Abstract
Robust CD8+ T-cell activation is vital for the recovery from many viral infections and is orchestrated via the integration of signals delivered through surface molecules, including the T-cell antigen receptors (TcRs) and cytokine receptors. Little is known about how virus-specific T cells interpret sequential or combined stimulation through these receptors, which must undoubtedly occur in vivo during antiviral immune responses. When measured in real time, peptide antigen and the cytokines, interleukin 12 (IL-12) and IL-18, independently regulate the on/off kinetics of protective (interferon γ, tumor necrosis factor α) and immunomodulatory (IL-2, CD40L) cytokine production by activated T cells and memory T cells. The remarkable differences in effector functions elicited by innate or adaptive signals (IL-12/ IL-18 or peptide, respectively) illustrate the complex and stringent regulation of cytokine expression by CD8+ T cells. Together, these results indicate how antiviral T cells incorporate multiple signals from their local microenvironment and tailor their cytokine responses accordingly.
Introduction
CD8+ T-cell activation is first initiated by engagement of the T-cell antigen receptor (TcR) with specific peptide presented in the context of major histocompatibility complex (MHC) class I, along with the interaction of costimulatory molecules. These events trigger the production of cytokines and the acquisition of cytolytic effector functions, and they initiate the clonal expansion of antigen-specific CD8+ T cells. Initial antigen encounter also programs CD8+ T-cell differentiation, so that after clearance of a primary viral infection, populations of memory cells will persist and be capable of a more effective secondary response upon reexposure to viral antigen and heightened long-term, adaptive immunity.1
Lymphocytic choriomeningitis virus (LCMV) infection of mice is a well-established model used for the analysis of CD8+ T-cell responses to viral infection.2 Several CD8+ T-cell epitopes have been identified in LCMV, making it possible to specifically monitor virus-specific T cells throughout the course of the antiviral response using enzyme-linked immunospot (ELISPOT), intracellular cytokine staining (ICCS), or peptide/MHC tetramer reagents.3 LCMV-specific T cells produce many immunomodulatory proteins during the course of infection, including interferon γ (IFNγ),3 tumor necrosis factor α (TNFα),4,5 interleukin 2 (IL-2),6-8 and CD40L.9 These proteins are rapidly produced by CD8+ T cells isolated from LCMV-infected hosts upon direct ex vivo stimulation with either peptide or virus-infected stimulator cells, although there are substantial differences in their expression patterns.4,5
Several studies have shown that CD8+ T cells not only participate in the adaptive immune response to cognate peptide antigen but can also function in a non–antigen-specific, or “innate” capacity by responding directly to cytokines secreted by damaged or infected antigen-presenting cells (APCs).10-13 IL-12 and IL-18 are examples of cytokines commonly produced by professional APCs such as macrophages and dendritic cells in response to host microbial infection and are potent inducers of natural killer (NK) cell activation and IFNγ production.14,15 Similar to the innate functions of NK cells, previously activated CD44hi CD8+ T cells are also responsive to IL-12 and IL-18, whereas naive CD44lo T cells are not.10-13,16 Thus, prior TcR-mediated activation enables T cells with “dual-function” capability, including both innate and adaptive effector functions. However, the direct ex vivo regulation of multiple innate and adaptive cytokine functions in virus-specific T cells at different stages of differentiation (ie, effector versus memory) has not been previously explored.
The observation that antigen-experienced CD8+ T cells produce IFNγ in response to IL-12 and IL-18 raises the question of whether these innate stimuli might also regulate the expression of other cytokines known to be induced by cognate antigen. The current studies were therefore undertaken to examine the immediate, ex vivo functions of virus-specific effector and memory T cells and to compare the expression patterns of 4 representative cytokines, including 2 antiviral/antimicrobial cytokines (IFNγ and TNFα) and 2 potent growth factors, IL-2 and CD40L. The results presented herein demonstrate that both peptide and the innate cytokines, IL-12 and IL-18, induce prolonged expression of IFNγ by 70% to 80% of virus-specific CD8+ T lymphocytes. Surprisingly, IL-12 plus IL-18–induced IFNγ production continues for many hours after IL-12 and IL-18 have been completely removed from the medium, a sharp contrast to what occurs after loss of stimulation through the TcR.17 Following acute viral infection, the majority of virus-specific CD8+ T cells will produce IFNγ after peptide stimulation, but this form of activation induces only transient expression of TNFα, IL-2, or CD40L, often in only a subset of cells. Unlike peptide stimulation, IL-12 and IL-18 did not induce appreciable levels of TNFα or IL-2, but they did induce CD40L expression, with production continuing long after peptide-induced production of this protein had ceased. Together, these results show that virus-specific T cells are capable of differential responses to exogenous signals mediated through the TcR or through innate cytokine receptors, resulting in distinct and selective secretion of inflammatory and/or immunomodulatory proteins.
Materials and methods
Mice and viral infection
BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME) or were bred at Oregon Health and Science University (OHSU). Mice between 6 and 12 weeks of age were infected intraperitoneally with 2 × 105 plaque-forming unit (PFU) LCMV-Armstrong (53b) and were used at the indicated time points after infection. All experimental procedures were approved by the OHSU Institutional Animal Care and Use Committee.
In vitro stimulation of T cells
Splenocytes isolated from LCMV-infected mice were depleted of red cells by NH4Cl lysis and were cultured in RPMI 1640 supplemented with 5% fetal bovine serum (FBS; HyClone, Logan, UT), 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), L-glutamine, and antibiotics. Cells were stimulated at a density of 1 × 106/150 μL/well in 96-well round-bottom plates and maintained at 37°C, 6% CO2. IL-12 (R&D Systems, Minneapolis, MN) and IL-18 (Medical & Biological Laboratories [MBL], Watertown, MA) were used at final concentrations of 10 ng/mL. Although a smaller subpopulation of CD8+ T cells could respond to either IL-12 or IL-18 alone (data not shown), optimal T-cell activation was accomplished when these 2 cytokines were used in combination. High-performance liquid chromatography (HPLC)–purified (> 95% pure) NP118-126 peptide (Alpha Diagnostic, San Antonio, TX) was used at 10–7 M. Brefeldin A (BFA; Sigma, St Louis, MO) was added for either the final hour of culture or the entire culture period at 2 μg/mL. Maintaining BFA for the entire culture period sharply inhibited T-cell activation by IL-12 plus IL-18 (data not shown), so optimal cytokine-stimulation occurred when cultures contained BFA for only the last hour of stimulation. We were unable to reproducibly identify direct ex vivo production of IL-5, IL-6, or IL-10 in CD8+ T cells by ICCS after stimulation with peptide or IL-12 and IL-18 (data not shown).
For “on-off-on” experiments, splenocytes were initially stimulated at a density of 10 × 106/500 μL/well in 24-well plates for 5 hours with 10 ng/mL each IL-12 and IL-18. Cells were then either maintained in these cytokines, treated with 100 μg/mL cycloheximide (CHX), or washed twice in medium and replated in medium, in 96-well plates as described in the paragraph above. To ensure that exogenous IL-12 and IL-18 had been completely removed from these direct ex vivo cultures, the IFNγ-inducing activity of the supernatants collected after each washing step was tested independently on fresh virus-specific CD8+ T cells. The wash steps diluted the exogenous cytokines in the initial cultures by approximately 225 000-fold (from 10 ng/mL to < 0.04 pg/mL), and the IFNγ-inducing activity of the final supernatant was indeed below the threshold required for T-cell activation (< 0.01 ng/mL), indicating that the exogenous cytokines had been removed. In some experiments, CD8+ T cells were purified by positive enrichment (∼ 90% pure) using CD8α microbeads (Miltenyi Biotec, Auburn, CA) to remove splenic APCs, stimulated for 5 hours with IL-12 and IL-18, washed, and immediately treated with neutralizing antibodies against IL-12 (10 μg/mL; R&D Systems) or against IL-18 (10 μg/mL, MBL) and the off-rate kinetics of IFNγ production were monitored. No difference was observed in the slow off-rate kinetics of purified CD8+ T cells in the presence or absence of APCs or these neutralizing antibodies (data not shown). After 5 hours or 14 hours of cytokine deprivation (without addition of neutralizing antibodies), IL-12 and IL-18 were replenished (10 ng/mL each, final concentration), and the cultures were incubated at 37°C as indicated with 2 μg/mL BFA added for the final hour of culture. Cell wash supernatants and supernatants from cytokine-depleted culture wells were tested for IFNγ-inducing activity by incubation with 1 × 106 fresh CD8+ T cells (day 8 after infection) for 5 hours, and these controls indicated that no residual stimulatory IL-12 or IL-18 activity remained.
Staining and flow cytometry
Cells were blocked with anti-FcγRII/III antibody (clone 2.4G2; 1.3 μg/mL) and mouse immunoglobulin G (IgG; 100 μg/mL; Sigma), and surface stained with anti-CD8α (clone 53-6.7; Pharmingen, San Diego, CA), and H-2Ld NP118-126 tetramer (National Institutes of Health [NIH] tetramer core facility, Atlanta, GA). Intracellular staining was subsequently carried out with antibodies specific for IFNγ (clone XMG1.2; CalTag, Burlingame, CA), TNFα (clone MP6-XT22; Pharmingen), IL-2 (clone JES6-5H4; Pharmingen), and CD40L (clone MR1; Pharmingen). Between 100 000 and 300 000 events were collected on a FacsCalibur flow cytometer and analyzed with CellQuest software (Becton Dickinson, San Jose, CA).
Statistical analysis
A 2-tailed Student t test with unequal variance was used to evaluate statistical significance of differences between groups except for cytokine mean fluorescence intensity (MFI) comparisons (Figure S1 on the Blood website; see the Supplemental Figure link at the top of the online article) in which a paired Student t test was used to compare the same sample under 2 different stimulation conditions. A value of P less than .05 was considered significant.
Results
Virus-specific T cells exhibit unique “on-off-on” regulation of IFNγ in response to IL-12 and IL-18
IFNγ arguably represents the most commonly measured antimicrobial protein secreted by T cells and is typically used to quantitate virus-specific T cells and to measure their effector function. Peptide-induced IFNγ production by virus-specific CD8+ T lymphocytes persists for as long as antigen is present but ceases almost immediately after peptide withdrawal.17 To determine whether IL-12– and IL-18–stimulated IFNγ production displays similarly stringent “on-off” regulation, T cells isolated from LCMV-infected mice at the peak of acute infection (8 days after infection) or after differentiation into resting memory cells (immune; > 120 days after infection) were stimulated directly ex vivo with these cytokines for up to 12 hours. Virus-specific CD8+ T cells were identified by binding of an MHC class I tetramer loaded with the LCMV nucleoprotein 118 to 126 (NP118) peptide, the dominant LCMV epitope that comprises approximately 90% of the CD8+ T-cell response in BALB/c mice.18,19 Stimulation with IL-12 plus IL-18 rapidly induced IFNγ production by virus-specific LdNP118 tetramer+ CD8+ T cells in both effector and memory T-cell populations (Figure 1A). Effector T cells reached maximal levels of IFNγ production within 5 hours, whereas memory T cells were substantially slower and required about 8 hours to reach full production. In both populations, approximately 70% to 80% of the virus-specific CD8+ T cells produced IFNγ, which persisted for at least 12 hours in the continuous presence of these cytokines. NP118 peptide stimulation in the presence of IL-12 and IL-18 did not improve the already rapid speed of peptide-induced IFNγ production,4,5,17,19 but it resulted in modestly higher levels of IFNγ expression on a per cell basis, most significantly in memory T cells (Figure S1).
Cytokine-induced IFNγ production by virus-specific CD8+ T cells. (A) Spleen cells isolated from mice at 8 days (day 8) or greater than 120 days (immune) after LCMV infection were activated directly ex vivo with the combination of IL-12 and IL-18 for up to 12 hours. After gating on CD8+LdNP118-Tetramer+ T cells, IFNγ production was detected by intracellular staining. Values are the average ± SD for 4 animals in each group. (B) Spleen cells from mice at day 8 after infection were activated directly ex vivo with IL-12 plus IL-18 for 5 hours to induce IFNγ production. Activated cells were then either maintained in cytokines, treated with the protein synthesis inhibitor CHX, or washed and replaced in medium without cytokines. After 5 hours of cytokine withdrawal (t = 10 hours), cells were restimulated with IL-12 plus IL-18 for 4 hours. IFNγ production by CD8+LdNP118-Tetramer+ cells was detected by intracellular staining. Values are averages ± SDs (n = 3). (C) Spleen cells from mice at day 8 after infection were activated directly ex vivo with IL-12 and IL-18 for 5 hours to induce IFNγ production. Activated cells were then either maintained in IL-12 and IL-18 or washed and replaced in medium without cytokines. After 14 hours of cytokine withdrawal (t = 19 hours), cells were restimulated with IL-12 and IL-18 for an additional 8 hours. IFNγ production by CD8+LdNP118-Tetramer+ cells was detected by intracellular staining. Values are averages ± SDs (n = 3).
Cytokine-induced IFNγ production by virus-specific CD8+ T cells. (A) Spleen cells isolated from mice at 8 days (day 8) or greater than 120 days (immune) after LCMV infection were activated directly ex vivo with the combination of IL-12 and IL-18 for up to 12 hours. After gating on CD8+LdNP118-Tetramer+ T cells, IFNγ production was detected by intracellular staining. Values are the average ± SD for 4 animals in each group. (B) Spleen cells from mice at day 8 after infection were activated directly ex vivo with IL-12 plus IL-18 for 5 hours to induce IFNγ production. Activated cells were then either maintained in cytokines, treated with the protein synthesis inhibitor CHX, or washed and replaced in medium without cytokines. After 5 hours of cytokine withdrawal (t = 10 hours), cells were restimulated with IL-12 plus IL-18 for 4 hours. IFNγ production by CD8+LdNP118-Tetramer+ cells was detected by intracellular staining. Values are averages ± SDs (n = 3). (C) Spleen cells from mice at day 8 after infection were activated directly ex vivo with IL-12 and IL-18 for 5 hours to induce IFNγ production. Activated cells were then either maintained in IL-12 and IL-18 or washed and replaced in medium without cytokines. After 14 hours of cytokine withdrawal (t = 19 hours), cells were restimulated with IL-12 and IL-18 for an additional 8 hours. IFNγ production by CD8+LdNP118-Tetramer+ cells was detected by intracellular staining. Values are averages ± SDs (n = 3).
IFNγ+ CD8+ T cells activated with IL-12 and IL-18 for 5 hours were then washed and deprived of further cytokine stimulation to determine how rapidly IFNγ synthesis would subside (Figure 1B). Cytokine withdrawal resulted in a surprisingly slow rate of decline in IFNγ production, with approximately 50% of the virus-specific cells still producing IFNγ after 7 hours of culture in the absence of further cytokine stimulation (t1/2 = 6.9 ± 1.1 hours). This result is in stark contrast to the rapid off-rate kinetics observed after removal of peptide stimulation (t1/2 < 1 hour17 ). As a control, the protein synthesis inhibitor, CHX, was added to parallel IL-12– and IL-18–stimulated cultures to directly block de novo cytokine production by activated CD8+ T cells. As expected, abrogation of protein synthesis by CHX caused the rapid disappearance of IFNγ protein within 1 hour (but with no affect on cell viability; data not shown and Slifka et al17 ). Residual IL-12 and IL-18 activity was not a factor in these on/off experiments because the washing steps removed more than 99.999% of IL-12 and IL-18, resulting in complete loss of IFNγ-inducing activity (see “Materials and methods”). Another explanation for the prolonged secretion of IFNγ observed in the absence of exogenously added cytokines was that IL-12 and IL-18 were possibly being produced by APCs in the cultures. This was unlikely, since the supernatants of activated/washed cell cultures did not contain any detectable IFNγ-inducing activity after 4 hours of incubation (data not shown). Moreover, the observation of prolonged IFNγ production after IL-12 and IL-18 withdrawal also occurred with purified CD8+ T cells (90% pure) that were washed and treated with saturating amounts of neutralizing anti–IL-12 and anti–IL-18 antibodies (10 μg/mL, each) to ensure complete blockade of this stimulatory pathway (data not shown). The production of IFNγ by purified CD8+ T cells following exposure to IL-12 and IL-18 indicates that other APC-derived cytokines are not required, and the continued IFNγ production by CD8+ T lymphocytes after IL-12 and IL-18 withdrawal is therefore not due to residual cytokines or exogenous production of IL-12 and IL-18 in the cultures. These results suggest that there is an intriguing and previously overlooked dichotomy in how virus-specific T cells respond to innate or adaptive signals in their environment; adaptive T-cell responses (triggered by peptide stimulation) are shut down rapidly after the loss of antigen contact, whereas innate activation of T cells (triggered by IL-12 and IL-18) induces cytokine production that continues for a prolonged period of time after the innate stimulus is removed.
One interesting paradigm of innate immunity is that initial activation often leads to transient hyporesponsiveness or desensitization to reactivation through the same ligand/receptor interaction. For example, a single exposure to lipopolysaccharide (LPS) can render monocytes hyporesponsive to subsequent LPS-induced activation.20 A similar tolerizing effect can be seen in the response of fibroblasts to secondary exposure to IFNα; up to 2 to 3 days of rest is required before the cells regain responsiveness to this cytokine21 (similar IFNα-specific desensitization to secondary exposure has been observed in macrophages; Dr Richard Pine, Public Health Research Institute, Newark, NJ; written personal communication, 2004). To determine whether the termination of IFNγ production by virus-specific CD8+ T cells following cytokine withdrawal also results in a similar state of hyporesponsiveness, IL-12– and IL-18–activated cultures were deprived of these cytokines and then reexposed to IL-12 and IL-18. Activated lymphocytes that had ceased IFNγ production at 5 hours (Figure 1B) or 14 hours (Figure 1C) after cytokine withdrawal, rapidly reinitiated IFNγ production upon reexposure to exogenous IL-12 and IL-18, with IFNγ levels (MFI) greatly exceeding those observed after primary stimulation (data not shown). Together, this indicates that IFNγ production is induced rapidly by IL-12 and IL-18 in the vast majority of both effector T cells and resting memory T cells and persists for as long as IL-12 and IL-18 are present (up to 27 hours; Figure 1C). Remarkably, IFNγ production ceases only gradually after ligand withdrawal (t1/2 = 6.9 ± 1.1 hours) and rapidly resumes upon reexposure to these cytokines, demonstrating that virus-specific T cells maintain the capacity to turn IFNγ on, off, and on again in direct response to their inflammatory environment.
Differential regulation of TNFα: transient expression induced by peptide, but no induction by cytokine-mediated activation
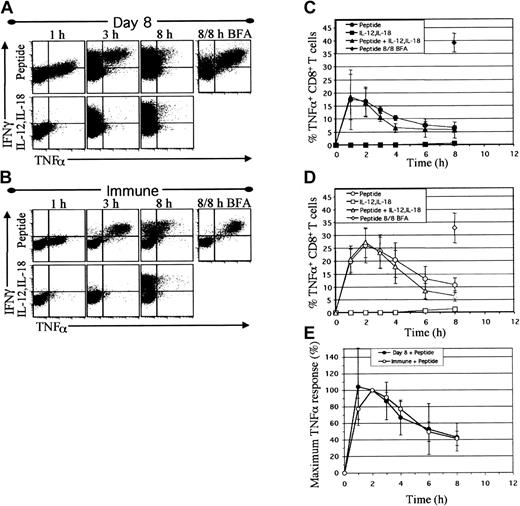
The observation that virus-specific effector and memory T cells both stably produced IFNγ in response to IL-12 and IL-18 raised the issue of whether other peptide-inducible secretory proteins might be similarly regulated by these innate cytokines. TNFα is a potent inflammatory molecule that has strong antiviral/antimicrobial activity but can be toxic or even lethal to the host when produced chronically or at high levels.22-24 TNFα production could be detected within 1 hour after direct ex vivo NP118 peptide stimulation of T cells examined at 8 days (Figure 2A) or greater than 200 days (Figure 2B) after LCMV infection, and, interestingly, TNFα induction briefly preceded the onset of IFNγ production. Peptide-induced TNFα expression peaked by approximately 2 hours and then declined markedly by 8 hours (Figure 2C-D) in agreement with previous observations.5 However, if Brefeldin A (BFA) was maintained in the cultures throughout the 8-hour incubation period to sequester intracellular TNFα, then 81% ± 11% of CD8+ T cells isolated at 8 days after infection whereas 98% ± 1% of the resting memory T cells, produced both IFNγ and TNFα (Figure 2A-B), similar to previous results.4
TNFα production is transient following peptide stimulation and is not induced by IL-12 and IL-18. Spleen cells from mice at day 8 (A,C) or day greater than 200 (B,D) after infection were activated directly ex vivo with NP118 peptide and/or IL-12 and IL-18. IFNγ and TNFα production by CD8+ T cells was detected by intracellular staining. Dotplots (A-B) are gated on CD8+ cells and depict IFNγ and TNFα production after 1, 3, and 8 hours of stimulation with peptide or IL-12 plus IL-18 in the presence of BFA added for the last hour, or 8 hours of peptide stimulation with continuous BFA (8 of 8 hours BFA) to fully sequester intracellular cytokines for comparison. Graphs (C-D) depict the percentage of CD8+ T cells producing TNFα versus time. Values are averages ± SDs (n = 4 day 8, n = 8 day> 200). (E) The relative rates of TNFα production by day 8 and day greater than 200 (immune) CD8+ T cells are represented as the percentage of maximum TNFα response at each time interval, with 100% maximum set at 2 hours.
TNFα production is transient following peptide stimulation and is not induced by IL-12 and IL-18. Spleen cells from mice at day 8 (A,C) or day greater than 200 (B,D) after infection were activated directly ex vivo with NP118 peptide and/or IL-12 and IL-18. IFNγ and TNFα production by CD8+ T cells was detected by intracellular staining. Dotplots (A-B) are gated on CD8+ cells and depict IFNγ and TNFα production after 1, 3, and 8 hours of stimulation with peptide or IL-12 plus IL-18 in the presence of BFA added for the last hour, or 8 hours of peptide stimulation with continuous BFA (8 of 8 hours BFA) to fully sequester intracellular cytokines for comparison. Graphs (C-D) depict the percentage of CD8+ T cells producing TNFα versus time. Values are averages ± SDs (n = 4 day 8, n = 8 day> 200). (E) The relative rates of TNFα production by day 8 and day greater than 200 (immune) CD8+ T cells are represented as the percentage of maximum TNFα response at each time interval, with 100% maximum set at 2 hours.
In stark contrast to peptide stimulation, IL-12 and IL-18 induced negligible levels of TNFα (1% TNFα+) in virus-specific T cells, even though copious amounts of IFNγ were produced (Figure 2A-B). The kinetics of TNFα production and the proportion of TNFα-producing cells induced by incubation with a combination of peptide and IL-12 plus IL-18 were not substantially different from that observed with peptide alone (Figure 2C-D). Interestingly, peptide stimulation in the presence of IL-12 and IL-18 resulted in decreased TNFα MFI levels at the peak of induction (Figure S1). Normalization of the maximum direct ex vivo TNFα response by CD8+ T cells revealed that effector and memory T-cell populations had similar spontaneous off rates of TNFα production (Figure 2E), with TNFα levels declining rapidly despite the presence of continued stimulation through the TcR and continued IFNγ production (Figure 1). Thus, in recently activated and resting memory CD8+ T cells, TNFα production is induced rapidly and transiently by peptide but is not induced by IL-12 and IL-18 stimulation and may even be partially inhibited by these cytokines.
IL-2 production by CD8+ T-cell subsets is differentially regulated by peptide or IL-12 plus IL-18
Unlike IFNγ and TNFα, IL-2 does not act directly as an antimicrobial agent, but instead is a T-cell growth factor that plays a role in driving the clonal expansion and survival of virus-specific T cells.6,8,25 Peptide-induced IL-2 production by virus-specific effector CD8+ T cells (Figure 3A) and resting memory CD8+ T cells (Figure 3B) exhibited slightly delayed kinetics compared with TNFα but was readily observed at 3 hours, before declining rapidly to baseline levels within 8 hours. At the peak of peptide induction, all of the IL-2–producing cells were contained within the virus-specific IFNγ+ CD8+ T-cell population, predominantly in cells producing high levels of IFNγ (Figure 3A-B). When the kinetics of peptide-stimulated IL-2 production were compared between effector and memory T-cell subsets (Figure 3E), the on-off rates of IL-2 expression were nearly identical and did not appear to be related to the differentiation state of the T-cell population. Similar to previous studies,26 peptide stimulation for 8 hours in the presence of BFA showed that IL-2–producing T cells comprised only 5.9% ± 0.1% of IFNγ+ effector CD8+ T cells, whereas IL-2 was produced by 45% ± 5% of the IFNγ+ memory CD8+ T cells.
IL-2 is transiently induced following peptide stimulation but is not induced by IL-12 and IL-18. Spleen cells from mice at day 8 (A,C) and day greater than 200 (B,D) after infection were activated as in Figure 2. IFNγ and IL-2 were detected by intracellular staining. Dotplots (A-B) are gated on CD8+ cells and show IFNγ and IL-2 produced after 1, 3, and 8 hours of direct ex vivo stimulation with peptide or IL-12 plus IL-18 in the presence of BFA for the last hour, or 8 hours of peptide stimulation with continuous BFA (8/8 h BFA). Graphs (C-D) show the percentage of CD8+ T cells producing IL-2 at each time interval. Values are averages ± SDs (n = 4 day 8, n = 8 day > 200). (E) The relative rates of IL-2 production by day 8 and day greater than 200 (immune) CD8+ T cells are represented as the percentage of maximum IL-2 response at each time interval, with 100% maximum at 3 hours.
IL-2 is transiently induced following peptide stimulation but is not induced by IL-12 and IL-18. Spleen cells from mice at day 8 (A,C) and day greater than 200 (B,D) after infection were activated as in Figure 2. IFNγ and IL-2 were detected by intracellular staining. Dotplots (A-B) are gated on CD8+ cells and show IFNγ and IL-2 produced after 1, 3, and 8 hours of direct ex vivo stimulation with peptide or IL-12 plus IL-18 in the presence of BFA for the last hour, or 8 hours of peptide stimulation with continuous BFA (8/8 h BFA). Graphs (C-D) show the percentage of CD8+ T cells producing IL-2 at each time interval. Values are averages ± SDs (n = 4 day 8, n = 8 day > 200). (E) The relative rates of IL-2 production by day 8 and day greater than 200 (immune) CD8+ T cells are represented as the percentage of maximum IL-2 response at each time interval, with 100% maximum at 3 hours.
In contrast to peptide stimulation, activation by IL-12 and IL-18 did not induce detectable levels of IL-2 (Figure 3A-B), nor did it change peptide-induced IL-2 responses when combined with NP118 peptide stimulation (Figure 3C-D) or change the relative levels of IL-2 that were produced (Figure S1). Although there appeared to be a mild enhancement of IL-2 production when memory T cells were stimulated with both peptide and cytokines, this was observed in only 5 of 8 mice tested and was not statistically significant (P = .06). Together, these results show that peptide induces IL-2 production only transiently, with kinetics slightly delayed relative to TNFα, and is largely unaffected by IL-12 and IL-18.
Unique CD40L expression kinetics; substantial skewing by innate cytokines or peptide stimulation
CD40L plays an important role in APC maturation, as well as the stimulation of B-cell proliferation and antibody production.27 Moreover, both CD4028 and CD40L9 have been identified on T cells and may be involved with T cell–T cell activation.28 CD40L is expressed by LCMV-specific CD8+ T cells at 8 days after infection,9 but little is known about the expression patterns of this immunomodulatory protein in memory T cells or after stimulation with inflammatory cytokines such as IL-12 and IL-18. The patterns of CD40L expression induced by peptide or IL-12 plus IL-18 (Figure 4) were substantially more complex than that observed with any of the previously examined cytokines (IFNγ, TNFα, and IL-2). Intriguingly, both forms of T-cell activation induced CD40L expression, albeit with very distinct response kinetics. Direct ex vivo peptide stimulation of effector (Figure 4A) or memory (Figure 4B) CD8+ T cells induced CD40L expression that slightly preceded IFNγ production at 1 hour. Similar to TNFα and IL-2, peptide-induced CD40L production was transient, peaking at 2 to 3 hours and then declining rapidly thereafter (Figure 4C-D). In contrast to the results observed with TNFα and IL-2, cytokine-mediated T-cell activation through IL-12 plus IL-18 stimulation induced delayed expression of CD40L that only began to appear after 3 to 4 hours, about the same time that peptide-induced CD40L expression had peaked and begun to wane (Figure 4A-B). By 12 hours, the peptide-stimulated CD40L+ population had declined substantially, but the cytokine-induced CD40L+ T-cell population was still rising. The combination of peptide plus cytokines generated a composite response of the 2 individual forms of stimulation; rapid induction of CD40L comparable to that seen with peptide alone, followed by maintenance of CD40L expression coincident with the delayed activation through the IL-12 and IL-18 pathways (Figure 4C-E).
CD40L is induced by either peptide or IL-12 and IL-18, but with independently regulated kinetics. Spleen cells from mice at day 8 (A-B) and day greater than 120 (B,D) after infection were activated as in Figure 2. IFNγ and CD40L were detected by intracellular staining. Dotplots (A-B) are gated on CD8+ T cells and show IFNγ and CD40L expression after 1, 3, and 12 hours of direct ex vivo stimulation with peptide or IL-12 and IL-18 in the presence of BFA for the last hour, or 12 hours of peptide stimulation with continuous BFA (12/12 h BFA). Graphs (C-D) show the percentage of virus-specific CD8+ T cells producing CD40L at each time interval. Values are averages ± SDs (n = 4). (E) The relative rates of CD40L production by day 8 and day greater than 120 (immune) virus-specific CD8+ T cells are represented as the percentage of maximum CD40L response at each time interval, with 100% maximum defined at 3 hours for peptide stimulation and 12 hours for IL-12 plus IL-18 stimulation.
CD40L is induced by either peptide or IL-12 and IL-18, but with independently regulated kinetics. Spleen cells from mice at day 8 (A-B) and day greater than 120 (B,D) after infection were activated as in Figure 2. IFNγ and CD40L were detected by intracellular staining. Dotplots (A-B) are gated on CD8+ T cells and show IFNγ and CD40L expression after 1, 3, and 12 hours of direct ex vivo stimulation with peptide or IL-12 and IL-18 in the presence of BFA for the last hour, or 12 hours of peptide stimulation with continuous BFA (12/12 h BFA). Graphs (C-D) show the percentage of virus-specific CD8+ T cells producing CD40L at each time interval. Values are averages ± SDs (n = 4). (E) The relative rates of CD40L production by day 8 and day greater than 120 (immune) virus-specific CD8+ T cells are represented as the percentage of maximum CD40L response at each time interval, with 100% maximum defined at 3 hours for peptide stimulation and 12 hours for IL-12 plus IL-18 stimulation.
The relative proportion of virus-specific CD8+ T cells capable of expressing CD40L after peptide or IL-12 plus IL-18 stimulation did not appear to change significantly with the differentiation state of the cells. With BFA maintained in the culture medium to sequester intracellular CD40L, an average of 42% ± 5% of IFNγ-producing CD8+ effector T cells and 45% ± 6% of memory T cells were capable of expressing this molecule during the 12-hour period of peptide stimulation (Figure 4C-D). Similarly, comparison of effector and memory CD8+ T cells under optimal stimulation for 12 hours with IL-12 and IL-18 (BFA added for the last hour of incubation) did not reveal significant differences in the percentages of virus-specific CD40L+ IFNγ+ T cells (12% ± 1% versus 18% ± 5% of IFNγ+ CD8+ T cells, respectively; P = .1). Interestingly, peptide-stimulated memory T cells expressed nearly twice as much CD40L as effector T cells, and these expression levels were unaltered by coincubation with IL-12 and IL-18 (data not shown). These results underscore the complexity of the T-cell responses to peptide or to IL-12 plus IL-18 and the remarkable ability of the responding CD8+ T-cell population to mediate distinct effector functions upon exposure to innate or adaptive stimuli.
Discussion
In this study, we have identified uniquely regulated cytokines in virus-specific CD8+ T cells that vary according to the differentiation state of the T-cell population and whether the stimulus is provided through the TcR or through cytokine receptors (or both). When comparing activated CD8+ T cells with memory T cells, we found several similarities as well as some interesting differences in cytokine expression. Kinetics of TNFα, IL-2, and CD40L were similar between these 2 subpopulations (Figures 2, 3, 4), whereas IFNγ production was markedly slower in memory cells compared with their highly activated counterparts following stimulation with IL-12 and IL-18 (Figure 1). In terms of cytokine expression levels, memory T cells expressed more CD40L but less IFNγ than activated T cells [Figure S1]. Overall, IFNγ was stably expressed in the presence of peptide or the cytokines, IL-12 and IL-18, but showed remarkably delayed off-rate kinetics after cytokine stimulation was removed. Unlike IFNγ, TNFα and IL-2 were only transiently expressed even under continuous peptide stimulation, and these cytokines were not readily induced by IL-12 and IL-18. Interestingly, CD40L production was highly divergent; it was expressed only briefly after peptide stimulation but was slowly up-regulated by IL-12 and IL-18 with peak production occurring at 12 hours after stimulation. These results demonstrate how T-cell activation through different surface molecule pathways (TcR versus cytokine receptors) results in fine-tuned cytokine responses that are strictly regulated by the environment of the stimulated T cell.
The majority of virus-specific CD8+ T cells exhibited prolonged synthesis of IFNγ upon exposure to peptide or innate cytokines, making it a useful marker of functional, antigen-experienced CD8+ T cells. The pattern of IFNγ production after IL-12 and IL-18 stimulation shows that the responsive cells are exquisitely sensitive to the surrounding cytokine milieu. Most notably, the “off” rate of IFNγ expression after cytokine withdrawal is slower (t1/2= ∼ 7 hours) than that previously described after peptide withdrawal from virus-specific cells (t1/2 < 1 hour).17 In the case of peptide-stimulated cells, antigen withdrawal resulted in nearly complete loss of IFNγ staining within 2 hours. By comparison, IL-12– and IL-18–deprived cells did not reach basal levels of IFNγ expression until 9 to 14 hours after removal of cytokines (Figure 1B-C). These results show that cytokine-activated T cells are slower to turn off signaling pathways upon ligand removal compared with TcR-stimulated cells, indicating that innate “bystander” activation and IFNγ production induced by IL-12 and IL-18 stimulation persists for a much longer period of time than that which occurs via stimulation through the TcR. These differences may be important in the immunopathology of sepsis, which is due to inflammatory cytokines that may involve T-cell activation/cytokine production but does not necessarily require further stimulation through the TcR.29 Nevertheless, T cells must produce sufficient amounts of cytokine to elicit protective immunity, but this must be balanced by the need to prevent severe cytokine-mediated immunopathology.22-24 Therefore, the ability of IL-12 and IL-18 to elicit some, but not all, peptide-inducible cytokines may be a clever mechanism for generating useful effector functions, while reducing the risk of hyperinflammation upon non–antigen-specific “bystander” activation. IL-12 and IL-18 induce virus-specific T cells to produce IFNγ, which has potent antiviral functions, and CD40L, which stimulates antigen presenting cell maturation, B-cell activation, and possibly T-cell activation as well.27,28 In contrast, IL-2, which is pivotal for T-cell clonal expansion,6,8,25 and the highly toxic inflammatory cytokine, TNFα, are both only transiently induced by peptide stimulation and are not induced by IL-12 and IL-18. This is an intriguing finding with potentially high biologic relevance. For instance, T cells from chronically infected mice lose effector functions sequentially, beginning with IL-2, TNFα, and then finally IFNγ.30,31 Thus, differential cytokine regulation appears to be an important mechanism for protecting the host from overt and potentially lethal immunopathology and this may explain why T cells appear hesitant to produce their full repertoire of potential cytokines for extended periods of time.
In contrast to the stable induction of IFNγ, peptide stimulation induced only transient expression of TNFα, IL-2, and CD40L. These cytokines reached peak levels after 2 to 3 hours and then declined even in the continued presence of peptide stimulation. One potential mechanism for terminating antigen-induced cytokine production involves internalization and/or degradation of the TcR and CD3 complex.32,33 However, TcR down-regulation would presumably result in the global attenuation of all peptide-inducible cytokines. This mechanism would not account for the observed selective loss of only some cytokines and the continued production of IFNγ. Moreover, even though antigenic stimulation triggers rapid TcR internalization and loss of surface TcR expression, the remaining surface TcR is sufficient to allow antigen-mediated signaling to persist for at least 10 hours4,5,17 and is consistent with previous reports of continuous signaling through the TcR.34,35 Thus, TcR down-regulation is unlikely to be a biologically relevant mechanism that could account for the selective inhibition of some, but not all, peptide-induced cytokines.
Differential down-modulation of cytokine production must therefore be affected by a more specific mechanism governing discrete regulatory pathways. This may be mediated by signaling feedback loops involving, for example, dephosphorylation of key signaling intermediates by specific phosphatases, phosphorylation of signaling proteins at negative regulatory sites, binding of inhibitory SH2 (src homology 2) domain-containing proteins to phosphotyrosine-docking residues, targeted proteosomal degradation of signaling proteins, and/or induction of transcriptional repressors.20,36-38 Notably, the 3′ untranslated regions of many cytokine genes contain AU-rich elements (AREs) that serve as binding sites for proteins that regulate mRNA stability and translation.39-41 Several families of ARE-binding proteins have been identified that can positively or negatively affect these processes, and these proteins demonstrate selectivity for different target transcripts.41 The importance of ARE-mediated cytokine down-regulation has been demonstrated by targeted genetic disruption of the TNFα ARE, which results in heightened and prolonged TNFα production, causing symptoms of chronic arthritis and inflammatory bowel disease.42 Clearly, the attenuation of inducible cytokine production is a complex, tightly regulated process, and the integrity of this mechanism is essential to prevent cytokine-mediated hyperinflammation and immunopathology.22-24
It is becoming clear that the induction of cytokine genes in antigen-experienced T cells can involve both innate and adaptive stimuli and therefore must be subject to complex, differential regulation of multiple signaling cascades. Surface receptors may be grouped into distinct structural families, which in turn couple to particular signal transduction pathways targeting specific transcription factors. These transcription factors act in a combinatorial fashion to induce expression of discrete target genes. These differences likely account for the ability of IL-12 and IL-18 to induce some (IFNγ and CD40L) but not other (TNFα, IL-2) peptide-inducible cytokines. The transcription factor families comprising nuclear factor of activated T cells (NFAT), activator protein 1 (AP1), and nuclear factor-κB (NFκB) are induced by the TcR and costimulatory receptors and are key inducers of many cytokine genes, including IFNγ, TNFα, IL-2, and CD40L.43-47 By comparison, the IL-12 receptor is a member of the hematopoietic cytokine receptor family, and couples to the Janus kinase/signal transducer and activator of transcription (Jak/STAT) signaling pathway, activating predominantly STAT4.14 STAT4 has been implicated in cytokine-mediated IFNγ induction.48 The IL-18 receptor is a member of the IL-1/Toll-like receptor family and signals via myd88 and IL-1 receptor activating kinases (IRAKs).15,49 NFκB is a key transcription factor activated by IL-18 and with IL-12–induced STAT4 contributes to the induction of the IFNγ gene.50 Thus, distinct surface receptors couple to specific signaling pathways and transcription factors and make unique contributions to the induction of cytokine genes. In cases of chronic viral infections or T-cell anergy models wherein TcR-mediated signaling pathways may be compromised, it may therefore be possible to use cytokines to activate T cells through alternate signaling pathways,8,51 although this could also result in substantial immunopathology.51
Together, these results indicate that virus-specific CD8+ T cells have the ability to differentially regulate both the initiation and termination of cytokine production in response to discrete innate and adaptive stimuli. Using real-time analysis of cytokine production at the protein level, we have demonstrated that virus-specific T cells independently regulate IFNγ, TNFα, IL-2, and CD40L production in response to peptide antigen or exposure to the innate cytokines, IL-12 and IL-18. These results demonstrate that the TcR and innate cytokine receptors play important but differentially regulated roles in T-cell activation. Each of these receptors therefore provides a unique target for potential therapeutic strategies to either enhance or attenuate inflammatory T-cell responses.
Prepublished online as Blood First Edition Paper, October 7, 2004; DOI 10.1182/blood-2004-07-2833.
Supported by grants from the National Institutes of Health (NIH) (AI54458) (M.K.S.) and Oregon National Primate Research Center (RR00163) (M.K.S.). H-2Ld NP118-126 tetramers were generously prepared by the NIH Tetramer Core Facility in Atlanta, GA.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal