-internalizing vehicles, such as the AP homeodomain 16-mer peptide, induce cell death. We previously reported that a synthetic peptide corresponding to residues 139-160 of p21 conjugated to a pentapeptide (RYIRS) caused apoptosis. In the present study we demonstrate that the mechanism of p21-IRS–induced apoptosis is distinct from that induced by other proapoptotic agents (Table 2).
Comparison of the features of apoptosis induced by p21-IRS, staurosporine, CH11, and granulysin
. | p21-IRS . | Staurosporine . | CH11 . | Granulysin . |
|---|---|---|---|---|
| Δψm | No change | ↓ | ↓ | ↓ |
| Apoptosis in cells overexpressing Bcl-2 | No change | ↓ | ↓ | ↓ |
| Apoptosis in cells overexpressing CrmA | No change | ↓ | ↓ | ↓ |
| Activation of caspase-9 | No | Yes | Yes | Yes |
| Apoptosis in cells treated with Z-VAD | No change | ↓ | ↓ | ↓ |
| Release of cytochrome C and AIF | Yes | Yes | Yes | Yes |
| Cytosolic Ca2+ | ↑ | ↑ | ↑ | ↑ |
| Mitochondrial Ca2+ | ↓ | ↑ | ↑ | ↑ |
| ROS | ↑ | ↑ | ↑ | ↑ |
| Apoptosis in cells treated with LNAC/MnTBAP | ↓ | ↓ | ↓ | ↓ |
| Apoptosis in cells treated with iberiotoxin | ↓ | No change | No change | No change |
| Apoptosis in cells treated with Ca2+ channel blockers | ↓ | ↓ | No change | ↓ |
| Apoptosis in cells treated with K+ channel blockers | ↓ | No change | No change | ↓ |
| Apoptosis in cells treated with Na+ channel blockers | ↓ | ↓ | No change | ↓ |
. | p21-IRS . | Staurosporine . | CH11 . | Granulysin . |
|---|---|---|---|---|
| Δψm | No change | ↓ | ↓ | ↓ |
| Apoptosis in cells overexpressing Bcl-2 | No change | ↓ | ↓ | ↓ |
| Apoptosis in cells overexpressing CrmA | No change | ↓ | ↓ | ↓ |
| Activation of caspase-9 | No | Yes | Yes | Yes |
| Apoptosis in cells treated with Z-VAD | No change | ↓ | ↓ | ↓ |
| Release of cytochrome C and AIF | Yes | Yes | Yes | Yes |
| Cytosolic Ca2+ | ↑ | ↑ | ↑ | ↑ |
| Mitochondrial Ca2+ | ↓ | ↑ | ↑ | ↑ |
| ROS | ↑ | ↑ | ↑ | ↑ |
| Apoptosis in cells treated with LNAC/MnTBAP | ↓ | ↓ | ↓ | ↓ |
| Apoptosis in cells treated with iberiotoxin | ↓ | No change | No change | No change |
| Apoptosis in cells treated with Ca2+ channel blockers | ↓ | ↓ | No change | ↓ |
| Apoptosis in cells treated with K+ channel blockers | ↓ | No change | No change | ↓ |
| Apoptosis in cells treated with Na+ channel blockers | ↓ | ↓ | No change | ↓ |
↓ indicates a decrease compared with medium; ↑, an increase compared with medium.
Similar to many other apoptosis-inducing factors, p21-IRS–induced apoptosis is rapid, cleaves DNA into a typical ladder, and is associated with release of AIF and cytochrome C from mitochondria as well as an increase in ROS production. However, p21-IRS–induced apoptosis does not involve depolarization of mitochondrial membranes, activation of caspases, or depletion of intracellular ATP. Goldstein et al37 reported that cytochrome C release from mitochondria was a very early event during apoptosis, and even preceded the exposure of phosphatidylserine and the reduction of Δψm. Moreover, the loss of mitochondrial membrane potential occurred later than caspase activation, and was not necessary for DNA fragmentation.38 Since ATP synthesis is driven by electrochemical gradient across mitochondria inner membrane, the integrity of mitochondrial inner membrane is required to maintain the level of ATP within the cells. ATP levels in cells treated with p21-IRS did not change and Δψm was stable, suggesting that p21-IRS only disrupts the outer mitochondrial membrane and leaves the inner membrane intact.
One of the major pathways for caspase activation involves cytochrome C.39 Once released from mitochondria, cytochrome C recruits proteins to assemble the apoptosome complex, which further initiates a proteolytic cascade resulting in the activation of caspase-9.1,40,41 However, cytochrome C release does not always result in the activation of caspases, 42 and conditions that elevate ROSs or nitric oxide can also lead to caspase inactivation.43
The observation that p21-IRS–induced apoptosis is not affected by the general caspase inhibitor Z-VAD implicates the caspase-independent effectors AIF and endonuclease G (EndoG). AIF induces chromatin condensation and large-scale DNA fragmentation. EndoG, like AIF, translocates to the nucleus where it causes oligonucleosomal DNA breakdown. The release of AIF and EndoG can be accompanied by a decrease or no change in mitochondrial membrane potential.
Bortner and Cidlowski44 studied apoptosis in Jurkat cells treated with various apoptosis-inducing agents. They concluded that cell shrinkage, K+ efflux, and a decrease in Δψm were tightly linked, but these events could occur independently of DNA fragmentation and did not require the activation of caspases. p21-IRS–induced apoptosis of U937 involves both K+ efflux and DNA degradation, but was not associated with a decrease in Δψm.
p21 has been reported to have both pro- and antiapoptotic effects. The majority of reports demonstrate that p21 inhibits apoptosis by a number of different mechanisms, including p21-dependent cell cycle arrest, inactivation of cyclin A/Cdk2, and inhibition of initiator caspase cleavage. There have been several reports that cleavage of p21 in vivo, or introduction of fragments of p21 into cells, results in apoptosis. Tchou et al45 found that lung carcinoma cells induced with the phorbol ester TPA underwent G2/M cell cycle arrest and cleavage of p21 at its carboxyl terminus. Levkau et al46 found that apoptosis of endothelial cells was associated with specific cleavage of the carboxyl terminus of p21, resulting in a decrease in its association with nuclear cyclin-cdk2 complexes. Zhang and coworkers47 reported that during apoptosis of cancer cells p21 was cleaved by caspase-3 and that the cleaved p21 no longer could interact with PCNA. Thus, a fragment similar to the p21 peptide may be generated in vivo under certain conditions and promote apoptosis.
Ball et al48 synthesized a panel of synthetic peptides corresponding to p21 and investigated their ability to bind to and inhibit cyclin D1-Cdk4. They identified a 20-mer peptide derived from residues 141-160 of p21 that inhibited cyclin D1-Cdk4 activity in vitro, and, when conjugated to a 16–amino acid sequence from the homeodomain of the AP, was translocated into cells where it blocked phosphorylation of the retinoblastoma protein Rb and caused G1/S growth arrest. Bonfanti and coworkers49 extended this observation to tumor cells. Mutoh et al10 optimized the p21 peptide inhibitory sequence to residues 139-164 and reported that the AP-p21 139-164 conjugate induced necrosis of a Burkitt lymphoma cell line, although the AP peptide alone also induced equivalent cell death at 10-fold higher concentrations.
Cell-penetrating peptides translocate across biologic membranes to enter cells in an apparently energy-independent manner. Prototypic cell-penetrating peptides include the basic sequence of the HIV-1 Tat protein50 and the third helix of the AP of Drosophila.51 The list of cell-penetrating peptides and the variety of molecules that they can deliver into cells has grown exponentially. The only consistent feature among cell-penetrating peptides is a high content of basic residues resulting in an overall positive charge. The theoretical isoelectric point (pI) of the p21 peptide is 11.84, and addition of the RYIRS tag increases the theoretical pI only slightly to 11.89. In addition, an alpha helical structure has been predicted for many cell-penetrating peptides.52 The predicted alpha helical content of the p21 peptide is 63%, and this value increases to 70% with the addition of the RYIRS sequence at the carboxyl terminus (http://www.embl-heidelberg.de/Services/serrano/agadir/agadir-start.html).
In summary, we demonstrate that a small peptide derived from the carboxyl terminus of p21, when conjugated to the pentapeptide RYIRS, enters cells and induces rapid apoptosis. Mitochondria are central to p21-IRS–induced apoptosis, but caspases and a decrease in Δψm are not involved. Methods to target the p21 peptide to specific cell populations may allow development of novel therapies to eliminate aberrant cells in human diseases.
Prepublished online as Blood First Edition Paper, October 5, 2004; DOI 10.1182/blood-2004-06-2188.
Supported by a grant from the National Institutes of Health (AI50187; C.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal