Abstract
Thymus-derived CD4+CD25+ regulatory T (Treg) cells are essential for the maintenance of immunologic self-tolerance. Despite their critical role in the active suppression of experimental autoimmune disorders, little is known about their involvement in human autoimmune diseases. Myasthenia gravis (MG) is a CD4+ T cell–dependent autoimmune disease and the thymus is assumed to be the initiation site. To identify possible defects in the Treg cells in MG, we analyzed CD4+CD25+ cells in thymi from patients with MG compared to those from healthy subjects. We found a normal CD4+CD25+ number but a severe functional defect in their regulatory activity together with a decreased expression of the transcription factor, Foxp3, which is essential for T-cell regulatory function. The phenotypic analysis of CD4+CD25+ thymocytes revealed an increased number of activated effector cells with strong Fas expression in patients with MG. However, whatever their level of Fas, CD4+CD25+ thymocytes from patients with MG remained unable to suppress the proliferation of responding cells, indicating that the impaired Treg cell function is not due to contamination by activated effector T cells. These data are the first to demonstrate a severe functional impairment of thymic Treg cells in MG, which could contribute to the onset of this autoimmune disease.
Introduction
The development of autoimmune diseases involves a breakdown in the mechanisms that control self-reactive lymphocytes. The primary mechanism that generally maintains self-tolerance is thymic deletion of autoreactive T cells with high affinity for self-antigens. However, this mechanism is not perfect and autoreactive T cells do escape to the periphery. Recent data suggest that in addition to the mechanisms of clonal deletion and anergy, regulatory T (Treg) cells play a significant role in the generation and maintenance of peripheral tolerance. Indeed, compelling evidence indicates that immune responsiveness is controlled by a subpopulation of Treg cells that are enriched in the naturally activated subset of CD4 T cells and that constitutively express the CD25 (interleukin 2 receptor α [IL-2Rα]) molecule. The thymus is a primary source of this regulatory CD4+CD25+ T-cell subset, which participates in the active suppression of potentially autoreactive T cells in the periphery.1,2 CD4+CD25+ Treg cells have been described in a variety of experimental systems to provide protection from T cell–mediated autoimmune disorders (for a review, see Shevach3 ). This Treg population has been also isolated from human thymus4,5 and the peripheral blood of healthy individuals.6-8 Similar to murine CD25+ T cells,9 they are naturally anergic in vitro and they inhibit the proliferation of cocultured conventional CD4+CD25- T cells in a contact-dependent manner. The possible role of the surface molecules, cytotoxic T lymphocyte associated-antigen 4 (CTLA-4) and transforming growth factor β (TGF-β), in the regulatory function of CD25+ Treg cells is controversial and their mechanisms of suppression remain to be determined.3
The forkhead transcription factor, FoxP3, was reported recently to be essential for the development and functional activity of mice and human CD25+ Treg cells.10-13 Moreover, FOXP3 transduction converts naïve CD4+CD25- T cells into CD25+ regulatory cells with suppressive activity. Despite a growing interest in CD4+CD25+ Treg cells and their role in the emergence of autoimmune diseases in animal models, only very limited and controversial information is available on the role of this T-cell population in the pathogenesis of human autoimmune diseases. Indeed, a decrease in the number of circulating CD4+CD25+ cells in autoimmune diabetes was reported.14 In multiple sclerosis, either an increase15 or no alteration16 was shown, although a very recent paper showed the loss of functional activity for circulating CD4+CD25+ T cells.17 In contrast, the CD4+CD25+ T-cell subset was enriched with functionally active regulatory cells in the inflamed joints of patients with rheumatoid arthritis.18 The deficiency in numbers of CD4+CD25+ T cells was reported in the peripheral blood leukocytes (PBLs) of patients with virus-associated autoimmunity hepatitis C-mixed cryoglobulinemia vasculitis, whereas suppressive activity of Treg cells was not changed.19 Therefore, additional studies are required to clarify the involvement of these cells in autoimmune diseases.
Myasthenia gravis (MG) is a prototypical CD4+ T cell–dependent autoimmune disease mediated by antiacetylcholine receptor (anti-AChR) autoantibodies.20 The anti-AChR antibodies affecting the neuromuscular transmission are found in more than 85% of patients. This autoimmune disorder is the only disease in which the thymus is believed to be the initiation organ of pathogenesis, and thymic ablation is a common therapy for many patients,21 making available thymic samples. MG is also characterized by a high incidence of morphologic thymic abnormalities.22 More than half of patients, especially young women with high titers of anti-AChR antibodies, present with thymic hyperplasia, characterized by numerous lymphoid follicles with germinal centers similar to those found in lymph nodes.23 The hyperplastic MG thymus contains activated anti-AChR autoreactive T cells24-28 and anti-AChR antibody-producing B cells.29 AChR expression has been demonstrated in both normal and MG thymi, enabling efficient autoantigen presentation.30-32 All together, these points strongly argue that the hyperplastic thymus is the effector site of the autoantibody response in MG.
In the present study, we analyzed thymi derived from patients with MG in an attempt to identify possible quantitative or qualitative defects in the regulatory T-cell population. Our results demonstrate a profound functional defect in CD4+CD25+ thymocytes from patients with MG characterized by high proliferation in response to mitogenic stimulation, severely impaired suppressive activity, as well as a significant decrease in expression of the FoxP3 transcription factor.
Patients, materials, and methods
Patients
Thymuses were obtained from 17 patients with MG (15 women and 2 men, aged 18-42 years) undergoing thymectomy at Marie Lannelongue Hospital. All the patients were selected using the following clinical criteria: thymic hyperplasia, high anti-AChR antibody titer (> 10 nM), and a generalized disease. Thymic hyperplasia was defined according to classification of Levine and Rosai33 as thymi containing germinal centers. Anti-AChR antibody titers were determined in serum as previously described.34 Patients on corticosteroid treatment and those with thymomas were excluded from the study.
Normal thymi, which would otherwise be discarded, were obtained from 12 newborn infants (aged 3 days to 8 months) and 11 young adults (aged 14-21 years) undergoing cardiac surgery. Controls older than 21 years were not included because of the very low number of cells recovered from their thymic samples. These investigations were approved by the local Ethics Committee, CCPPRB (Comité Consultatif de Protection des Personnes dans la Recherche Biomédical, Kremlin Bicêtre, France). Informed consent was provided according to the Declaration of Helsinki.
Antibodies
Fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, or biotin-conjugated anti-CD25 (M-A251), anti-CD71 (M-A712), anti-CD80 (BB1), anti-CD86 (FUN-1), anti-CD95 (DX2), anti-CD122 (Mik-β2), anti-CTLA-4 (BNI3.1) monoclonal antibodies (mAbs) as well as isotype controls, IgG1-FITC, IgG1-PE, biotinylated IgG1, IgG2a, IgM, and streptavidin-coupled PE, were purchased from Becton Dickinson (Le Pont de Claix, France). Peridinin chlorophyll protein (PerCP)–conjugated anti-CD4 (SK3) and IgG1 were from Becton Dickinson. The FITC-conjugated anti-CD8 (DK25) was from DakoCytomation (Trappes, France). The FITC-conjugated anti-HLA-DR was from Beckman Coulter France (Ville-pinte, France). The biotin-conjugated anti-LAP (TGF-β1, goat IgG) and goat IgG control were from R&D Systems Europe (Lille, France).
Thymocyte isolation
Thymocytes were mechanically isolated by gentle scraping of fresh thymic tissue. The cells were filtered through sterile gauze and washed once with Hanks balanced salt solution (HBSS). For depletion of CD8-expressing cells, total thymocytes were mixed with anti-CD8 magnetic beads (Immunotech, Marseille, France) in phosphate-buffered saline (PBS) plus 30% fetal calf serum (FCS) to separate CD8+ cells. The percentage of residual CD8+ cells after depletion, determined by fluorescence-activated cell sorting (FACS) analysis, was always less than 0.5%, and the remaining population consisted of 70% to 90% CD4+ cells and 10% to 30% CD4-CD8- cells.
The isolation of CD4+CD25+ and CD4+CD25- thymocytes was performed as follows. Positive selection of the CD4+ population among the CD8-depleted thymocytes was performed using CD4-MultiSort magnetic microbeads (Miltenyi Biotec, Paris, France). After isolation and release of microbeads, CD4+ thymocytes were stained with FITC-conjugated anti-CD25 mAb and separated into CD25+ and CD25- cells by positive selection using anti-FITC magnetically activated cell sorting (MACS) microbeads (Miltenyi Biotec). The purity of the resulting population was more than 90% for CD4+CD25+ and 95% for CD4+CD25- thymocytes.
For cell sorting experiments, CD8-depleted thymocytes from normal or hyperplastic myasthenic thymi were stained with anti-CD4, anti-CD25, and anti-Fas mAbs and Faslo and Fashi were separated on a FACS Vantage SE (Becton Dickinson) cell sorter. Fasneg cells were not sorted. The purity of sorted Faslo and Fashi subsets was more than 95%.
Flow cytometry analysis
For immunofluorescence staining, 106 cells were incubated with biotinylated mAbs for 20 minutes at 4°C. After washing with HBSS/5% FCS the biotin-labeled cells were incubated with streptavidin-coupled PE for 20 minutes at 4°C, washed twice with PBS/0.5% BSA, and analyzed on FACScalibur using CellQuest software (Becton Dickinson). To assess the frequency of CD4+CD25+ cells, the data were collected on 2 × 105 total thymocytes (gated by forward and side scatter properties). For the analysis of intracellular CTLA-4 expression, cells were stained for CD4 and CD25 surface expression, fixed and permeabilized using IntraPrep Permeabilization reagents (Immunotech), and labeled with biotinylated anti-CTLA-4 and streptavidin-coupled PE.
Proliferation assays
To assess the proliferative response of purified CD4+CD25- and CD4+CD25+ thymocytes, 5 × 104 to 1 × 105 responder cells were incubated in X-VIVO-15 (Cambrex, Emerainville, France) supplemented with 20 μg/mL gentamicin (Merck, Eurolab, Fontenay-sous-bais, France) and 2 mM glutamine (Cambrex) in 96-well U-bottom plates (Costar, VWR, Fortenay-sous-bais, France) with 2.5 × 105 allogenic accessory cells and 5 μg/mL phytohemagglutinin-protein (PHA-P; Sigma, Aldrich, Lyon, France). T cell–depleted accessory cells were isolated by incubation of allogenic peripheral blood mononuclear cells (PBMCs) with M-450 anti-CD3 Dynabeads (Dynal, Compiègne, France) to remove T cells, followed by irradiation at 50 Gy. On day 5, after an 18-hour pulse with 1 μCi (0.037 MBq) 3[H]-thymidine, cells were harvested and proliferation measured using a liquid scintillation counter. The results were calculated as the mean ± SD of triplicate wells. To test the suppressive activity of CD4+CD25+ cells, 5 × 104 CD4+CD25- thymocytes were cocultured with increasing numbers of CD4+CD25+ or CD4+CD25- thymocytes in the presence of the fixed dose of accessory cells and PHA, as indicated.
Immunohistochemical analysis of thymic sections
Acetone-fixed 6-μm–thick frozen sections were incubated for 30 minutes at room temperature with peroxidase-blocking reagent (DakoCytomation) followed by 30 minutes staining at room temperature with 20 μg/mL anti-CD25 mAb (M-A251; PharMingen). For visualization, peroxidase-based EnVision+ reagents with 3-amino-9-ethylcarbazole (AEC+) chromogenic substrate (Dako) were used according to the manufacturer's instructions. After 3 washes in PBS, the sections were counterstained with Mayer hematoxylin (Dako) and mounted in aqueous mounting medium (Dako paramount from DakoCytomation). Control sections were incubated with mouse IgG1 (DakoCytomation).
Sections were viewed with a Leica DMRB microscope. All observations were performed using a 10 × eyepiece objective (aperture 0.3) or 40 × objective (aperture 0.7). Images were captured with a Sony DXC-930P color CCD camera connected to a Leica MSP 930 Image analysis system. The acquisition software was Cyberview 4.0.
Real-time quantitative polymerase chain reaction
Total RNA was extracted from CD4+CD25- and CD4+CD25+ thymocytes using Trizol reagent (Gibco BRL, Karlsruhe, Germany). cDNA was synthesized from 1 μg total RNA with specific primers designed using LC Probe software (Roche Diagnostics, Meylan, France). Quantitative cDNA amplification was performed according to manufacturer's instructions. The products of polymerase chain reaction (PCR) LightCycler amplification were detected with SYBR green I dye (Roche Diagnostics). PCR cycling conditions were 95°C for 10 minutes and 34 cycles of 95°C for 10 seconds, 64°C for 5 seconds, 72°C for 20 seconds, followed by final melting curve program. The melting curve analysis of PCRs together with their electro-phoresis separation revealed the presence of one amplicon of the expected size. Each sample was run in duplicate and mean values were used for quantitation. The FOXP3 mRNA levels were normalized to the 28S rRNA expression in each sample. The primers were as follows. FoxP3: 5′-GGAGTTCCGCAAGAAACGG-3′, 5′-CTGAAGTAATCTGTGCGAGC-3′; 28S: 5′-CGGGTAAACGGCGGGAGTAA-3′, 5′-GGTAGGGACAGTGGGAATCT-3′. An external standard curve was obtained with serial dilutions of a reference cDNA sample amplified at the same time as the unknown samples. FOXP3 mRNA levels were determined by comparing experimental levels to the standard curves and expressed as arbitrary units.
Statistical analysis
Differences between groups were evaluated by the nonparametric Mann-Whitney t test (Instat; Graph Pad Software, San Diego, CA) with a level of significance set at P less than .05.
Results
Number of CD4+CD25+ thymocytes in patients with MG is unchanged
To identify possibly regulatory T-cell defects in MG, CD25 expression was investigated in single-positive mature CD4+ thymocytes from patients with MG with positive anti-AChR antibody titers and from healthy young adult or newborn controls. The unfractionated thymocyte population was analyzed for the expression of CD4, CD8, and CD25. The percentage of CD4+CD8-CD25+ cells was not significantly different in patients with MG (mean, 12%; range, 9%-30%) as compared to the 2 control groups, young adult individuals (mean, 14%; range, 9%-19%) and newborns (mean, 13%; range, 10%-16%; Figure 1A). The regulatory activity of CD4+CD25+ T cells in peripheral blood was associated with a high CD25 expression.18,35 However, no differences were detectable in either abundance or intensity of CD4+CD25hi thymocytes of patients with MG versus healthy controls (Figure 1B). CD4+CD25+ Treg cells have been purified from postnatal human thymi,4,5 but they have not been assessed in adult thymic tissue. Our analysis of the CD4+CD25+ adult thymocyte population among CD4+ cells indicates that it is similar to that reported in normal human peripheral blood, representing about 13% of the CD4+ population.6,7,36
The frequency of CD4+CD8-CD25+ thymocytes in patients with MG is similar to healthy newborn and young adult thymi. Freshly isolated human thymocytes from hyperplastic thymi of 15 patients with MG as well as the thymocytes from 10 young adult and 10 newborn thymi were analyzed by 3-color flow cytometry for the expression of CD4, CD8, and CD25. Each point represents 1 patient or 1 control. The bars represent mean values. The proportion of CD4+CD8- cells with CD25+ (A) as well as CD25hi (B) expression was calculated. The differences between newborns, adults, and patients with MG are not significant (NS).
The frequency of CD4+CD8-CD25+ thymocytes in patients with MG is similar to healthy newborn and young adult thymi. Freshly isolated human thymocytes from hyperplastic thymi of 15 patients with MG as well as the thymocytes from 10 young adult and 10 newborn thymi were analyzed by 3-color flow cytometry for the expression of CD4, CD8, and CD25. Each point represents 1 patient or 1 control. The bars represent mean values. The proportion of CD4+CD8- cells with CD25+ (A) as well as CD25hi (B) expression was calculated. The differences between newborns, adults, and patients with MG are not significant (NS).
Suppressive activity of myasthenic CD4+CD25+ thymocytes is severely impaired
CD4+CD25+ and CD4+CD25- thymocytes from patients and healthy subjects were purified (Figure 2A) and tested for their ability to proliferate in response to mitogenic stimulation in the presence of T cell–depleted PBMCs alone or in coculture. CD4+CD25+ newborn thymocytes were hyporesponsive to stimulation with PHA/mixed lymphocyte culture (MLC) and the proliferation of CD4+CD25+ adult thymocytes was also very low, representing up to 10% of CD4+CD25- cell response (Figure 2B). Unlike the healthy subjects, CD4+CD25+ thymocytes from patients with MG did not show the anergic phenotype of Treg cells and were able to proliferate, with a response of about 60% of the CD4+CD25- cell response to allogenic stimulation (Figure 2B). CD4+CD25+ newborn thymocytes suppressed the proliferation of the cocultured autologous CD4+CD25- population in a dose-dependent manner (Figure 2C) as previously reported.4,5 CD4+CD25+ adult thymocytes showed similar dose-dependent suppressive capacity with up to 80% inhibition of their CD4+CD25- counterparts' response at a 1:1 ratio (Figure 2C). In contrast to the normal thymus populations, the suppressive activity of myasthenic CD4+CD25+ thymocytes was severely reduced, at all CD4+CD25+/CD4+CD25- cell ratios tested (Figure 2C). Even at a 1:1 ratio, CD4+CD25+ thymocytes from patients with MG were unable to suppress the proliferation of their CD4+CD25- counterparts, whereas in healthy young adult subjects 10-fold fewer CD4+CD25+ thymocytes resulted in a 30% reduction of the CD4+CD25- cell response.
Myasthenic CD4+CD25+ thymocytes are responsive to mitogenic stimulation and their suppressive activity is impaired. (A) CD8-depleted thymocytes were purified by positive selection of CD4+ cells followed by positive or negative selection for CD25 expression using MACS magnetic microbeads. A representative experiment using newborn and myasthenic thymocytes is shown. (B) CD4+CD25+ thymocytes from 5 controls and 3 patients with MG were stimulated in the presence of irradiated allogenic T cell–depleted PBMCs and 5 μg/mL PHA. The results are expressed as the percent of proliferative response of CD4+CD25- thymocytes, with proliferation in the absence of CD4+CD25+ cells corresponding to 100%. Mean values (± SD) of triplicate wells are reported. (C) The CD4+CD25- thymocytes were stimulated in the presence of different doses of CD4+CD25+ thymocytes. Mean values (± SD) of 3 separate experiments are shown.
Myasthenic CD4+CD25+ thymocytes are responsive to mitogenic stimulation and their suppressive activity is impaired. (A) CD8-depleted thymocytes were purified by positive selection of CD4+ cells followed by positive or negative selection for CD25 expression using MACS magnetic microbeads. A representative experiment using newborn and myasthenic thymocytes is shown. (B) CD4+CD25+ thymocytes from 5 controls and 3 patients with MG were stimulated in the presence of irradiated allogenic T cell–depleted PBMCs and 5 μg/mL PHA. The results are expressed as the percent of proliferative response of CD4+CD25- thymocytes, with proliferation in the absence of CD4+CD25+ cells corresponding to 100%. Mean values (± SD) of triplicate wells are reported. (C) The CD4+CD25- thymocytes were stimulated in the presence of different doses of CD4+CD25+ thymocytes. Mean values (± SD) of 3 separate experiments are shown.
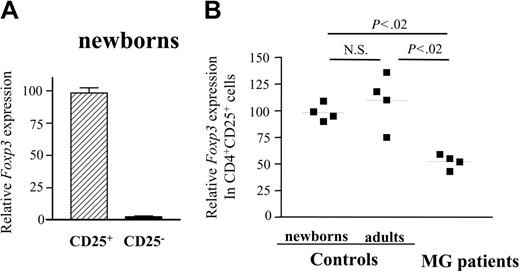
FOXP3 expression is decreased in CD4+CD25+ thymocytes of patients with MG
The transcription factor, FoxP3, was shown to be required for the suppressive activity of CD4+CD25+ regulatory cells in mice10-12 and the selective expression of FOXP3 was demonstrated in CD4+CD25+ cells from human thymi.13 To further evaluate possible alterations in expression of this factor in MG, we assessed FOXP3 mRNA expression in the CD4+CD25- and CD4+CD25+ thymocytes from patients with MG and healthy subjects using real-time quantitative PCR. Indeed, in normal newborn thymi, FOXP3 expression was confined to the CD4+CD25+ population, being 50-fold higher as compared to CD4+CD25- cells (Figure 3A). The amount of FOXP3 mRNA produced by CD4+CD25+ thymocytes from patients was 2-fold reduced (Figure 3B) compared to young adults and newborn CD4+CD25+ thymocytes, whereas their CD25- counterparts expressed similarly low levels of FOXP3 as the control CD25- cells (not shown). It is noteworthy that despite the partial expression of FOXP3 in MG CD4+CD25+ thymocytes, their suppressive activity was null (Figure 2C).
CD4+CD25+ thymocytes of patients with MG show decreased expression of Foxp3 mRNA. cDNA samples were obtained from CD4+CD25- and CD4+CD25+ thymocytes that had been purified using MACS magnetic microbeads and were subjected to real-time quantitative PCR using primers specific to FoxP3. The Foxp3 mRNA values were normalized to the 28S rRNA expression in each sample. (A) Quantification of relative Foxp3 mRNA levels for CD4+CD25- and CD4+CD25+ thymocytes from newborns. Error bars indicate SD value. (B) Quantification of relative Foxp3 mRNA levels in CD4+CD25+ thymocytes from newborns, young adults, and patients with MG.9. ▪ indicates 1 patient or 1 control. Bars represent mean values.
CD4+CD25+ thymocytes of patients with MG show decreased expression of Foxp3 mRNA. cDNA samples were obtained from CD4+CD25- and CD4+CD25+ thymocytes that had been purified using MACS magnetic microbeads and were subjected to real-time quantitative PCR using primers specific to FoxP3. The Foxp3 mRNA values were normalized to the 28S rRNA expression in each sample. (A) Quantification of relative Foxp3 mRNA levels for CD4+CD25- and CD4+CD25+ thymocytes from newborns. Error bars indicate SD value. (B) Quantification of relative Foxp3 mRNA levels in CD4+CD25+ thymocytes from newborns, young adults, and patients with MG.9. ▪ indicates 1 patient or 1 control. Bars represent mean values.
Localization of CD25+ cells in normal and hyperplastic thymi
The in vitro functional defect of thymic regulatory cells in MG suggests that despite the unchanged number of CD4+CD25+ cells in patients with MG, this subset could be altered either in number or function. To further investigate this cell population in patients, we localized the CD25-expressing cells in the thymus using immunohistochemical staining with anti-CD25 mAb. CD25-expressing cells were mainly detected in the medulla of newborn and young adult control thymus with minor localization to the cortex (Figure 4A-B). In myasthenic hyperplastic thymus, CD25-expressing cells were similarly found in the medulla (Figure 4C-D) but also in the areas surrounding the germinal centers (Figure 4C,E) with some cells located inside the lymphoid follicles (Figure 4E).
Distribution of CD25+ cells in normal and hyperplastic MG thymi. Frozen sections of normal (A-B) and hyperplastic MG (C-E) thymi were stained with anti-CD25 mAb (red color) and counterstained with Gill hematoxylin. The left photomicrographs (original magnification × 100) show that CD25 expression is observed in the medullary area of normal (A) and hyperplastic MG thymus (C-D) and, in addition, around the germinal centers of hyperplastic MG thymus (C,E). The right photomicrographs (original magnification × 400) are enlargements of the framed areas corresponding to medullary area of normal (B) and MG (D) thymus and germinal center of MG thymus (E).
Distribution of CD25+ cells in normal and hyperplastic MG thymi. Frozen sections of normal (A-B) and hyperplastic MG (C-E) thymi were stained with anti-CD25 mAb (red color) and counterstained with Gill hematoxylin. The left photomicrographs (original magnification × 100) show that CD25 expression is observed in the medullary area of normal (A) and hyperplastic MG thymus (C-D) and, in addition, around the germinal centers of hyperplastic MG thymus (C,E). The right photomicrographs (original magnification × 400) are enlargements of the framed areas corresponding to medullary area of normal (B) and MG (D) thymus and germinal center of MG thymus (E).
Although the expression of CD25 delineates a population of regulatory CD4+ T cells, it is also a marker of recent cellular activation. The localization of myasthenic CD25+ cells in the areas of antibody production involved in the pathologic process suggests that this population includes the conventional activated effector CD25+ T cells.
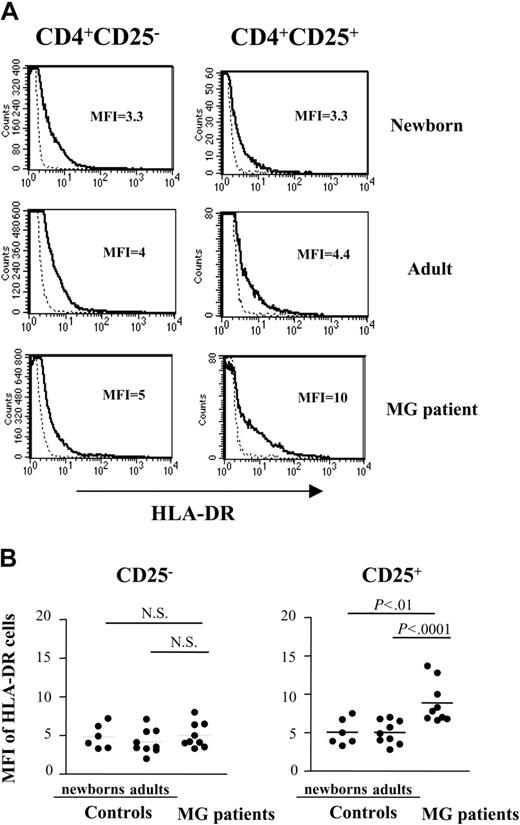
CD4+CD25+ thymocytes of patients with MG are enriched in HLA-DR and Fashi-expressing cells
Because the regulatory CD4+CD25+ cells share most activation/memory antigens with recently activated effector CD4+ T cells, it seems difficult to discriminate between these 2 cell subsets on the basis of surface marker expression. Nevertheless, several phenotypic markers have been used to identify the CD25+ regulatory cells.5-7,36 Therefore, we compared the expression of some of these markers on CD8-depleted, gated CD4+CD25-, and CD4+CD25+ thymocytes from patients versus those from healthy newborn and young adults subjects. TGF-β1, CD80 (B7-1), and CD86 (B7-2), the activation markers induced on regulatory CD4+CD25+ cells after in vitro activation,37 were not detected on freshly isolated CD4+CD25+ thymocytes from normal or MG samples. The expression of CD122 and CD71 was similar in patients and healthy subjects (not shown), and no surface expression of CTLA-4 was observed in either population (not shown). Finally, the frequency of CD4+CD25+ thymocytes expressing cytoplasmic CTLA-4 was moderately higher in newborns compared to patients and healthy adults, whereas there was no difference between patients and healthy young adults (not shown).
In contrast, CD4+CD25+ thymocytes from patients showed an increased expression of HLA-DR on the cell surface, compared to newborn and young adult cells, whereas there was no difference between CD4+CD25- thymocytes. The mean fluorescence intensity (MFI) values were significantly higher in patients with MG (8.9 ± 1.7) compared to healthy adults (5.0 ± 1.5, n = 9, P less than .0001) and newborns (5.1 ± 2.7, n = 6, P less than .001; Figure 5A-B).
Expression of HLA-DR is increased in CD4+CD25+ T cells from patients with MG. (A) Representative HLA-DR expression profile for newborn, young adult, and myasthenic CD8-depleted thymocytes stained with anti-HLA-DR, anti-CD25, anti-CD4 mAbs and analyzed by flow cytometry. HLA-DR expression is shown with a solid line and the staining with an isotype IgG control with a dotted line. (B) The percentage of HLA-DR was determined among CD4+CD25- and CD4+CD25+ cells in 9 MG patient, 9 young adult and 6 newborn thymi. • represents 1 patient or 1 control. Bars indicate mean values.
Expression of HLA-DR is increased in CD4+CD25+ T cells from patients with MG. (A) Representative HLA-DR expression profile for newborn, young adult, and myasthenic CD8-depleted thymocytes stained with anti-HLA-DR, anti-CD25, anti-CD4 mAbs and analyzed by flow cytometry. HLA-DR expression is shown with a solid line and the staining with an isotype IgG control with a dotted line. (B) The percentage of HLA-DR was determined among CD4+CD25- and CD4+CD25+ cells in 9 MG patient, 9 young adult and 6 newborn thymi. • represents 1 patient or 1 control. Bars indicate mean values.
We previously found that the apoptosis-regulated antigen, Fas (CD95), is overexpressed on autoreactive T cells from patients with MG.28 Therefore, we analyzed the surface expression of Fas on CD25+ cells. CD4+CD25- thymocytes expressed low levels of Fas in both patients and controls (Figure 6A-B). CD4+CD25+ thymocytes from healthy subjects mainly displayed low Fas expression with a small proportion of cells showing high Fas expression (Fashi; Figure 6A). The proportion of Fashi-expressing cells increased strikingly in myasthenic CD4+CD25+ thymocytes. Analysis of cells from several subjects confirmed the considerably higher Fashi frequency among the myasthenic CD4+CD25+ cells (55% ± 6.4% in patients versus 23% ± 4.5% in adults, P less than .0001; Figure 6B). Interestingly, the frequency of Fashi increased with age (23% ± 4.5% in young adults versus 10% ± 3.8% in newborns, P less than .0001).
CD4+CD25+ thymocytes from patients with MG are enriched in Fashi cells. (A) Representative Fas expression profile for newborn, young adult normal, and myasthenic CD8-depleted thymocytes stained with anti-CD25, anti-CD95, anti-CD4 mAbs and analyzed by flow cytometry. The IgG isotype control staining is shown with a dotted line. The percentage of Fashi cells is indicated above the brackets delimiting this cell subset. (B) The percentage of Fas expression was determined among CD4+CD25- and CD4+CD25+ cells in thymi from 11 patients with MG, 9 young adults, and 10 newborns.
CD4+CD25+ thymocytes from patients with MG are enriched in Fashi cells. (A) Representative Fas expression profile for newborn, young adult normal, and myasthenic CD8-depleted thymocytes stained with anti-CD25, anti-CD95, anti-CD4 mAbs and analyzed by flow cytometry. The IgG isotype control staining is shown with a dotted line. The percentage of Fashi cells is indicated above the brackets delimiting this cell subset. (B) The percentage of Fas expression was determined among CD4+CD25- and CD4+CD25+ cells in thymi from 11 patients with MG, 9 young adults, and 10 newborns.
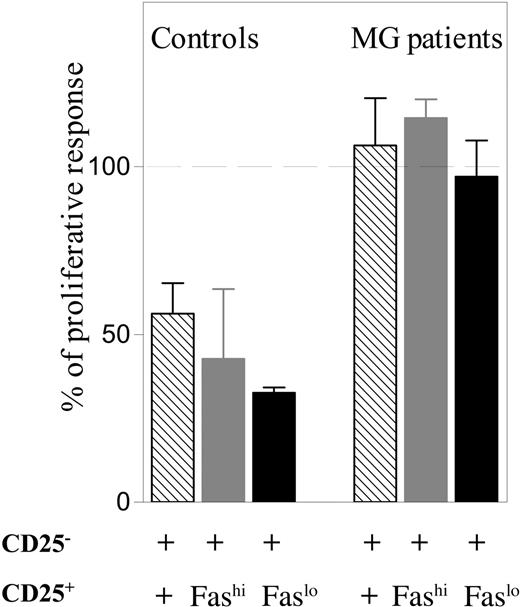
The suppressive function of CD25+Faslo thymocytes of patients with MG is severely impaired
Because the CD4+CD25+ population from myasthenic thymus contains a large proportion of Fashi cells, we wondered whether the defect in suppressive activity of CD25+ T cells was due to this population, which is thought to include activated effector cells. Therefore CD4+CD25+Faslo and CD4+CD25+Fashi cells from MG thymi were sorted and their suppressive activity was tested. We showed that not only did patient-derived CD4+CD25+Fashi cells lack suppressive activity, but also the CD4+CD25+Faslo population was unable to suppress the proliferation of CD4+CD25- cells, whereas both CD4+CD25+Fashi and CD4+CD25+Faslo populations from normal thymi suppressed the proliferation of their CD4+CD25- counterparts (Figure 7). In one of the controls, FOXP3 expression was tested by real-time PCR and was found to be highly expressed in both CD4+CD25+Fashi and CD4+CD25+Faslo populations (data not shown).
The suppressive function of both CD4+CD25+Faslo and CD4+CD25+Fashi thymocytes of patients with MG is severely impaired. CD4+CD25- T cells from MG or normal thymi were stimulated in the presence of irradiated allogenic T cell–depleted PBMCs in coculture with sorted autologous unfractionated CD4+CD25+ (▧) , CD4+CD25+Fashi (▦ ), or CD4+CD25+Faslo (▪) thymocytes at a ratio of 1:1. The results are expressed as a percentage of the proliferative response versus that obtained when adding the 2 separate populations (defined as 100%). The experiments were done with thymic cells from 2 patients with MG and 2 healthy controls. The results are expressed as the mean ± SEM of these 2 experiments.
The suppressive function of both CD4+CD25+Faslo and CD4+CD25+Fashi thymocytes of patients with MG is severely impaired. CD4+CD25- T cells from MG or normal thymi were stimulated in the presence of irradiated allogenic T cell–depleted PBMCs in coculture with sorted autologous unfractionated CD4+CD25+ (▧) , CD4+CD25+Fashi (▦ ), or CD4+CD25+Faslo (▪) thymocytes at a ratio of 1:1. The results are expressed as a percentage of the proliferative response versus that obtained when adding the 2 separate populations (defined as 100%). The experiments were done with thymic cells from 2 patients with MG and 2 healthy controls. The results are expressed as the mean ± SEM of these 2 experiments.
Thus, even in the absence of cells expressing high level of Fas, the suppressive function of the CD25+Faslo population in thymi from patients with MG is severely impaired.
Discussion
Thymus-derived CD4+CD25+ Treg cells inhibit the development of autoimmune disorders in rodent models and this regulatory cell subset has been identified in the thymus and peripheral blood of healthy humans. CD4+CD25+ Treg cells are naturally anergic and suppress the proliferation of their CD4+CD25- counterparts in a dose-dependent manner. Little is still known about the possible link between the defect of regulatory cell subset and the development of human autoimmune diseases, and the few published results are rather controversial.14-19
We proposed that a defect in the thymic regulatory function could contribute to the onset of MG, a T cell–dependent human autoimmune disease mediated by autoantibodies. We did not find any significant difference in number of CD4+CD8-CD25+ thymocytes from patients with MG versus that in healthy newborn and young adult controls. Despite the unchanged number/proportion of myasthenic CD4+CD25+ thymocytes, the functional properties of this population were significantly different from those of the CD4+CD25+ thymocytes from newborn and young adult thymi. Whereas this population from healthy subjects exhibited the anergic/suppressive phenotype of regulatory T cells, CD4+CD25+ thymocytes from patients with MG were responsive to mitogenic stimulation and their suppressive activity was severely impaired. Myasthenic CD4+CD25+ thymocytes were unable to suppress the proliferation of their CD4+CD25- counterparts even at a ratio of 1:1.
The lack of suppressive capacity of CD4+CD25+ thymocytes from patients with MG may be explained either by a defect in functional activity of CD25+ Treg cells or by the presence of a high number of activated effector cells in the CD4+CD25+ population, or both. Indeed, the CD4+CD25+ cell population from patients with MG differed from CD4+CD25+ regulatory thymocytes from healthy subjects in several respects.
First, we detected the CD25+ myasthenic thymocytes not only in the medulla as in thymi of healthy subjects, but also in the areas surrounding the germinal centers of hyperplastic MG thymus. The thymus of patients with MG with follicular hyperplasia is a site of cellular activation containing activated anti-AChR autoreactive T cells together with activated B cells.25,28,29 The localization of myasthenic CD25+ thymocytes in the areas of antibody production together with the lack of suppressive activity indicates that this population includes activated effector T cells.
Second, myasthenic CD4+CD25+ thymocytes expressed higher levels of major histocompatibility complex (MHC) class II molecules (HLA-DR) and were enriched in cells with strong Fas expression compared to newborn and young adult healthy subjects. Up-regulation of expression of the apoptosis-related Fas protein is associated with peripheral CD4+ lymphocyte activation in vivo in patients with systemic lupus erythematosus and multiple sclerosis.38,39 Furthermore, in our previous study, we identified CD4+Fashi cells from patients with MG as autoreactive-activated T cells.28 Here we report that the frequency of CD4+CD25+Fashi cells was strikingly elevated (up to 55% of CD4+CD25+ cells) in the thymus of patients with MG, whereas the cells with high Fas expression constituted only 23% and 10% of CD4+CD25+ thymocytes from young adult and newborn thymi, respectively. Of note, the frequency of cells expressing the high level of Fas among the CD4+CD25+ population was also augmented in healthy young adults compared to newborns, which could reflect the accumulation of activated/memory T cells throughout adult life. Thus, the CD4+CD25+ population from MG thymus is heterogeneous and seems to contain many activated effector T cells.
Therefore, we hypothesized that the presence of Fashi cells with strong proliferative capacity could contribute to the lack of suppression by CD4+CD25+ cell subsets from MG thymi. To test this hypothesis, we purified CD4+CD25+Faslo and CD4+CD25+Fashi cells and showed that both populations from MG thymi were devoid of suppressive function, whereas the same purified populations from normal thymi display strong suppressive activity and express high levels of FOXP3. Thus, the functional defect of CD4+CD25+ T cells could not be explained by the presence of CD4+CD25+Fashi activated effector cells. Our results stress that both purified CD4+CD25+Faslo and CD4+CD25+Fashi cells have a defective suppressive activity, suggesting that MG is associated with a central defect in immune suppression.
Limited information is available on the role of cytokines in CD4+CD25+ Treg cell activity. IL-2 is required for the development and homeostasis of CD4+CD25+ Treg cells and their anergic state as well as suppressive capacity are abrogated in the presence of exogenous IL-2.3 It was also shown recently that CD4+CD25+ Treg cells lose their ability to suppress the proliferation of their CD4+CD25- counterparts in the presence of IL-6 together with an unknown factors.40 A possible reason for the lack of regulatory function of CD4+CD25+ thymocytes from patients with MG is that the cytokine environment in the hyperplastic myasthenic thymus is unfavorable for the suppressive activity of Treg cells. We have previously demonstrated the presence of cells producing proinflammatory cytokines such as IL-6 in hyperplastic thymus of patients with MG.41 Nevertheless, our results demonstrating that Treg cells remain nonfunctional even after their isolation from the context of a hyperplastic thymus do not support this hypothesis.
The expression of the transcription factor FoxP3 was shown to be necessary and sufficient for Treg cell generation and function in mice and in healthy humans.10-13 Mutations of the FOXP3 gene in humans result in syndromes characterized by immune dysregulation, polyendocrinopathy, enteropathy, and X-linked syndrome (IPEX), which presents early in life with multiorgan autoimmunity and allergic inflammation.42-44 We demonstrate here that FOXP3 expression was decreased 2-fold in myasthenic CD4+CD25+ thymocytes. The impaired suppressive activity of CD4+CD25+ T cells together with the decreased level of FOXP3 in patients with MG could be due to a mutation of FOXP3 or to a polymorphism in the promoter region of FOXP3 gene. Interestingly, a polymorphism in this region, associated with a significant difference in the enhancer activity between distinct alleles, has been recently reported in patients with autoimmune diabetes.45
In conclusion, we demonstrate for the first time a functional defect of regulatory thymic cells that could explain the loss of self-tolerance in human autoimmune MG. Multiple factors contribute to the induction of MG pathogenesis and the defective suppressive function of CD4+CD25+ Treg cells could play an essential role in the onset or maintenance of this autoimmune disease.
Prepublished online as Blood First Edition Paper, September 28, 2004; DOI 10.1182/blood-2003-11-3900.
Supported by grants from the National Institute of Health (NS39869) and the European Community (QLG1-CT-2001-01918).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Michel Hours for his professional help in cell sorting experiments and Shelley Schwarzbaum for editing the manuscript.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal