Abstract
To better understand the influence of cytoskeletal regulation on dendritic cell (DC) function in vivo, the Rho guanosine triphosphatase (GTPase) Rac1 was selectively inhibited in DCs in transgenic (Tg) mice. Although transgene expression did not interfere with the migratory capacities of DC in vivo, a decreased uptake of fluorescent probes was observed. Interestingly, the absence of full Rac1 function most strongly affected the development and function of CD8+ DCs. Apoptotic cell uptake was severely reduced in Tg mice, impairing subsequent DC-mediated cross-presentation and priming of bacteria-specific T-cell responses. These findings highlight a special role for Rac1 in the capacity of CD8+ DCs to endocytose apoptotic cells and prime T cells via cross-presentation.
Introduction
The actin cytoskeleton is essential for a wide variety of cellular activities, from cytokinesis and intracellular trafficking to cell motility and endocytosis. Regulation of these processes falls largely to the Rho family of guanosine triphosphatases (GTPases).1 Investigation of the more than 20 known Rho family members, of which Cdc42 and Rac1 are among the best characterized, has demonstrated their specific roles in the formation of migratory structures.1,2 The Rho GTPases are also involved in phagocytosis in various cell types,1 and both Cdc42 and Rac1 have been implicated in constitutive macropinocytosis by dendritic cells (DCs) cultured in vitro.3,4
The crucial role of cytoskeletal regulation in immune surveillance has been demonstrated in Wiskott-Aldrich syndrome (WAS), a complex disease characterized by recurrent infection, tumors, and autoimmunity. WAS results from a variety of naturally occurring mutations in WAS protein (WASp), an effector molecule downstream of Cdc42.5 Although traditionally attributed to impaired T- and B-cell function, immune dysregulation in patients with WAS may also reflect deficiencies in the function of antigen-presenting cells (APCs), because recent studies have revealed that DCs from patients with WAS display defects in migration.6-8
DCs are unique among APCs in their immunostimulatory capacity, with a particularly critical role in the activation of naive T cells.9,10 Various characteristics of DCs account for their efficiency as APCs. Immature DCs in the periphery constantly sample the environment, endocytosing large amounts of antigen while remaining alert for signs of infection. Inflammatory signals induce DC maturation, stimulating their migration to lymphoid organs and up-regulation of major histocompatibility complex (MHC)/peptide complexes and costimulatory molecules. Modulation of the actin cytoskeleton would appear to be central to many, if not all, of the specialized functions characteristic of the different DC developmental stages.
What roles do the Rho GTPases play in DC function? The migratory defects in DCs generated from patients with WAS might suggest that cytoskeletal function in DCs is crucial for establishment of an efficient immune response, but in such a case of ubiquitous deficiency, it is impossible to pinpoint the cellular source of immunodysregulation. It is not possible to analyze functions of DCs in mice deficient in Rac1 or Cdc42 because genetic targeting of these molecules results in early embryonic lethality.11,12 Microinjection of large amounts of dominant-negative (DN) Cdc42 or Rac1 protein into DCs cultured in vitro resulted in complete abrogation of endocytosis, leading to the conclusion that these Rho GTPases are crucial for the constitutive antigen uptake characteristic of DCs.3,4 However, such artificially generated DCs are, at best, representative of only a fraction of DCs in vivo, which are highly heterogeneous in cell surface phenotype, function, and location13 and might be similarly heterogeneous in their need for Rho GTPase function. Alone in the spleens of mice, DCs can be divided into several subpopulations; CD8+ and CD8- DCs, for example, are localized to the T-cell areas and marginal zones, respectively, and have been reported to further differ in their preferential induction of Th1-versus Th2-biased responses.13 CD8+ DCs have a special capacity for the uptake of apoptotic cells14 and cross-presentation,15 the processing and presentation of exogenous antigen on MHC class I molecules, which seems to be important for both tolerance and the induction of CD8+ T-cell responses to tumor, viral, and bacterial antigens.16
To study the importance of Rac1 in DC function in vivo, we generated transgenic (Tg) mice expressing the DN form of the protein, Rac1(N17), under the control of the DC-selective murine CD11c promoter.17 Our studies reveal a particular sensitivity of CD8+ DCs to Rac1 inhibition; substantially decreased frequencies of CD8+ DCs were detected in CD11c-Rac1(N17) Tg+ mice, and the remaining CD8+ DCs demonstrated a severely reduced capacity for the uptake of apoptotic cells in vivo. This defect corresponded with decreased efficiency of cross-presentation and markedly impaired T-cell responses to a bacterial pathogen. Thus, DC-specific inhibition of Rac1 revealed a fundamental role for this Rho GTPase in the development and function of CD8+ DCs in vivo.
Materials and methods
Mice and infections
DN Rac1(N17) was excised by EcoRI/HindIII restriction digestion from the pGEM1-Myc-Rac1N17#6 plasmid18 and, following blunt ending with Klenow fragment, ligated into the blunt-ended EcoRI site of the previously described CD11c-pDOI-5 vector.17 cDNA orientation was controlled by restriction digestion and DNA sequence analysis. The linearized Tg construct devoid of vector sequences was injected into fertilized oocytes from (BDF1 × BDF1)F1 mice, and 8 Tg founders were identified by Southern blotting and polymerase chain reaction (PCR). Unless otherwise indicated, experiments were performed using founder line 4038. For transfer experiments, mice were backcrossed to C57BL/6 for at least 7 generations. C57BL/6, BALB/c, OT-1 (CD8+ TCR-Tg specific for the ovalbumin [OVA]–derived SIINFEKL peptide), and β2-microglobin (β2m)-/- mice were originally obtained from the Jackson Laboratory (Bar Harbor, ME). All mice were bred and maintained at the animal facilities of the Institute for Immunology (LMU, Munich, Germany) or the Institute for Medical Microbiology, Immunology and Hygiene (TU, Munich, Germany) in accordance with established guidelines.
Listeria monocytogenes (Lm)–OVA (kindly provided by H. Shen, University of Pennsylvania School of Medicine, Philadelphia, PA19 ) was grown in brain-heart infusion broth. Mice were infected intravenously into the tail vein with the indicated dose of Lm-OVA in 200 μL phosphate-buffered saline (PBS). Lipopolysaccharide (LPS; 30 μg/mouse; Sigma, St. Louis, MO) and LPS/anti-CD40 (25/100 μg/mouse) injections were also performed intravenously in 200 μL PBS.
Antibodies and multimers
The following antibodies or reagents from BD PharMingen (San Diego, CA) were used for flow cytometry or fluorescence microscopy or both: CD4-peridinin chlorophyll protein (PerCP; RM4-5), CD8α-phycoerythrin (PE) and -fluorescein isothiocyanate (FITC; 53-6.7), CD11b-PE and -biotin (M1/70), CD11c-allophycocyanin and -PE (HL3), CD43-biotin (S7), CD45R/B220-FITC (RA3-6B2), CD62L-FITC and -APC (MEL-14), CD86 (B7.2)–PE (GL1), Ly-6G/Ly-6C (Gr-1)–PE (RB6-8C5), NK1.1-PE (PK136), H2-Kb/Db-biotin (28-8-6), I-Ab-FITC (AF6-120.1), Vα2-PE (B20.1), Vβ5-FITC (MR9-4), mouse IgG1-allophycocyanin (X56), interferon γ (IFN-γ)–FITC (XMG1.2), isotype control rat IgG1 (R3-34), and SA-PerCP. The following antibodies were also used: Thy1.2-FITC (Becton Dickinson, Heidelberg, Germany); CD8-APC (CT-CD8α), SA-APC, and streptavidin (SA)–cyanine 5 (Cy5; Caltag, Burlingame, CA); IgM-FITC (Southern Biotechnology, Birmingham, AL); Moma-biotin (Bachem Biochemica, Heidelberg, Germany); c-myc (9E10, biotin-conjugated and raw ascites fluid; Covance, Richmond, CA); and CD40-biotin and purified (FGK-45; generated in-house). For Western blots, mouse monoclonal antibody (mAb) specific for Rac1 (clone 23A8) and c-myc (clone 9E10) were purchased from UBI (Lake Placid, NY) and Roche (Meylan, France), respectively. Horseradish peroxidase (HRP)–coupled anti–mouse IgG antibody came from Jackson ImmunoResearch Laboratories (West Grove, PA).
MHC class I multimer reagents (H2-Kb/SIINFEKL and H2-M3/fMIGWII) were generated as described previously.20
Preparation of DCs and lymphocytes
For DC analysis, spleens or lymph nodes (LNs) were digested in RPMI 1640 culture medium (5% fetal bovine serum [FBS], 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μM β-mercaptoethanol) containing 0.5 mg/mL collagenase (Worthington Biochemical, Freehold, NJ) and 40 μg/mL DNaseI (Sigma) for 30 minutes at 37°C. For lymphocyte analysis, spleens were dissociated directly into staining buffer (PBS/0.01% NaN3/2% FBS). Erythrocytes were lysed with ammonium chloride-Tris (tris(hydroxymethyl)aminomethane).
DC culture and Western blot
Total bone marrow was plated in 90-mm plates at 5 × 106 cells/mL in 10 mL RPMI 1640 culture medium containing 25 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF). DCs (day 6 to day 9) were further purified using anti-CD11c microbeads (magnetically activated cell sorting [MACS], Miltenyi Biotec, Bergisch-Gladbach, Germany) and mass spectometry (MS) MACS columns. The positively selected fraction was more than 95% CD11c+. CD11c+ cells sorted by MACS were lysed on ice in lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate [SDS]) supplemented with protease inhibitors (complete EDTA [ethylenediaminetetraacetic acid]–free; Roche) and 1 mM sodium orthovanadate. The lysate supernatant was loaded onto a 10% polyacrylamide gel and transferred overnight onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA). Immunoblot was performed and revealed by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Saclay, France). The membranes were stripped in 0.2 M glycine for 30 minutes at room temperature. Short exposure-time films were scanned (Epson Perfection 3200, Long Beach, CA) and analyzed with National Institutes of Health (NIH) Image software.
Staining of cells for flow cytometry
Staining of surface molecules was performed with 1 × 106 to 6 × 106 cells in cold staining buffer (+2 mM EDTA for DC analysis) for 30 minutes at 4°C (60 minutes when MHC multimers used). Dead cell exclusion was attained by incubation with 2 μg/mL ethidium monoazide bromide (EMA; Molecular Probes, Eugene, OR) prior to surface staining or addition of 0.8 μg/mL propidium iodide (PI; Sigma) just prior to acquisition. Intracellular staining for c-myc or cytokines was performed using the Cytofix/Cytoperm kit (PharMingen, San Diego, CA). Flow cytometry was performed with a FACSCalibur (Becton Dickinson), and data were analyzed with FlowJo software (Tree Star, Ashland, OR).
Endocytosis assay
For analysis of in vivo endocytosis, mice were injected intravenously with 3 mg FITC-OVA (Molecular Probes) and humanely killed 1 hour later for DC analysis. Preparation of DCs and staining of surface molecules was performed as described (see “Preparation of DCs and lymphocytes” and “Staining of cells for flow cytometry,” respectively).
Stimulation of cytokine production by epitope-specific T cells
Splenocytes (2 × 107/well in 2 mL RPMI medium) were incubated with 10-6 M SIINFEKL, fMIGWII, or listeriolysin (LLO)190-201 peptide (Affina, Berlin, Germany), in dimethyl sulfoxide (DMSO) alone as a control in a 24-well plate for 5 hours at 37°C. After 2 hours, 1 μL/mL GolgiPlug (PharMingen) was added to each well. Stimulated cells (6 × 106) were washed and then incubated for 30 minutes at 4°C with 1 μg/mL Fc block (PharMingen) and EMA. Following washing, surface molecules (CD8 and CD62L) and intracellular IFN-γ were stained as described (see “Staining of cells for flow cytometry”).
Fluorescence microscopy
Spleens were embedded in OCT, snap-frozen, and cut into 5-μm cryosections. Acetone-fixed slides were stained for 30 minutes with primary (B220-FITC, CD11c-PE, Moma-biotin) and secondary (SA-Cy5) antibody mixes prepared in PBS/0.25% bovine serum albumin (BSA)/10% normal mouse serum. The coverslip was mounted with Fluoromount G (Southern Biotechnology). Sections were analyzed on a Leica DMXA-RF8 microscope (Leica acquisition program QFISH) equipped with a Sensys CCD camera (Photometrix, Tucson, AZ).
Skin painting
FITC (Sigma) was dissolved at a concentration of 5 mg/mL in equal volumes of dibutyl phthalate and acetone, and 0.4 mL was applied to the shaved abdomens (below the rib cage) of mice. After 24 hours, pooled inguinal LNs and axillary LNs from each mouse were digested with collagenase/DNaseI and stained with allophycocyanin-conjugated anti-CD11c mAb.
Enrichment and CFSE labeling of allogeneic B cells and OT-1 cells
CD43+ cells were removed from single-cell suspensions of erythrocyte-depleted BALB/c splenocytes using anti–CD43-biotin, SA microbeads (Miltenyi Biotec), and MS columns. The negatively selected fraction was 75% to 90% B cells. Spleen and LN suspensions from OT-1 mice were enriched for Tg T cells using a CD8 subset column kit (R&D Systems, Minneapolis, MN), resulting in purities of more than 90% CD8+, Vβ5/Vα2+ (OT-1) T cells. Enriched cells were labeled with 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE, 5 μM; Molecular Probes) for 10 minutes at 37°C. Approximately 1 × 107 B cells or 1 to 2.5 × 106 OT-1 cells in 200 μL PBS were injected.
Cross-priming with β2m-/- cells
Single-cell suspensions from spleen and LNs of β2m-/- mice were loaded with OVA (Sigma) or BSA (Carl Roth GmbH, Karlsruhe, Germany) by osmotic shock as described previously,15 irradiated (1500 rad), and injected (1 × 106 cells) intravenously in 200 μL PBS.
Results
Selective expression of DN Rac1 in DCs
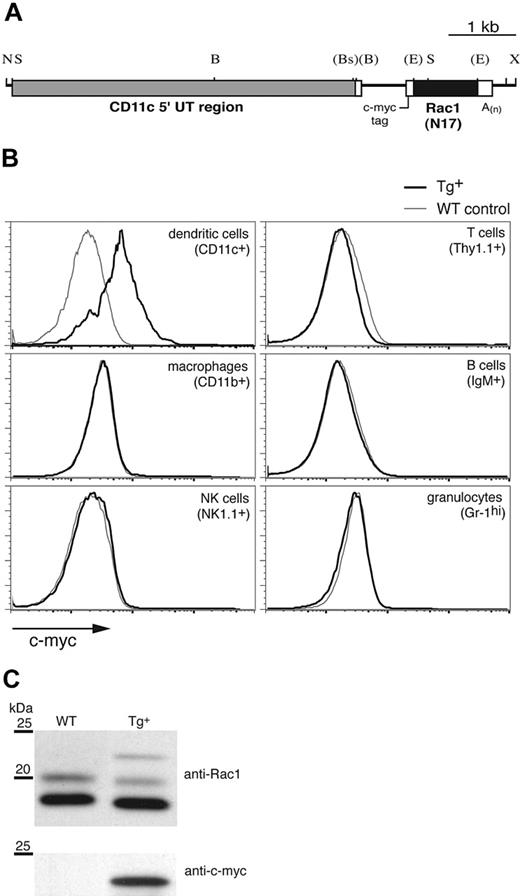
To investigate the function of the Rho GTPase Rac1 specifically in DCs, we expressed the DN form of the molecule, Rac1(N17), under control of the murine CD11c promoter (Figure 1A), which has been used previously to target Tg expression to DCs.17,21 CD11c-Rac1(N17) Tg founders were identified by Southern blot and PCR (not shown). Intracellular staining for the c-myc tag included within the construct (Figure 1A) revealed Rac1(N17) expression in DCs (Figure 1B). Double staining failed to reveal any CD11chi populations positive for markers of other cell lineages (data not shown), and no c-myc expression in macrophages, natural killer (NK) cells, T cells, B cells, or granulocytes was detected in any of the founder lines (Figure 1B). Western blotting of lysates from cultured DCs revealed 3 bands specific for Rac1 (Figure 1C upper panel); the upper band, detectable only in Tg+ mice, corresponds to the higher molecular weight Rac1(N17) protein encoded by the Tg construct, whereas the double bands of the endogenous protein, which appear under some experimental conditions,22 may represent 2 maturation forms of Rac1 (P.C., unpublished data, April 2002). Stripping and reprobing of the blot with anti–c-myc mAb confirmed the Tg nature of the upper band (Figure 1C lower panel). Quantification of the signal strength indicated the presence of approximately 20-fold more endogenous Rac1 than Tg DN Rac1 in DCs from Tg+ mice (data not shown).
Generation of CD11c-Rac1(N17) Tg+ mice and selective Tg expression in DCs. To inhibit Rac1 function selectively in DCs, the DN form of the GTPase, Rac1(N17), was expressed under the control of the murine CD11c promoter in Tg mice. (A) Restriction map of the Tg construct (B indicates BamHI; Bs, BspHI; E, EcoRI; N, NotI; S, SstI; X, XhoI). Restriction sites (in parentheses) were destroyed by blunt-end cloning. (B) Splenocytes from Tg+ mice (black) and WT controls (gray) were stained to distinguish different cell types, followed by intracellular staining to detect the c-myc tag contained within the Rac1(N17) construct (x-axis). Histograms are gated on cell populations positive for the cell surface markers specified in parenthesis. (C) In vitro–cultured DCs from Tg+ mice and WT controls were analyzed for Rac1 and c-myc expression by Western blot. Lysates of purified CD11c+ cells were probed with anti-Rac1 (upper panel) and, after stripping of the membrane, anti–c-myc mAb (lower panel). The 25- and 20-kDa molecular weight markers are shown. Blots are representative of 7 WT and 8 Tg+ mice tested.
Generation of CD11c-Rac1(N17) Tg+ mice and selective Tg expression in DCs. To inhibit Rac1 function selectively in DCs, the DN form of the GTPase, Rac1(N17), was expressed under the control of the murine CD11c promoter in Tg mice. (A) Restriction map of the Tg construct (B indicates BamHI; Bs, BspHI; E, EcoRI; N, NotI; S, SstI; X, XhoI). Restriction sites (in parentheses) were destroyed by blunt-end cloning. (B) Splenocytes from Tg+ mice (black) and WT controls (gray) were stained to distinguish different cell types, followed by intracellular staining to detect the c-myc tag contained within the Rac1(N17) construct (x-axis). Histograms are gated on cell populations positive for the cell surface markers specified in parenthesis. (C) In vitro–cultured DCs from Tg+ mice and WT controls were analyzed for Rac1 and c-myc expression by Western blot. Lysates of purified CD11c+ cells were probed with anti-Rac1 (upper panel) and, after stripping of the membrane, anti–c-myc mAb (lower panel). The 25- and 20-kDa molecular weight markers are shown. Blots are representative of 7 WT and 8 Tg+ mice tested.
Reduced frequencies of CD8+ DCs in CD11c-Rac1(N17) Tg+ mice
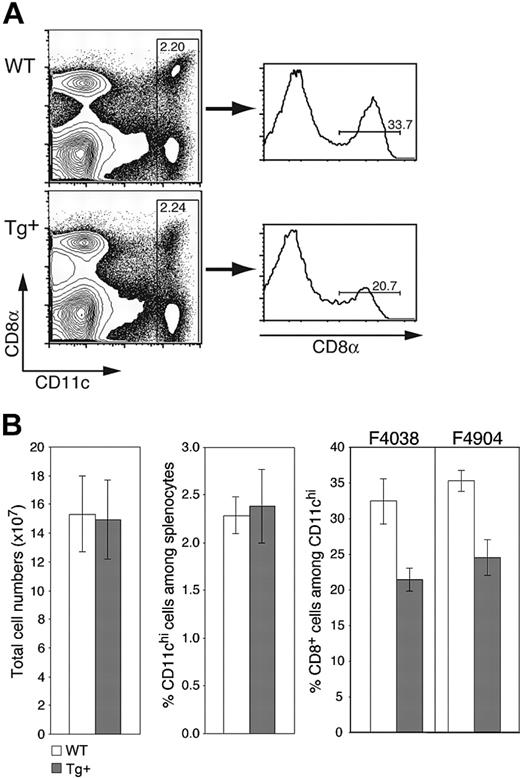
As expected from the selective nature of Tg expression, CD11c-Rac1(N17) mice exhibited no gross deformities or deficiencies, gave rise to Tg offspring with the expected frequencies, and had lymphoid compartments with normal cell numbers and ratios of T cells, B cells, macrophages, and other cell populations (Figure 2B and data not shown). The frequency of CD11chi DCs in the spleens of both Tg+ and wild-type (WT) mice was within the expected range of 2% to 2.5% of total cells (Figure 2A-B). However, the percentage of CD8+ DCs was reduced by approximately one third in Tg+ mice, composing 20% to 25% of CD11chi DCs as compared to 30% to 35% in WT mice (Figure 2A-B); a corresponding increase in CD8-CD11b+ DCs was observed (data not shown). As expected from the equivalent total cell numbers in Tg+ and WT mice (Figure 2B), Rac1 inhibition also resulted in decreased numbers of splenic CD8+ DCs. Similar decreases in the proportion of CD8+ DCs were detected in different Tg founders (Figure 2B), supporting the Tg-specific nature of the phenotype. Thus, inhibition of Rac1 in DCs does not affect overall DC numbers in the spleen but has a selective negative effect on the development of CD8+ DCs.
CD11c-Rac1(N17) mice have normal DC numbers but reduced frequencies of CD8+ splenic DCs. Splenocytes from Tg+ mice and WT controls were prepared as described in “Materials and methods” and stained with mAb specific for CD11c and CD8α as well as PI to exclude dead cells. (A) Representative dot plots are gated on live (PI-) cells, with staining for CD11c and CD8 as indicated. Histograms are gated on CD11chi cells. Cell frequencies within the specified gates are shown. (B) Mean (± SD) values for total splenic cell numbers, DC frequencies, and CD8+ DC percentages are shown (n = 7, representative of more than 5 experiments with similar results). Analyses from founder lines F4038 (n = 7) and F4904 (n = 3) are shown. Populations were defined as indicated in panel A.
CD11c-Rac1(N17) mice have normal DC numbers but reduced frequencies of CD8+ splenic DCs. Splenocytes from Tg+ mice and WT controls were prepared as described in “Materials and methods” and stained with mAb specific for CD11c and CD8α as well as PI to exclude dead cells. (A) Representative dot plots are gated on live (PI-) cells, with staining for CD11c and CD8 as indicated. Histograms are gated on CD11chi cells. Cell frequencies within the specified gates are shown. (B) Mean (± SD) values for total splenic cell numbers, DC frequencies, and CD8+ DC percentages are shown (n = 7, representative of more than 5 experiments with similar results). Analyses from founder lines F4038 (n = 7) and F4904 (n = 3) are shown. Populations were defined as indicated in panel A.
Normal maturation of DCs in CD11c-Rac1(N17) Tg+ mice
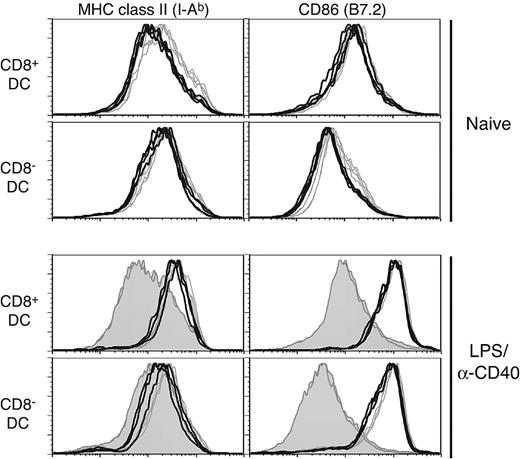
Selective effects on splenic DC subpopulations have been noted in analyses of various knockout mice23 ; for example, a loss of interferon consensus sequence binding protein (ICSBP)24,25 or inhibitor of DNA binding 2 (Id2)26 affects development of CD8+ DCs. Interestingly, ICSBP-/- mice also have a general deficiency in DC maturation. Given the impact of Rac1 inhibition on development of CD8+ DCs, we next analyzed in vivo phenotypic maturation of DCs, which has important consequences for their immunostimulatory capabilities. In naive Tg+ mice, both CD8+ DCs and CD8- DCs had lower MHC class II levels than controls (Figure 3 upper histograms; mean fluorescent intensity [MFI]: CD8+ DCs, 263 versus 377 and CD8- DCs, 281 versus 383 for Tg+ and WT, respectively; n = 4). Somewhat reduced B7.2 levels were also noted in naive Tg+ CD8- DCs (MFI: 96.5 versus 123 for Tg+ and WT, respectively), whereas MHC class I and CD40 expression were not significantly different (data not shown). However, both CD8+ and CD8- DCs in Tg+ mice were fully capable of up-regulating MHC class II and B7.2 following in vivo stimulation with LPS and anti-CD40 antibody (Figure 3 lower histograms) or either stimulus alone (data not shown). Stimulation of in vitro–cultured Tg+ DCs also revealed no deficiency in up-regulation of maturation markers or cytokine secretion (interleukin 12 [IL-12]p40, IL-12p70, IL-6) in response to inflammatory stimuli (data not shown).
DC in CD11c-Rac1(N17) mice exhibit no maturation deficiency when stimulated in vivo. Splenic DCs from naive (upper histograms) or LPS/anti-CD40–injected (4 hours after injection, lower histograms) Tg+ mice (black lines) and WT controls (gray lines) were stained with mAb specific for CD11c, CD8α, and MHC class II (I-Ab) or CD86. The filled histograms represent naive controls analyzed in parallel with LPS/anti-CD40–injected animals. Histograms are gated on live CD11c+ cells, with further separation based on expression of CD8 (CD8+ DCs, top panels; CD8- DCs, bottom panels).
DC in CD11c-Rac1(N17) mice exhibit no maturation deficiency when stimulated in vivo. Splenic DCs from naive (upper histograms) or LPS/anti-CD40–injected (4 hours after injection, lower histograms) Tg+ mice (black lines) and WT controls (gray lines) were stained with mAb specific for CD11c, CD8α, and MHC class II (I-Ab) or CD86. The filled histograms represent naive controls analyzed in parallel with LPS/anti-CD40–injected animals. Histograms are gated on live CD11c+ cells, with further separation based on expression of CD8 (CD8+ DCs, top panels; CD8- DCs, bottom panels).
Inhibition of Rac1 function in DCs inhibits endocytosis but not migratory capabilities
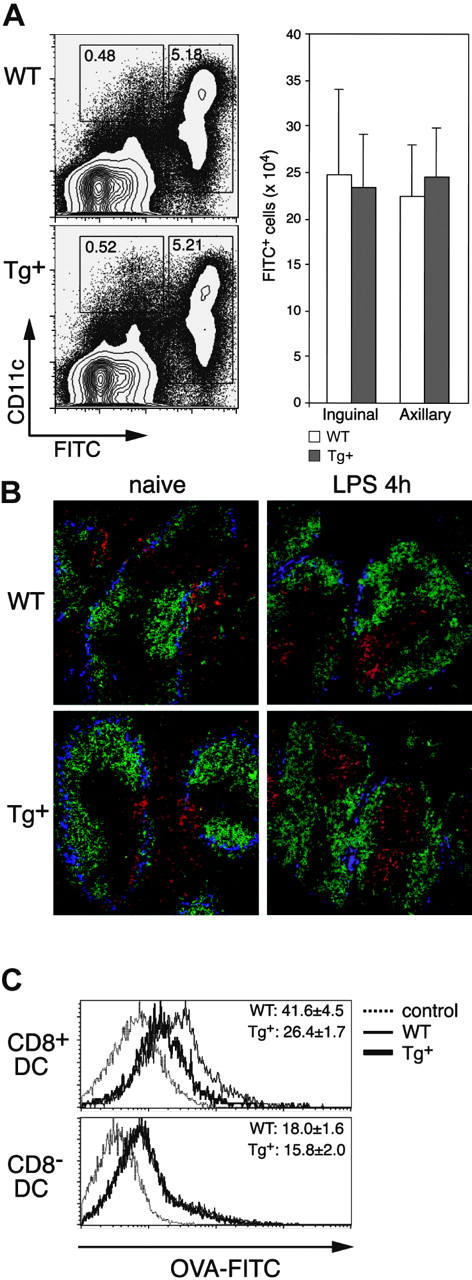
Endocytosis and migration require modification of the actin cytoskeleton, and DCs have been shown to involve Rac1 in both functions.4,7 We first analyzed in vivo DC migration in CD11c-Rac1(N17) mice following FITC skin painting, which results in the rapid maturation and mobilization of skin DCs into the draining LNs. Four hours following application of FITC to the abdomen, most CD11c+ cells in the draining inguinal and axillary LNs were FITC+, and the same frequencies and overall numbers of FITC+ DCs were detected in LNs of Tg+ mice and WT controls (Figure 4A). Only negligible numbers of FITC+ cells were found in the nondraining brachial LNs (data not shown). However, with the concern that low CD11c levels (and subsequent low Tg expression) on some skin DCs might account for the normal migration in Tg+ mice, we performed an in vivo assay to test the migratory capabilities of DCs in the spleen; exposure to inflammatory stimuli results in rapid maturation and migration of splenic DCs into T-cell areas.27 As depicted in Figure 4B (left), naive Tg+ mice have a normal distribution of DCs in the spleen; the majority of DCs (red) are located outside the T-cell areas (unstained/black, surrounded by green-stained B-cell areas), diffusely distributed in the red pulp and along the marginal zone (blue). The location of the T-cell area was confirmed by direct staining of serial sections with anti-Thy1.1 mAb (Figure S2, available on the Blood website; see the Supplemental Figures link at the top of the online article). After intravenous injection of LPS, nearly all DCs in both Tg+ mice and controls migrated into the T-cell areas (Figure 4B right). Additional analyses of in vitro-cultured CD11chi DCs also failed to reveal an effect of Rac1 inhibition on migration; no deficiencies in the ability of DCs from Tg+ mice to form podosomes (Figure S3) or to migrate in response to CCL21 or stromal cell-derived factor 1α (SDF-1α; transwell assay) were detected (data not shown). Thus, inhibition of Rac1 activity in CD11c-Rac1(N17) Tg+ mice did not diminish maturation- or chemokine-induced migration by DCs in commonly used assays.
Impact of DC-selective DN Rac1 expression on DC migration and macropinocytosis in vivo. (A) FITC (2 mg) was applied to the shaved abdomens of Tg+ and WT mice, and the draining (inguinal and axillary) LNs were harvested 24 hours later. Inguinal LNs and axillary LNs from each mouse were combined, subjected to collagenase/DNase digestion, and stained with anti-CD11c mAb and PI. Dot plots of axillary LNs from representative mice are gated on PI- cells, with FITC and CD11c staining as indicated. The mean (± SD) numbers of FITC+ cells in the combined inguinal and combined axillary LNs from single mice are shown for 3 mice per group. Data are from one experiment of 3 performed, all with similar results. (B) Immunofluorescence staining of cryostat sections from spleens of naive (left) or LPS-injected (4 hours, right) mice. WT and Tg+ mice were stained with the following mAbs: B220-FITC (green), CD11c-PE (red), and Moma-biotin (+ SA-Cy5, blue). Photographs were taken with a × 20 objective. (C) Mice were injected with FITC-OVA, and analysis of CD8+ DCs (upper histograms) and CD8- DCs (lower histograms) from spleens of control (injected with unlabeled OVA, dotted lines), WT (thin line), and Tg+ (thick line) mice was performed 1 hour after injection. Numbers indicate the mean (± SD, n = 3) FITC fluorescence from one experiment. Similar differences were found in 3 independently performed experiments.
Impact of DC-selective DN Rac1 expression on DC migration and macropinocytosis in vivo. (A) FITC (2 mg) was applied to the shaved abdomens of Tg+ and WT mice, and the draining (inguinal and axillary) LNs were harvested 24 hours later. Inguinal LNs and axillary LNs from each mouse were combined, subjected to collagenase/DNase digestion, and stained with anti-CD11c mAb and PI. Dot plots of axillary LNs from representative mice are gated on PI- cells, with FITC and CD11c staining as indicated. The mean (± SD) numbers of FITC+ cells in the combined inguinal and combined axillary LNs from single mice are shown for 3 mice per group. Data are from one experiment of 3 performed, all with similar results. (B) Immunofluorescence staining of cryostat sections from spleens of naive (left) or LPS-injected (4 hours, right) mice. WT and Tg+ mice were stained with the following mAbs: B220-FITC (green), CD11c-PE (red), and Moma-biotin (+ SA-Cy5, blue). Photographs were taken with a × 20 objective. (C) Mice were injected with FITC-OVA, and analysis of CD8+ DCs (upper histograms) and CD8- DCs (lower histograms) from spleens of control (injected with unlabeled OVA, dotted lines), WT (thin line), and Tg+ (thick line) mice was performed 1 hour after injection. Numbers indicate the mean (± SD, n = 3) FITC fluorescence from one experiment. Similar differences were found in 3 independently performed experiments.
Roles for Rac1 and Cdc42 in macropinocytosis by (cultured) DCs were previously shown through analysis of the uptake of fluorescent probes.3,4 Using flow cytometry, we analyzed the capacity of DCs in vivo to internalize FITC-OVA from the external milieu (Figure 4C). CD8+ DCs in Tg+ mice displayed a significant decrease in the uptake of FITC-OVA as compared to control DCs. Interestingly, no defect in FITC-OVA uptake by CD8- DCs in Tg+ mice was detected. These data indicate that the degree of Rac1 inhibition in CD11c-Rac1(N17) Tg+ mice is sufficient to interfere with DC uptake of soluble protein but not with migration.
DC-selective inhibition of Rac1 activity reduces apoptotic cell uptake and cross-presentation
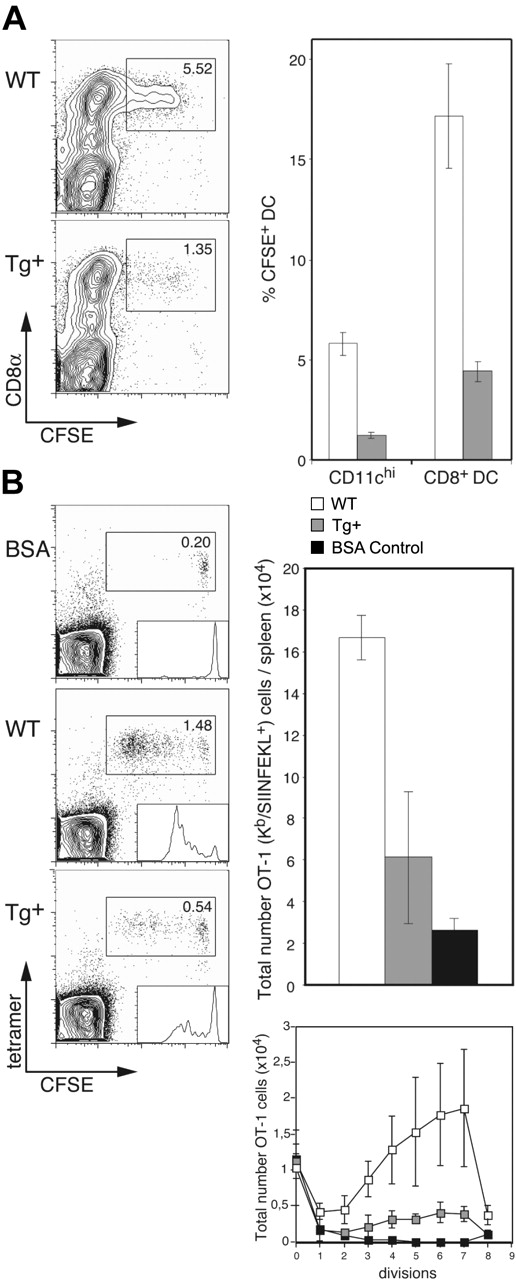
Having established that DC-selective Rac1 inhibition interferes with development of CD8+ DCs and leads to a reduction in endocytosis, we were interested in determining the capacity of DCs in CD11c-Rac1(N17) Tg+ mice for internalization of apoptotic cells and subsequent cross-presentation. To investigate apoptotic cell uptake, live CFSE-labeled allogeneic (BALB/c) B cells were injected into Tg+ and WT mice (C57BL/6 background); the absence of self-MHC molecules leads to rapid killing of allogeneic cells by host NK cells, followed by efficient uptake by CD8+ DCs.14 Injection of enriched B cells was undertaken to prevent induction of T cell–mediated graft-versus-host reaction. Only CD8+ DCs were observed to take up allogeneic B cells, and DC-specific Rac1 inhibition resulted in a 3- to 4-fold reduction in uptake (∼5.5% versus 1.5% of CD11chi cells in WT and Tg+ mice, respectively; Figure 5A). This effect reflects more than the decrease in the overall number of CD8+ DCs; of the existing CD8+ DCs in Tg+ mice, only 5% were capable of ingesting apoptotic cells, whereas 15% to 20% of CD8+ DCs in controls demonstrated uptake of the injected cells (Figure 5A).
Impaired apoptotic cell uptake and cross-presentation by CD8+ DCs in CD11c-Rac1(N17) mice. (A) Tg+ mice and WT controls were injected with 1 × 107 CFSE-labeled allogeneic (BALB/c) B cells, and spleens were harvested 14 hours later. Following collagenase/DNase digestion, splenocytes were stained with mAb specific for CD11c and CD8α. Dot plots are gated on CD11chi cells, with CFSE labeling and CD8 staining as indicated. The frequencies of CFSE+ cells among all DCs (CD11chi) and CD8+ DCs are shown (mean ± SD, n = 3). Data are representative of 2 independent experiments with similar outcome. (B) CFSE-labeled OT-1 cells (1 × 106) were transferred into Tg+ mice and WT controls, followed by injection of 1 × 106 OVA- or BSA (control)–loaded β2m-/- cells 1 day later. Spleens were harvested 3 days after the second injection, digested with collagenase/DNase, and stained with anti-CD8α mAb, H2-Kb/SIINFEKL tetramers, and PI. Dot plots are gated on CD8+ cells, with CFSE labeling and tetramer staining as indicated. Histogram analysis of CFSE+ OT-1 cells is shown as inserts in the dot plots. Total numbers of OT-1 (Kb/SIINFEKL tetramer-positive) cells per spleen (upper graph) and OT-1 cells per cell division (lower graph) were calculated; the mean (± SD) is shown for 3 mice per group (BSA, n = 2). Results from one experiment are shown and are representative 3 independent experiments performed.
Impaired apoptotic cell uptake and cross-presentation by CD8+ DCs in CD11c-Rac1(N17) mice. (A) Tg+ mice and WT controls were injected with 1 × 107 CFSE-labeled allogeneic (BALB/c) B cells, and spleens were harvested 14 hours later. Following collagenase/DNase digestion, splenocytes were stained with mAb specific for CD11c and CD8α. Dot plots are gated on CD11chi cells, with CFSE labeling and CD8 staining as indicated. The frequencies of CFSE+ cells among all DCs (CD11chi) and CD8+ DCs are shown (mean ± SD, n = 3). Data are representative of 2 independent experiments with similar outcome. (B) CFSE-labeled OT-1 cells (1 × 106) were transferred into Tg+ mice and WT controls, followed by injection of 1 × 106 OVA- or BSA (control)–loaded β2m-/- cells 1 day later. Spleens were harvested 3 days after the second injection, digested with collagenase/DNase, and stained with anti-CD8α mAb, H2-Kb/SIINFEKL tetramers, and PI. Dot plots are gated on CD8+ cells, with CFSE labeling and tetramer staining as indicated. Histogram analysis of CFSE+ OT-1 cells is shown as inserts in the dot plots. Total numbers of OT-1 (Kb/SIINFEKL tetramer-positive) cells per spleen (upper graph) and OT-1 cells per cell division (lower graph) were calculated; the mean (± SD) is shown for 3 mice per group (BSA, n = 2). Results from one experiment are shown and are representative 3 independent experiments performed.
We next analyzed the impact of this deficiency in apoptotic cell uptake on cross-presentation in vivo. CFSE-labeled OT-1 cells were transferred into Tg+ mice and controls, followed by injection of OVA-loaded β2m-/- splenocytes as described previously15 ; β2m-/- cells lack expression of cell surface MHC class I and are therefore unable to present antigen directly to CD8+ T cells, limiting the route of cytotoxic T lymphocyte (CTL) activation to cross-presentation. Inhibition of Rac1 in DCs resulted in strong inhibition of cross-presentation; the frequency and overall number of expanded OT-1 cells was approximately 3-fold lower in CD11c-Rac1(N17) Tg+ mice than in WT littermates (Figure 5B). In addition, analysis of the total numbers of OT-1 cells per cell division according to the level of their CFSE label revealed substantial inhibition of OT-1 priming by Tg+ DCs (Figure 5B). Thus, DC-specific Rac1 inhibition results in a significant reduction in the capacity of CD8+ DCs for uptake of apoptotic cells, with serious consequences for the efficiency of cross-presentation.
DC-specific Rac1 inhibition results in reduced induction of bacteria-specific T-cell responses
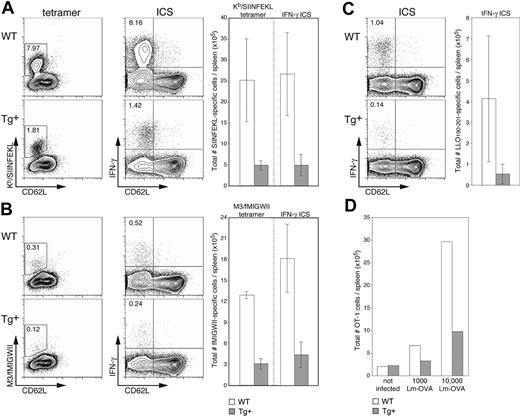
We next wanted to investigate the in vivo consequences of DC-selective Rac1 inhibition on the initiation of immune responses in a more physiologic setting. Sublethal infection with the bacterium L monocytogenes (Lm) is a widely used murine model for examining T-cell responses to an intracellular pathogen. Lm is rapidly cleared from the target organs (spleen and liver) and stimulates expansion of both CD8+ and CD4+ T-cell populations, resulting in long-lasting T cell-mediated immunity. It was previously shown that bone marrow–derived APCs,28 and more specifically DCs,21 are necessary for priming of Lm-specific CD8+ T cells. These data, in combination with the finding that DCs are unfit hosts for bacterial replication,29 led to the conclusion that cross-presentation of Lm antigen by (uninfected) DCs is crucial for establishment of effective Lm-specific CTL responses.21,28 Because no immunodominant H2b-restricted CTL population expands upon infection of C57BL/6 mice with WT Lm, we examined induction of endogenous T-cell responses using recombinant OVA-expressing Lm (Lm-OVA), which stimulates expansion of an easily detectable H2-Kb-restricted, SIINFEKL-specific population.19,20 At the peak of expansion (7 days after infection), an average of 5-fold fewer SIINFEKL-specific CTLs were found in Tg+ mice than in WT littermates (Figure 6A). The differences in frequency (dot plots, left) and absolute cell numbers (graph, right) detected by staining with H2-Kb/SIINFEKL tetramers and for intracellular IFN-γ production were equivalent, indicating that although expansion is significantly reduced in Tg+ mice, the induced CTLs are functional. Examination of CTL responses restricted by a highly conserved MHC class Ib molecule (H2-M3) gave similar results; approximately 4-fold fewer M3-restricted T cells specific for the Lm-derived fMIGWII peptide were detected (by tetramer staining and IFN-γ production) in Tg+ mice than in WT controls (Figure 6B). Finally, to round out our investigation of Lm-specific T-cell responses, we examined the immunodominant CD4+ T-cell response specific for the Lm-derived LLO190-201 peptide20,30 ; only analysis by IFN-γ production was carried out because the necessary MHC class II tetramers have not yet been developed. Although variation in the CD4+ T-cell response was greater than that in the CD8+ T-cell compartment, induction of CD4+ T-cell responses in CD11c-Rac1(N17) Tg+ mice was substantially reduced (approximately 4-fold) as compared to controls (Figure 6C). Determination of bacterial numbers in the spleen and liver on day 3 after infection revealed no significant differences between Tg+ and WT mice, and bacteria were completely cleared in all mice by day 7 (data not shown).
Reduced expansion of epitope-specific T cells in Lm-infected CD11c-Rac1(N17) mice. Spleens from Tg+ mice and WT controls were harvested 7 days after infection with 2000 Lm-OVA, and the frequencies of T cells specific for SIINFEKL (A), fMIGWII (B), and LLO190-201 (C) were analyzed. A portion of cells was stained with PE-conjugated tetramers (H2-Kb/SIINFEKL or H2-M3/fMIGWII) in combination with anti-CD62L and anti-CD8α mAb and EMA to stain dead cells. Other cells were stimulated briefly (5 hours) in vitro in the presence of 10-6 M SIINFEKL, fMIGWII, or LLO190-201 peptide and brefeldin A. Intracellular staining (ICS) for IFN-γ followed staining with EMA and mAb specific for CD4, CD8α, and CD62L. Dot plots are gated on live CD8+ cells (SIINFEKL and fMIGWII) or CD4+ cells (LLO190-201), with the frequency of activated (CD62Llow, x-axis) epitope-specific T cells as determined by tetramer or intracellular IFN-γ staining (y-axis, left and right dot plots, respectively) indicated. Total numbers of epitope-specific T cells are shown in the graphs to the right of each set of dot plots (n = 3 ± SD). Results are representative of 2 independent experiments. (D) CFSE-labeled OT-1 cells (2.5 × 106) were transferred into Tg+ mice and WT controls, and mice were infected 1 day later with 1000 or 10 000 Lm-OVA. A group of control mice received OT-1 cells but was not infected. Splenocytes were harvested 3 days after Lm-OVA infection and stained with PI, anti-CD8α mAb, and Kb/SIINFEKL tetramers. The graph shows total numbers of OT-1 cells (positive for CFSE and tetramer) for 2 mice per group. Results are representative of 2 independent experiments.
Reduced expansion of epitope-specific T cells in Lm-infected CD11c-Rac1(N17) mice. Spleens from Tg+ mice and WT controls were harvested 7 days after infection with 2000 Lm-OVA, and the frequencies of T cells specific for SIINFEKL (A), fMIGWII (B), and LLO190-201 (C) were analyzed. A portion of cells was stained with PE-conjugated tetramers (H2-Kb/SIINFEKL or H2-M3/fMIGWII) in combination with anti-CD62L and anti-CD8α mAb and EMA to stain dead cells. Other cells were stimulated briefly (5 hours) in vitro in the presence of 10-6 M SIINFEKL, fMIGWII, or LLO190-201 peptide and brefeldin A. Intracellular staining (ICS) for IFN-γ followed staining with EMA and mAb specific for CD4, CD8α, and CD62L. Dot plots are gated on live CD8+ cells (SIINFEKL and fMIGWII) or CD4+ cells (LLO190-201), with the frequency of activated (CD62Llow, x-axis) epitope-specific T cells as determined by tetramer or intracellular IFN-γ staining (y-axis, left and right dot plots, respectively) indicated. Total numbers of epitope-specific T cells are shown in the graphs to the right of each set of dot plots (n = 3 ± SD). Results are representative of 2 independent experiments. (D) CFSE-labeled OT-1 cells (2.5 × 106) were transferred into Tg+ mice and WT controls, and mice were infected 1 day later with 1000 or 10 000 Lm-OVA. A group of control mice received OT-1 cells but was not infected. Splenocytes were harvested 3 days after Lm-OVA infection and stained with PI, anti-CD8α mAb, and Kb/SIINFEKL tetramers. The graph shows total numbers of OT-1 cells (positive for CFSE and tetramer) for 2 mice per group. Results are representative of 2 independent experiments.
It has been reported that activated CD8+ T cells express CD11c.21,31 Although we failed to detect Tg expression in T cells (Figure 1) or a negative impact on the in vitro activation of CD8+ T cells (data not shown), we were concerned that the reduced induction of endogenous Lm-specific T-cell responses in CD11c-Rac1(N17) Tg+ mice might be due to a T cell-specific effect of the Tg. We therefore adoptively transferred (SIINFEKL-specific) OT-1 T cells into Tg+ mice and controls. After infection with either 1000 or 10 000 Lm-OVA, the antigen-specific OT-1 T-cell expansion detected in Tg+ mice was 2- to 3-fold lower than that in WT controls (Figure 6D), a level of reduction comparable to that observed with endogenous T cells. These results strongly indicate that the reduced expansion of Lm-specific T cells in CD11c-Rac1(N17) Tg+ mice is not due to a direct negative effect of Tg expression on T cells but can be attributed instead to the selective inhibition of Rac1 activity in DCs.
Discussion
Many questions remain as to the mechanisms that make DCs such effective APCs. To more closely analyze the function of DCs in vivo, we selectively expressed a DN form of Rac1, a Rho family GTPase, under control of the CD11c promoter in Tg mice. For many GTPases, a single amino acid mutation at codon 17 creates a specific DN form that interferes with activation of the GTPase by competing for the downstream guanine nucleotide exchange factors (GEFs) involved in the exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP).32 Although distinct phenotypes have resulted from expression of DN Rho family members in cells, the specificity of these DN proteins is of concern because many GEFs associate with more than one Rho GTPase. We cannot exclude an effect of DN Rac1 on other GTPases, but a recently reported Rac1 conditional knockout33 has a comparable reduction in the frequency of CD8+ DCs, strongly supporting a dominant role of Rac1 (inhibition) in the phenotype of CD11c-Rac(N17) Tg+ mice. This similarity also indicates that the relatively low level of DN expression in CD11c-Rac(N17) Tg+ mice is sufficient to impair at least some mechanisms mediated by Rac1; we were unable to directly confirm inhibition of endogenous Rac1 activation (Figure S1). The dramatic effect on apoptotic cell uptake suggests that this important biologic activity is especially dependent on Rac1. In contrast, although clear roles for Rac1 in both endocytosis and migration have been demonstrated in vitro through microinjection of DN protein into cultured cells,2 including DCs,4,7 we detected no effect of DN Rac1 expression on DC migration. Benvenuti et al33 reported that DC migration in Rac1/2 double-deficient mice is impaired, but the effect of Rac1 deletion alone was not reported. It is possible that we found no effect of DN Rac1 expression on migration because the assays lacked the required sensitivity. Alternatively, nucleotide exchange occurring without the need for GEFs could explain intact migration in CD11c-Rac(N17) Tg+ mice; it has been shown that nitric oxide can induce exchange on the GTPase Ras.34 However, we favor the hypothesis that different biologic functions, that is, endocytosis and migration, require different levels of Rac1, and apoptotic cell uptake is simply extremely sensitive to Rac1 levels. A distinct dose dependency of diverse biologic effects on GTPase activity has been demonstrated by expressing different amounts of DN Ras1 (N17Ras1).35
Although significant focus has been placed on DC subpopulations in recent years, the origin and development of the various subsets are still unclear. A linear model of differentiation was suggested by a study in which CD8+ DCs developed from adoptively transferred CD8- DCs,36 but these findings have been recently challenged.37 In contrast, gene array analysis revealed a close relationship between CD4+ and double-negative (CD8-) DCs, whereas the origins of CD8- and CD8+ DCs appeared more distant,38 supporting a lineage model for differentiation of the DC subsets. The selective deficiency in CD8+ or CD8- DC subsets observed in various knockout mice is also generally considered to be supportive of a lineage model.23 However, in contrast to ICSBP-/- mice, in which the reduction in splenic CD8+ DCs does not result in increased CD8- DC numbers (as might be expected if CD8+ DCs arise from CD8- precursors),24 such an increase in CD8- DCs was observed in Id2-/- mice26 and CD11c-Rac1(N17) Tg+ mice (Figure 2), resulting in maintenance of overall DC numbers. We hypothesized that the lower percentage of CD8+ DCs in CD11c-Rac1(N17) Tg+ mice could be the result of defective maturation or migration (or both) of CD8- DCs; deficient DC maturation was observed in ICSBP-/- and Id2-/- mice. Although our further studies failed to detect an effect of DC-selective DN Rac1 expression on inflammation-induced maturation or migration (Figures 3 and 4B), we cannot exclude the possibility that subtle differences in maturation or migration could not be detected following injection of such a strong inflammatory stimulus or with the experimental methods used. These data nevertheless indicate that a linear model of CD8- and CD8+ DC differentiation should not yet be abandoned.
It has become increasingly clear that cross-presentation, which enables the loading of exogenous (extracellular) antigen onto MHC class I molecules, plays an important role in the establishment of CTL responses to some pathogens and tumors and may be crucial in inducing peripheral tolerance.16,39 The intracellular pathway that enables cross-presentation to occur has recently been elucidated,40-42 but these studies, which used cultured DCs, DC lines, and macrophages, do not explain the unique role attributed to CD8+ DCs in cross-presentation.15 Our findings suggest a crucial role for Rac1 in apoptotic cell uptake by CD8+ DCs (Figure 5A), and the corresponding reduction in cross-presentation (Figure 5B) provides strong in vivo evidence to support the hypothesis that it is the unique capacity of CD8+ DCs for uptake of apoptotic cells14,43 that accounts for their importance in cross-presentation. Indeed, studies in Caenorhabditis elegans and mammalian cells have indicated that Rac1 is part of an evolutionarily conserved pathway for the identification and endocytosis of apoptotic material44,45 that is connected to the αvβ5-integrin receptor. Endocytosis of apoptotic cells and cross-presentation in DCs was linked relatively early to the αvβ5 integrin and the associated CD36 molecule,46,47 and CD36 is preferentially expressed on CD8+ DCs,14 but the absence of either αvβ5 or CD36 was reported to have no negative impact on cross-presentation.48,49
Cross-presentation of antigen from apoptotic cells infected with Salmonella and Mycobacterium tuberculosis is critical for the priming of CD8+ T-cell populations specific for these phagosomally localized intracellular bacteria.50,51 Although Lm quickly gains access to the cytosol of infected cells, evidence indicates that cross-presentation may also be important for the in vivo generation of T-cell responses specific for this bacterium: Lm replicates poorly in DC,29 and intracellular bacteria were not detected within DC from Lm-infected mice although the DCs could present Lm-derived antigen ex vivo.52 Thus, endocytosis of antigen from infected (apoptotic) cells and presentation by DCs may be important for the priming of CD8+ and CD4+ T-cell populations specific for Lm.52 The diminished apoptotic cell uptake, cross-presentation, and induction of Lm-specific T-cell responses in CD11c-Rac1(N17) Tg+ mice are in accordance with a role for cross-priming in the generation of Lm-specific T-cell responses. Although Jung et al21 previously implicated DCs in cross-presentation of Lm-derived antigen in their system of DC ablation, our studies point more specifically at the role of CD8+ DCs in this process.
Through DC-specific inhibition of Rac1 activity in vivo, we have established a role for this GTPase in the development and function of CD8+ DCs. CD11c-Rac1(N17) Tg+ mice will be a valuable tool for further evaluation of the role of cross-presentation by DCs in a variety of immunologic settings, including tumors, vaccines, infections, and the maintenance of peripheral tolerance in vivo.
Prepublished online as Blood First Edition Paper, September 21, 2004; DOI 10.1182/blood-2004-05-1891.
Supported by grants from the Bavarian BSE-Forschungsverbund (no. LMU11; T.B.) and the Deutsche Forschungsgemeinschaft (DG) SFB571-B5 (T.B.), in part by a stipend from the Humboldt Foundation (K.M.K.), and in part by the Gerhard Hess Program of the DFG (D.H.B.).
The online version of the article includes a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Y. Hirokami for initial genotyping and A. Bol and W. Mertl for maintenance of the CD11cRac1(N17) mice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal