Abstract
CD4+CD25+ T regulatory (Treg) cells have been shown to critically regulate self and allograft tolerance in mice. Studies of human Treg cells have been hindered by low numbers present in peripheral blood and difficult purification. We found that cord blood was a superior source for Treg-cell isolation and cell line generation compared with adult blood. Cord blood CD4+CD25+ cells were readily purified and generated cell lines that consistently exhibited potent suppressor activity, with more than 95% suppression of allogeneic mixed lymphocyte reactions (MLRs) (29 of 30 donors). Cultured Treg cells blocked cytokine accumulation in MLRs, with a less robust inhibition of chemokine production. These cell lines uniformly expressed CD25, CD62L, CCR7, CD27, and intracellular cytotoxic T-lymphocyte antigen-4 (CTLA4). FoxP3 protein, but not mRNA, was specifically expressed. Upon restimulation with anti-CD3/CD28 beads, the cultured Treg cells produced minimal cytokines (interleukin-2 [IL-2], interferon-γ [IFN-γ], and IL-10) and preferentially expressed tumor growth factor-β (TGF-β) latency associated protein. Cytokine production, however, was restored to normal levels by restimulation with phorbol myristate acetate (PMA)/ionomycin. Cord blood–derived cultured suppressor cell function was predominantly independent of IL-10 and TGF-β. These results demonstrate cord blood contains a significant number of Treg precursor cells capable of potent suppressor function after culture activation. Banked cord blood specimens may serve as a readily available source of Treg cells for immunotherapy.
Introduction
Naturally arising CD4+CD25+ T regulatory (Treg) cells can restrict or alter most types of immune responses.1 Initially they were described to be critical for the control of autoimmunity2,3 and were found on adoptive transfer to prevent experimental autoimmune diseases. More recently, Treg cells have been shown to suppress allogeneic immune responses and can prevent transplant rejection.4,5 In addition, these cells can restrain antitumor6,7 and antimicrobial immune responses.8 Thus, CD4+CD25+ Treg cells appear to be central control elements of immunoregulation, and understanding their biology will be important for efforts aimed at therapeutically manipulating immune responses.
Treg cells are best characterized in mice, where they constitute 5% to 10% of lymph node and spleen CD4+ T-cell populations. They are generated both through central thymic developmental mechanisms in pathogen-free mice and also arise by poorly defined peripheral generation or expansion mechanisms.9,10 To date they have primarily been defined by coexpression of CD4+ and CD25+ antigens on fresh isolation. CD25 as well as other markers of murine Treg cells, cytotoxic T-lymphocyte antigen-4 (CTLA4 [CD152]) and glucocorticoid-induced tumor necrosis factor (TNF)–like receptor (GITR), are all activation antigens on conventional T cells and therefore are not specific. FoxP3, a nuclear protein thought to function as a transcriptional repressor, is a newer marker considered to be more specific for Treg cells.11 It has been demonstrated that after activation (T-cell receptor–based, antigen-specific, or anti-CD3), Treg cells can nonspecifically suppress proliferation of both CD4+ and CD8+ T cells. The mechanism of suppression is unclear and in vitro appears to require cell-cell contact. A functional result of suppression is impaired production of interleukin-2 (IL-2).12,13 In vivo, the suppression mechanism is more controversial, with some studies showing dependence on immunosuppressive cytokines,14 which are not required for in vitro suppression.
Studies in mouse models of bone marrow transplantation (BMT) have shown that fresh or culture-expanded CD4+CD25+ cells can delay or prevent disease.15-17 Previous studies have shown that ex vivo polyclonally expanded Treg cells, with anti-CD3 plus IL-2 (for 10 days), can be effective in preventing graft versus host disease (GVHD).15 Ex vivo expansion of Treg cells with irradiated allogeneic antigen-presenting cells (APCs) plus exogenous IL-2 is also effective at suppressing GVHD.17 In some model systems, Treg cells can prevent GVHD and still allow for graft versus leukemia (GVL) effects.18-20 Consequently, Treg cells have a potential role in clinical immunosuppressive therapy in transplantation, provided human Treg cells could be isolated and expanded in culture to generate sufficient numbers for in vivo infusion.
While the murine data are very promising, there still remains a practical problem of isolating pure Treg cells from human blood. In young mice, CD4+CD25+ cells are moderately abundant and the CD25+ subset is readily apparent. In humans the CD25+ cells are not as discrete a population, because there exists a large and overlapping population of CD25dim cells. We hypothesize that this copurification (of conventional T cells with Treg cells) is the basis for the modest or variable suppressor activity observed in studies of human CD4+CD25+ cells.21 Fluorescence-activated cell sorting (FACS) of the highest 1.7% of CD25+ expressors (CD25high cells) has been reported to enable suppressor cell isolation.22 We have recently shown that a stringent magnetic bead–based approach was required to isolate populations of adult blood–derived Treg cells pure enough for CD4+CD25+ cells to generate potent suppressor cell lines. Even so, strongly suppressive cell lines could only be generated in a subset (approximately one third) of donors, and potency correlated with cell line purity.23 FACS sorting of CD25high cells (top 2.1%) has been reported to enable more consistent suppressor cell line generation from adult blood.24
It is unknown to what extent conventional activated T cells contribute to the CD4+CD25+ populations. The presence of memory T cells expressing CD25 at low to moderate levels (CD25dim cells) and conventional activated T cells (CD25+) that interfere with Treg-cell purification confounds human Treg-cell studies. However, both of these types of T cells (memory and activated) should be lacking from cord blood–derived cell populations, because cord blood cells develop in a protected environment and are immunologically naive. Therefore, we hypothesized that if cord blood contained CD25+ Treg cells, they should be more readily purified in comparison with adult blood.
Cord blood has previously been shown to contain CD4+CD25+ cells by FACS.25-27 However, minimal data are reported on the function of these cells. One report has inferred suppressive function based on limiting-dilution frequency analysis.26 The only report evaluating functional activity of freshly isolated CD4+CD25+ cells revealed no suppression of antigen-specific responses. In addition, there was no increased antigen-specific reactivity of CD4+ cells after CD25+ cell depletion. However, modest suppression was noted in anti-CD3–based T-cell coculture assays (60% at 1:1 responder-suppressor cell ratio).28 Thus, it was suggested that most cord blood–derived CD25+ cells were not yet mature enough to be suppressive.28
In the present studies, we were able to demonstrate that cord blood CD4+CD25+ cells can form potent suppressor cells after isolation and culture. A straightforward direct magnetic-activated cell separation (MACS)–based purification was sufficient for Treg-cell isolation from nearly every donor. After activation with anti-CD3/CD28 beads and culture in IL-2, cord blood–derived CD4+CD25+ cells acquired potent suppressor function, which was maintained for several weeks. We used these cell lines to better characterize CD4+CD25+ Treg-cell phenotype and function. These cell lines exhibit a profound bioactivity and form a reproducible model system for the study of Treg-cell biology.
Materials and methods
MACS purification of CD25+ and CD25-CD4+ T cells
CD25+ and CD25-CD4+ T cells were isolated from umbilical cord blood (Saint Louis Cord Blood Bank, St. Louis, MO, and Red Cross, Saint Paul, MN). Mononuclear cell preparations were prepared by centrifugation over Ficoll-Hypaque. After CD34+ depletion with magnetic microbeads (Miltenyi Biotec, Auburn, CA), CD25+ cells were isolated by positive selection with directly conjugated anti-CD25 magnetic microbeads (4 μL per 107 cells; Miltenyi Biotec). Cells were then applied to a second magnetic column, washed, and re-eluted. After the double column procedure, cells were routinely more than 90% pure (for CD4/CD25) by FACS analysis. The non-CD25 fraction was then applied to another magnetic column to deplete any remaining CD25+ cells before isolation of CD4+CD25- cells by positive selection with anti-CD4 monoclonal antibody (mAb)–coated microbeads (Miltenyi Biotec). Stringent purification of adult CD25+ cells used anti-CD25–fluorescein isothiocyanate (FITC) and anti-FITC microbeads (2 μL per 107 cells) and passage over magnetic column and elution for 2 cycles. This was followed by releasing the magnetic beads and subsequent lineage depletion with anti-CD8, CD14, CD19, and CD56 directly conjugated microbeads (CD4+CD25++ lineage- [CD4+CD25++lin-]).23
Culture of Treg cells
Isolated CD4+CD25+ cells or control CD4+CD25- cells were cultured as previously described23 with anti-CD3/CD28 mAb-coated dynabeads (University of Pennsylvania, Philadelphia)29 at a 3:1 bead-to-cell ratio. Cells were cultured at 1 × 106 total cells per milliliter in 24-well plates. IL-2 was added on day 3 at 50 IU/mL (Chiron, Emeryville, CA). In contrast to the prior study, all lines were cultured without feeder cells. For cord blood cultures, the addition of irradiated feeder cells (CD4+CD25- cells)23 was neither helpful for expansion nor preservation of suppressor function; therefore, feeders were omitted to simplify the culture protocol. Cell cultures were split as needed, approximately 1/3 every 3 days during the fast-growth phase. Culture media was RPMI 1640 (Invitrogen-Gibco, Carlsbad, CA) supplemented with 10% fetal calf serum (FCS) (Invitrogen-Gibco), l-glutamine, penicillin, and streptomycin.
Responder and stimulator cells for MLR cultures
CD4+CD25- responder T cells were isolated from buffy coat preparations derived from the whole blood of healthy volunteer donors (Memorial Blood Centers, Minneapolis, MN). Cells were centrifuged over Ficoll-Hypaque to collect peripheral blood mononuclear cells (PBMCs). PBMCs were first depleted of CD25+ cells with anti-CD25 mAb-coated microbeads (Miltenyi Biotec) before CD4+ T cells were isolated by positive selection with anti-CD4 mAb-coated magnetic microbeads (Miltenyi Biotec). Cells were routinely 96% to 98% pure by FACS analysis. Immature dendritic cells (DCs) were generated from CD14+ monocytes,30 isolated from PBMCs by magnetic bead–based purification (Miltenyi Biotec), and cultured in X-vivo-15 (BioWhittaker, Walkersville, MD) media at 1 × 106 cells per milliliter supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF) (50 ng/mL final) and IL-4 (20 ng/mL final) (both cytokines from R&D Systems, Minneapolis, MN). Cells were cultured for 5 to 10 days before use as stimulators in mixed lymphocyte reactions (MLRs). For some experiments, DCs were matured with lipopolysaccharide (LPS) (Sigma, St Louis, MO) (100 ng/mL) or a combination of TNF-α (20 ng/mL final) and poly I:C, a Toll-like receptor-3 (TLR-3) agonist ligand (20 μg/mL final) (Sigma) for 2 days.31,32 DC stimulators were irradiated at 30 Gy.
MLR assay culture
A total of 5 × 104 responding CD4+CD25- T cells and 5 × 103 DC stimulator APCs were cultured per well in 96-well U-bottom plates. Cultured suppressor or conventional T-cell lines were added at 2.5 × 104 per well for standard assays or in graded numbers for titration experiments. For antibody-blocking experiments, 104 or 5 × 103 suppressor cells were used. Culture media was RPMI 1640 supplemented with 10% FCS. Wells were pulsed on days 3, 5, 6, and 7 with 3H-thymidine for the last 16 hours of culture. Each time point had 6 replicates. Results were expressed in counts per minute. Data were collected with a direct β counter (no liquid scintillation); thus, the magnitude of the results was lower but proportionally correct.
Real-time PCR
Total RNA was extracted using TRI-reagent (Molecular Research Center, Cincinnati, OH) or RNAeasy (Qiagen, Valencia, CA). Complementary DNA was synthesized from 1 μg of each RNA sample using Taqman universal master mix (Applied Biosystems, Foster City, CA); 10 ng was used in each quantitative polymerase chain reaction (qPCR). All samples were run in duplicates. Primers and probes for FoxP3 and cyclophilin A were purchased from Applied Biosystems. Real-time PCR was performed using the ABI Prism 7900 (Applied Biosystems). FoxP3 message levels were determined after normalizing data to cyclophilin A.
Western blotting
Nuclear extracts were prepared (Active Motif, Carlsbad, CA), and 70 μg protein was loaded per lane. Samples were run on NuPage 10% Bis-Tris minigels (Invitrogen, San Diego, CA). Proteins were transferred to polyvinylidene fluoride (PVDF) membranes and incubated with goat anti-FoxP3 antibody (AB2481) (Abcam, Cambridge, MA), followed by rabbit antigoat immunoglobulin G (IgG) horseradish peroxidase. Blots were developed with SuperSignal WestPico Chemiluminescense substrate (Pierce, Rockford, IL).
Cytokine analysis
MLR culture supernatants were spun free of cells, and aliquots were frozen at -80°C. For restimulations, anti-CD3/CD28 beads were used at 1:1 bead to cell ratio, or phorbol myristate acetate (PMA) at 2 ng/mL and ionomycin at 500 ng/mL were used (Sigma). Cells were cultured at 1 × 106/mL media in 24-well plates. Supernatants were evaluated by the Luminex assay system with a latex bead–based multianalyte system (R&D Systems).
Monoclonal antibodies
To evaluate purification, cells were stained with anti-CD25–phycoerythrin (PE) (clone M-A251) (BD Pharmingen, San Diego, CA), which is not blocked by anti-CD25 microbeads. Other antibodies for flow cytometry included anti-CD4–peridinin chlorophyll protein (PerCP) (clone SK3) (Becton Dickinson Immunocytometry Systems, San Jose, CA); anti-CD152–PE (BNI3), anti-CD27–FITC (M-T271), anti-CD62L–allophycocyanin (Dreg56), anti-CD69–FITC (FN50), anti-CD134 (ACT35) (BD Pharmingen); and anti-GITR–PE (110416) and biotinylated anti–latency associated protein (anti-LAP) (27240) (R&D Systems). In functional experiments designed for blocking suppression, neutralizing antibodies were used at 10 μg/mL. Antibodies included anti-CTLA4 (BNI3) (BD Pharmingen); anti-PD1 (J116) (eBioscience, San Diego, CA); anti-OX40 (L106) (Becton Dickinson); and anti-GITR (MAB689), anti-GITR ligand (anti–GITR-L) (MAB6941), anti-OX40L (MAB10541), anti–IL-10 (MAB217), anti–IL-10 receptorα (MAB274), anti-TGFβ-1,2,3 (1D11), anti-TGFβ-1 (MAB1835), polyclonal chicken anti-TGFβ-1/1.2 (AF-101-NA), polyclonal goat anti-TGFβRII (AF-241-NA), anti-LAP (MAB246), polyclonal goat anti-LAP (AF-246-NA), recombinant LAP (246-LP), and recombinant TGFβRII-Ig (1003-RT) (R&D Systems).
Flow cytometry
For immunofluorescence staining, cells were stained for 30 minutes at 4°C. Cells were washed and run on a FACSCalibur cytometer (Becton Dickinson). Data were analyzed by FlowJo software version 4.5 (Treestar, Ashland, OR). Intracellular staining was done using 2% paraformaldehyde-fixed cells, followed by permeabilization and staining in 0.1% saponin-containing buffer.
Statistics
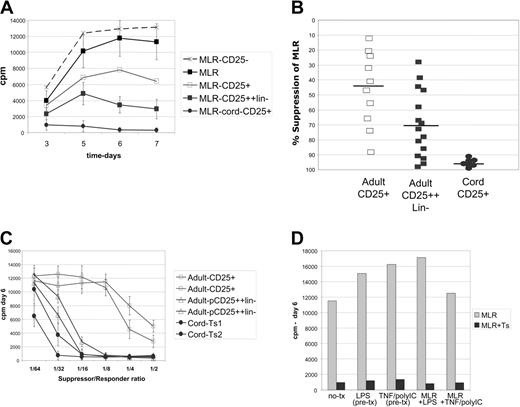
Cultured cord blood–derived CD4+CD25+ cells markedly suppress MLRs. Analysis of suppressor cell function in MLR assays by proliferative inhibition. (A) Kinetic curves of proliferation over a 1-week MLR. Control MLR (▴) cord blood–derived cells essentially block MLRs (•); directly MACS-selected adult cell lines had weak suppressor function (□); and stringently selected adult cells (CD25++lineage-) had moderate potency (▪). CD25- cells are not suppressive (▵). Results representative of 10 experiments. Cultures are pulsed daily with 3H-thymidine for the last 16 hours of culture. (B) Scatter plot showing the consistency of suppression (at day 6 of MLR) of individual cell lines; cord blood–derived (•) versus direct MACS adult cell lines (□) or stringently purified adult lines (CD25++lineage-) (▪). (C) Graded numbers of cultured Treg cells were added to the MLR reaction to determine the minimum number needed for potent inhibition. Up to a 1:32 dilution (roughly 1560 suppressors per 50 000 responders) still would markedly impair MLRs when using cord blood–derived suppressor cell lines (•), versus 1:16 for a selected potent subset of stringently purified adult lines (pCD25++lin-) (▵). Two lines each are shown, representative of 6 adult- and cord blood–derived suppressor cell lines. (D) Maturation of DCs prior to MLR, by LPS or TNF–poly I:C combination, or inclusion of these stimulating factors in the MLR fails to bypass suppression. Representative of 3 experiments.
Cultured cord blood–derived CD4+CD25+ cells markedly suppress MLRs. Analysis of suppressor cell function in MLR assays by proliferative inhibition. (A) Kinetic curves of proliferation over a 1-week MLR. Control MLR (▴) cord blood–derived cells essentially block MLRs (•); directly MACS-selected adult cell lines had weak suppressor function (□); and stringently selected adult cells (CD25++lineage-) had moderate potency (▪). CD25- cells are not suppressive (▵). Results representative of 10 experiments. Cultures are pulsed daily with 3H-thymidine for the last 16 hours of culture. (B) Scatter plot showing the consistency of suppression (at day 6 of MLR) of individual cell lines; cord blood–derived (•) versus direct MACS adult cell lines (□) or stringently purified adult lines (CD25++lineage-) (▪). (C) Graded numbers of cultured Treg cells were added to the MLR reaction to determine the minimum number needed for potent inhibition. Up to a 1:32 dilution (roughly 1560 suppressors per 50 000 responders) still would markedly impair MLRs when using cord blood–derived suppressor cell lines (•), versus 1:16 for a selected potent subset of stringently purified adult lines (pCD25++lin-) (▵). Two lines each are shown, representative of 6 adult- and cord blood–derived suppressor cell lines. (D) Maturation of DCs prior to MLR, by LPS or TNF–poly I:C combination, or inclusion of these stimulating factors in the MLR fails to bypass suppression. Representative of 3 experiments.
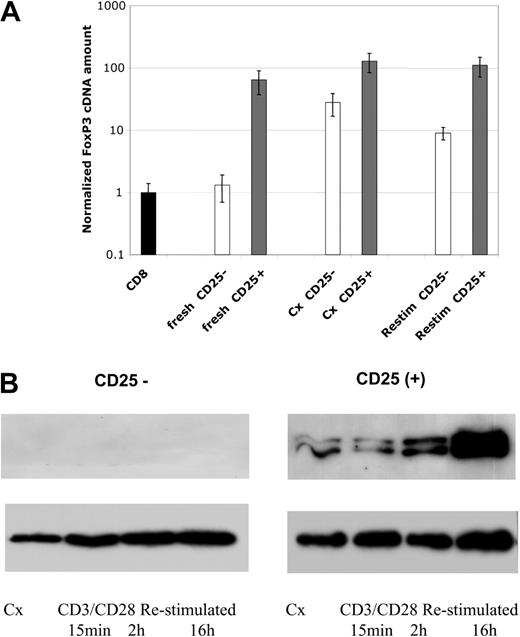
FoxP3 mRNA and protein expression. (A) Levels of FoxP3 mRNA were assessed by real-time PCR analysis. Samples include freshly isolated cells, cell lines after 4 weeks of culture, and 4 week–cultured cell lines 24 hours after restimulation with anti-CD3/CD28 beads, as indicated. Data are plotted as fold comparison to mRNA levels present in freshly isolated CD8+ T cells. CD4+CD25--derived cells and lines are indicated by blue bars and CD4+CD25+ cells and lines by burgundy bars. (B) Western blot analysis of FoxP3 protein expression in cultured cells. Cell lines were cultured for 4 weeks and restimulated with anti-CD3/CD28 beads for 15 minutes, 2 hours, or overnight. The level of histone H3 was used as a loading control for nuclear proteins. Results representative of 4 independent experiments.
FoxP3 mRNA and protein expression. (A) Levels of FoxP3 mRNA were assessed by real-time PCR analysis. Samples include freshly isolated cells, cell lines after 4 weeks of culture, and 4 week–cultured cell lines 24 hours after restimulation with anti-CD3/CD28 beads, as indicated. Data are plotted as fold comparison to mRNA levels present in freshly isolated CD8+ T cells. CD4+CD25--derived cells and lines are indicated by blue bars and CD4+CD25+ cells and lines by burgundy bars. (B) Western blot analysis of FoxP3 protein expression in cultured cells. Cell lines were cultured for 4 weeks and restimulated with anti-CD3/CD28 beads for 15 minutes, 2 hours, or overnight. The level of histone H3 was used as a loading control for nuclear proteins. Results representative of 4 independent experiments.
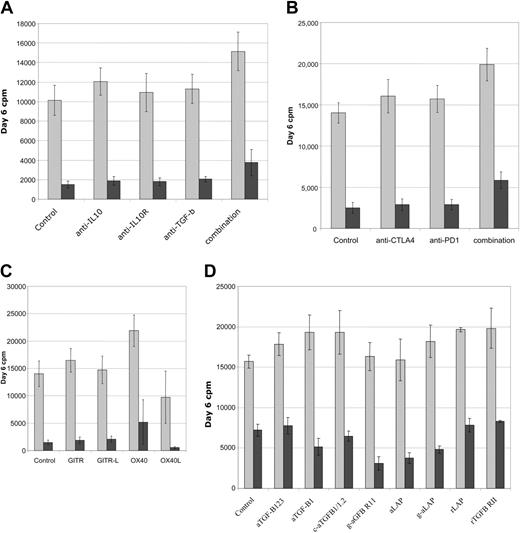
Functional analysis of suppression in MLRs. Multiple neutralizing antibodies and fusion proteins were screened for functional effects on suppression in CD4+ T-cell DC-MLR. (A) Antibodies to immunosuppressive factors IL-10 and TGF-β as well as anti–IL-10R or combinations of all 3 fail to reverse suppression mediated by cultured Treg cell lines. (B) Antibodies to negative signaling molecules, CTLA4 and PD1, do not reverse suppression. (C) Antibodies to GITR or GITR-L do not effect suppression. Agonist antibodies to OX40 partially reverse suppression while antibody to OX40L partially enhances suppression. (D) Multiple TGF-β–neutralizing antibodies and soluble receptors do not prevent suppressor cell effector function. Treated MLRs are indicated by gray bars and treated MLRs plus Ts cultures by burgundy bars. Representative of 6 independent experiments for panels A-C and 3 independent experiments for panel D.
Functional analysis of suppression in MLRs. Multiple neutralizing antibodies and fusion proteins were screened for functional effects on suppression in CD4+ T-cell DC-MLR. (A) Antibodies to immunosuppressive factors IL-10 and TGF-β as well as anti–IL-10R or combinations of all 3 fail to reverse suppression mediated by cultured Treg cell lines. (B) Antibodies to negative signaling molecules, CTLA4 and PD1, do not reverse suppression. (C) Antibodies to GITR or GITR-L do not effect suppression. Agonist antibodies to OX40 partially reverse suppression while antibody to OX40L partially enhances suppression. (D) Multiple TGF-β–neutralizing antibodies and soluble receptors do not prevent suppressor cell effector function. Treated MLRs are indicated by gray bars and treated MLRs plus Ts cultures by burgundy bars. Representative of 6 independent experiments for panels A-C and 3 independent experiments for panel D.
Results
Cord blood contains a distinct population of CD4+CD25+ cells
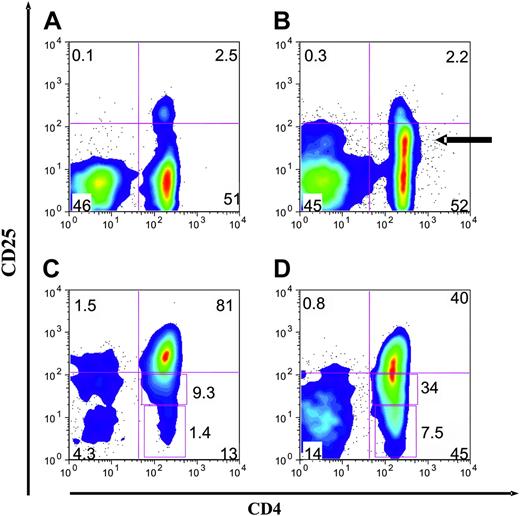
Because cord blood mononuclear cells (CBMCs) have been shown to contain CD4+ T cells that coexpress CD25, we sought to evaluate the potential of these cells for suppressor function and suppressor cell line generation. By FACS analysis we confirmed that CBMCs contain a significant population of CD4+ T cells that coexpress CD25+25-27 and, more important, that this was a discrete population27 (Figure 1A). In contrast, adult blood contains CD4+ T cells with a broad spectrum of levels of CD25, including a large population of nonsuppressive CD25dim cells (Figure 1B). Approximately 5% of the cord blood CD4+ T cells distinctly expressed CD25+ (mean, 5.2%; range, 2.3% to 9.5%; n = 20), a slightly higher percentage than present in adult peripheral blood CD4+ T cells (mean, 3.8% of CD4+).23,24 For direct comparison of purification, CD25+ Treg cells were isolated from both adult and cord blood using an identical MACS-based protocol (direct anti-CD25–based selection). The CD25+ cells purified from cord blood contained a more focused population of CD25+bright cells (mean fluorescence intensity [MFI], 320 versus 130) and fewer CD25dim or CD25- cells (Figure 1C); mean, 9% (range, 5% to 21%; n = 10) and 3% (range, 1% to 9%; n = 10), respectively. In comparison, the CD25+ cells derived from adult blood were found to contain more CD25dim and CD25- cells (Figure 1D); mean, 30% (range, 25% to 38%; n = 10) and 10% (range, 2% to 24%; n = 10), respectively.
Cord blood CD4+CD25+ cells are a distinct population. Representative 2-color FACS plots of PBMCs, CBMCs, and purified CD4+CD25+ cells from both adult and cord blood sources. (A) Examination of cord blood reveals a distinct population of CD4+ and CD25+ cells. There is a wide separation of the CD25+ cells from the CD25- cells. (B) Examination of peripheral blood reveals CD4+CD25+ cells constitute 1% to 2% of PBMCs. There are many CD25dim cells (arrow). (C) CD25+ cells purified from cord blood by direct anti-CD25 microbeads are a more pure population. (D) CD25+ cells purified from adult blood by direct anti-CD25 microbeads. Representative of 10 donors and 10 cell purification experiments.
Cord blood CD4+CD25+ cells are a distinct population. Representative 2-color FACS plots of PBMCs, CBMCs, and purified CD4+CD25+ cells from both adult and cord blood sources. (A) Examination of cord blood reveals a distinct population of CD4+ and CD25+ cells. There is a wide separation of the CD25+ cells from the CD25- cells. (B) Examination of peripheral blood reveals CD4+CD25+ cells constitute 1% to 2% of PBMCs. There are many CD25dim cells (arrow). (C) CD25+ cells purified from cord blood by direct anti-CD25 microbeads are a more pure population. (D) CD25+ cells purified from adult blood by direct anti-CD25 microbeads. Representative of 10 donors and 10 cell purification experiments.
MACS-selected cord blood CD4+CD25+-derived cell lines consistently have potent suppressor function
We and others have previously reported that stimulation with anti-CD3/CD28–coated beads and supplementation with IL-2 induced significant expansion of stringently purified adult-derived CD25+ Treg cells.23,24 Thus, we used this culture strategy to generate cell lines from the purified adult or cord blood CD4+CD25+ cells as well as cord blood CD4+CD25- cells for comparison. The cord blood–derived CD4+CD25+ cells expanded readily in culture—approximately 100-fold over 3 weeks with a single initial stimulation. After 3 to 4 weeks, the cell lines stopped expanding in number and were maintained in IL-2. Thus, the growth curves of these cell lines were similar to those of the adult blood–derived CD4+CD25+ cell lines.23
To evaluate suppressor function of the cell lines, we utilized an HLA-mismatched allo-MLR assay as a functional readout. The CD4+ responder and DC-stimulated MLR assays are very robust and consistent among donors and therefore served as our standard measure of suppression.23 All cell lines were initially screened for suppressor activity in MLRs after 2 to 3 weeks of culture and then further analyzed over the next 3 to 4 weeks. Strikingly, the cell lines derived from cord blood CD25+ cells were consistently and potently suppressive (Figure 2A). Potent suppressive cell lines were isolated from 29 of 30 donors, where inhibition of proliferation was typically more than 95% (Figure 2B). Control cell lines derived from adult or cord blood CD25- cells were not suppressive (Figure 2A). Cell lines derived from adult CD25+ cells (directly isolated) manifested weak and variable suppressive activity (Figure 2A and B). We previously reported that stringent MACS-based selection was required to generate significant suppressor cell lines potent in a subset of adult donors.23 The suppression mediated by these lines (CD25++lineage-) is also shown (Figure 2A and B). This plot shows the remarkable consistency of the cord blood–derived cell lines.
To further evaluate suppression potency, titration experiments were undertaken. Lowering the number of suppressors added to standard DC-stimulated MLR cultures revealed that nearly full inhibitory activity of cord blood–derived suppressors was maintained out to a ratio of 1:16 or 1:32 (as few as 1500 suppressors to 50 000 responders). This is slightly more potent (about 2-fold) than the selected most potent adult-derived cell lines (pCD25+lin-) (Figure 2C). Cell lines derived from directly selected adult CD25+ cells were poorly suppressive upon titration.
As a further assessment of potency, suppressor cell lines were evaluated in MLRs where the DC stimulators had been matured. Activation/maturation of DCs with lipopolysaccharide (LPS) (TLR-4 ligand) or the combination of TNF and poly I:C (double-stranded RNA analog: TLR-3 ligand)33 did not lead to bypass of suppression (Figure 2D). In addition, inclusion of LPS or TNF–poly I:C in the MLR culture also did not bypass suppression. Thus, the cord-derived Treg cells were as potent as the best-selected adult-derived cell lines,23 and activated DCs, which abundantly express costimulatory molecules and cytokines, were not able to bypass their suppressive effect.
Cord blood suppressor cells impair cytokine production with less of an effect on chemokine production
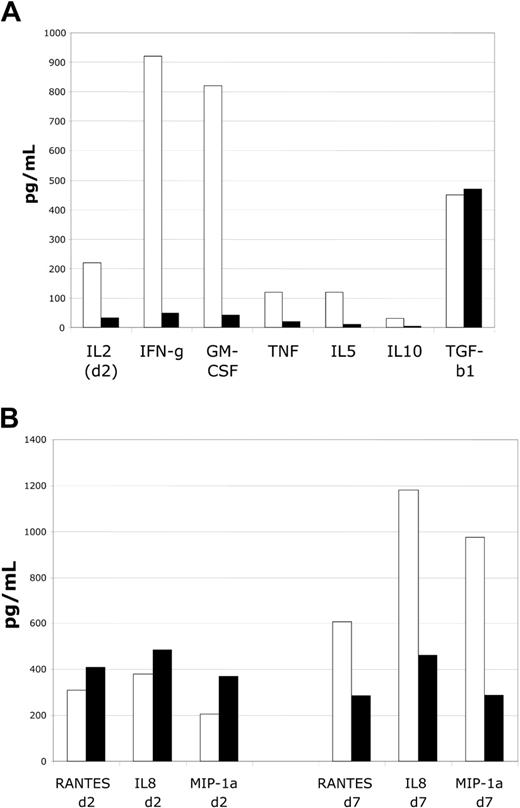
To determine the effects of the suppressor cell lines on responder T cells in MLR assays, we evaluated supernatants for the presence and magnitude of multiple cytokines. Suppressor cell addition to MLR assays markedly reduced accumulation of T-cell activation–dependent cytokines, including IL-2, interferon-γ (IFN-γ), GM-CSF, TNF-α, and IL-10. Importantly, levels of TGFβ-1 accumulation did not seem to be altered. Activation-dependent cytokine accumulation was minimal at all time points during the suppressed MLR, with results at time of peak detection in control DC-MLR shown (Figure 3A). These data suggest a marked impairment of T-cell activation. To determine the effects of suppression on selected chemokine expression, MLR supernantants were evaluated for the expression of IL-8 (CXCL8), macrophage inflammatory protein-1α (MIP-1α) (CCL3), and RANTES (regulated on activation normal T cells expressed and secreted) (CCL5)— proinflammatory chemokines. At early time points no effect on accumulation was noted (Figure 3B). However, at late time points in MLRs, accumulation was diminished by approximately by 50% to 75%. Thus, chemokine production appears to be less affected by suppression, and there is some degree of selectivity in the effects of suppressor cells.
Cultured CD4+CD25+ cells markedly suppress cytokine accumulation in MLRs. Analysis of suppressor cell function in MLR assays by assessment of cytokine levels. (A) Assessment of cytokines produced by activated T cells reveals marked impairment of accumulation. Inhibition of IL-2, IFN-γ, GM-CSF, TNF-α, IL-5, and IL-10 is observed. No alteration in TGF-β1 accumulation is noted. Shown are IL-2 levels on day 2 and other cytokines on day 6, respective times of peak of accumulation in control MLR cultures. (B) Assessment of chemokine levels reveals minimal alterations at early time points (day 2) and modest decrements in levels at late time points (day 7) for RANTES, IL-8, and MIP-1α. Representative of 4 MLR experiments.
Cultured CD4+CD25+ cells markedly suppress cytokine accumulation in MLRs. Analysis of suppressor cell function in MLR assays by assessment of cytokine levels. (A) Assessment of cytokines produced by activated T cells reveals marked impairment of accumulation. Inhibition of IL-2, IFN-γ, GM-CSF, TNF-α, IL-5, and IL-10 is observed. No alteration in TGF-β1 accumulation is noted. Shown are IL-2 levels on day 2 and other cytokines on day 6, respective times of peak of accumulation in control MLR cultures. (B) Assessment of chemokine levels reveals minimal alterations at early time points (day 2) and modest decrements in levels at late time points (day 7) for RANTES, IL-8, and MIP-1α. Representative of 4 MLR experiments.
Phenotype and FoxP3 expression of cord blood–derived suppressor lines
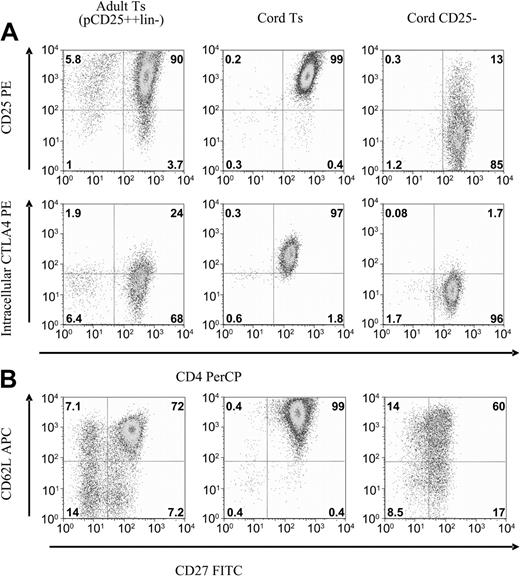
Cell lines were analyzed by flow cytometry for antigens associated with suppressor cell phenotype. Suppressor cell lines were cultured in parallel with CD4+CD25--derived cell lines, which served as conventional T-cell controls. Cell lines were evaluated after 3 weeks of culture, at which time the bead-based activation had nearly resolved and the cells had returned to a more quiescent state. The cell lines maintained a relatively stable phenotype and function for the next 3 to 5 weeks. The cord-derived CD25+ cell lines maintain remarkably uniform expression of multiple antigens, including CD25, intracellular CTLA4, CD27, and CD62L (Figure 4A). In comparison, the best adult-derived suppressor cell lines, generated by the most stringent purification, were slightly heterogeneous for these antigens (Figure 4).
Flow cytometric comparison of CD25+ versus CD25- cell lines. After 3 to 4 weeks of culture expansion, antigen expression was profiled by FACS. Shown are representative plots of CD25+-derived suppressor cell lines compared with CD25--derived cell lines. Also compared are potent adult-derived cell lines. (A) CD25 and intracellular CTLA4 expression remains high on cord T suppressor line, less so for the adult-derived Ts, and largely returns to baseline for the CD25--derived cell lines. (B) CD62L and CD27 expression remains uniformly high on cord Ts lines, on a subset of the adult lines, and diminishes on the CD25--derived cell lines. Representative of 10 cell lines each, analyzed at 4 weeks of culture.
Flow cytometric comparison of CD25+ versus CD25- cell lines. After 3 to 4 weeks of culture expansion, antigen expression was profiled by FACS. Shown are representative plots of CD25+-derived suppressor cell lines compared with CD25--derived cell lines. Also compared are potent adult-derived cell lines. (A) CD25 and intracellular CTLA4 expression remains high on cord T suppressor line, less so for the adult-derived Ts, and largely returns to baseline for the CD25--derived cell lines. (B) CD62L and CD27 expression remains uniformly high on cord Ts lines, on a subset of the adult lines, and diminishes on the CD25--derived cell lines. Representative of 10 cell lines each, analyzed at 4 weeks of culture.
The cord-derived CD25+ cell lines maintained high expression of cell surface CD25 and intracellular CTLA4, an expression pattern considered typical of the Treg-cell phenotype (Figure 4A). This occurs despite the fact that other activation antigens such as CD69, CD71, and OX40 have returned to baseline expression. The cord-derived suppressor cell lines also uniformly express both CD62L and CD27 (Figure 4B). We have previously shown that, within potent adult-derived suppressor cell lines, the cells with suppressor function reside within this double-positive subset.23 CD25-derived cells lines also maintain CD27 expression but at a lower level than CD25+-derived cell lines. This CD27dim expression pattern was also noted with adult-derived CD25-derived cell lines.23
Expression of the transcription factor FoxP3 has been proposed to be the most specific marker for regulatory cells in mice.11 We found FoxP3 message to be more highly expressed in CD25+ cells and lines as compared with CD25- T cells and lines. Freshly isolated CD25+ cells from cord blood contained approximately 64-fold more message than CD4+CD25- cells or fresh CD8+ T cells (Figure 5A). Cultured CD25+-derived cell lines contained 2- to 4-fold more message than freshly isolated CD25+ cells. Although message levels are low in CD25- cells on isolation, they increase approximately 25- to 30-fold on culture. This occurred even with exhaustive depletion of CD25dim/+ cells prior to culture (Figure 5A). Further restimulation of the 3- to 6-week-old cell cultures with anti-CD3/CD28 beads did not increase message expression but, rather, slightly decreased it in both types of cell lines.
In contrast to the data with mRNA message levels, Western blotting with a polyclonal antibody revealed FoxP3 protein expression to be primarily expressed in the CD25+-derived cell lines. Despite expressing message, the CD25--derived cell lines expressed minimal background levels of FoxP3 protein (Figure 5B). Importantly, restimulation of the CD25+ cell lines markedly increased FoxP3 protein expression. The increase in FoxP3 protein level occurred despite the minimal change (actual decrease) in message levels. Restimulation of CD25- cells still did not induce FoxP3 protein in CD25-derived cell lines.
Reactivation of suppressor cell lines induces minimal cytokine production and enhanced surface TGF-β LAP expression
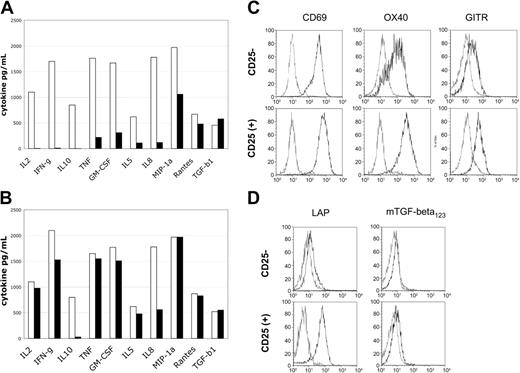
To determine the functional capabilities of the suppressive versus conventional T-cell lines, we evaluated their potential for cytokine production and cell surface molecule expression after restimulation. Cell lines were restimulated with anti-CD3/CD28 beads for potent reactivation and supernatants harvested at defined time points for analysis of cytokine content by Luminex bead-based assay. The CD25+-derived cell lines produced essentially no IL-2, IFN-γ, or IL-10 (Figure 6A), while control CD25-derived cell lines produced high levels of these cytokines. The accumulation of TNF, GM-CSF, IL-5, and the chemokine IL-8 was also markedly reduced as compared with control cell lines (Figure 6A). In contrast, the accumulation of transforming growth factor-β (TGF-β) and the chemokines MIP-1α and RANTES was not significantly different between different cell lines.
Restimulation of suppressor lines reveals cytokine production defects and cell surface LAP expression. Levels of cytokine produced in supernatants 48 hours after restimulation. (A) Cell lines were reactivated with anti-CD3/CD28 beads. (B) Cells were reactivated with PMA/ionomycin. Control CD25--derived cell lines are indicated by blue bars and CD25+-derived cell lines by burgundy bars. Cell surface activation antigen expression 24 hours after restimulation with anti-CD3/CD28 beads. (C) Expression of CD69, OX40 (CD134), and GITR is intact. (D) Cell surface LAP expression is specific for CD25+-derived cell lines. Resting cell lines are indicated by blue lines and restimulated cell lines by red lines. Resting cell lines were negative for these activation antigens prior to restimulation.
Restimulation of suppressor lines reveals cytokine production defects and cell surface LAP expression. Levels of cytokine produced in supernatants 48 hours after restimulation. (A) Cell lines were reactivated with anti-CD3/CD28 beads. (B) Cells were reactivated with PMA/ionomycin. Control CD25--derived cell lines are indicated by blue bars and CD25+-derived cell lines by burgundy bars. Cell surface activation antigen expression 24 hours after restimulation with anti-CD3/CD28 beads. (C) Expression of CD69, OX40 (CD134), and GITR is intact. (D) Cell surface LAP expression is specific for CD25+-derived cell lines. Resting cell lines are indicated by blue lines and restimulated cell lines by red lines. Resting cell lines were negative for these activation antigens prior to restimulation.
Restimulation of the cell lines with pharmacologic agents PMA and ionomycin, which can bypass proximal signaling pathways, led to nearly equivalent levels of cytokine production by both the CD25+- and CD25--derived cell lines (Figure 6B). Thus, the CD25+-derived cell lines appear to have proximal T-cell receptor (TCR) and CD28 signaling impairments that preclude the normal production of multiple cytokines. In addition, the CD25+-derived cell lines had impaired production of the immunosuppressive cytokine IL-10, which was not restored by PMA/ionomycin stimulation. This suggests that production of IL-10 is probably not a major mechanism of suppression effected by the CD25+-derived cell lines.
To determine if general activation of cord blood–derived CD25+ cell lines was impaired, we evaluated the expression of cell surface activation antigens by flow cytometric analysis. Cell lines were restimulated with anti-CD3/CD28 beads and evaluated after overnight culture for CD69, OX40 (CD134), and GITR expression. All 3 antigens were reexpressed on both the CD25+- and CD25--derived cell lines (Figure 6C). The expression of OX40 and GITR appeared slightly enhanced on the CD25+-derived cell lines versus the reactivated CD25- cell lines.34 Importantly, reactivation of the CD25+ cell lines was relatively intact by cell surface activation antigen expression analysis, in contrast to the results shown for cytokine accumulation.
Cell surface expression of the TGF-β latency associated protein (LAP), the TGF-β precursor protein, has been reported to be associated with Treg cells.35 In addition, recombinant forms of this protein have been recently reported to partially neutralize suppressor function.35 Thus, we evaluated the expression of LAP on the CD25+- and CD25--derived cell lines. Neither CD25+ nor CD25- lines expressed this protein after culture for 3 to 6 weeks; however, after restimulation with anti-CD3/CD28 beads there was a distinct expression of cell surface LAP on the CD25+-derived cell lines (Figure 6D) but not the CD25--derived cell lines. Cell surface expression of TGF-β was not detectable.
Cultured suppressor cells function by an unknown mechanism independent of IL-10, TGF-β, and multiple costimulatory molecules
To determine if the cultured suppressor cell lines work through known soluble immunosuppressive or cell surface–negative regulatory proteins, DC-MLR suppressor assays were treated with neutralizing or blocking monoclonal antibodies. Assays were evaluated for the reversal of suppression by resumption of proliferation. Initially antibodies to IL-10, IL-10 receptor (IL-10R), and TGFβ-1,2,3 were tested alone or in various combinations with minimal effect. Only by combining all 3 antibodies at high doses (30 μg/mL) were modest and probably nonspecific effects seen (Figure 7A); treated control cultures also evidenced increased proliferation. Antibodies to the negative costimulatory signaling molecules CTLA4 and PD1 were also tested and found to have essentially no effect on their own but, again, only in combination was a marginal effect on suppression noted (Figure 7B). Agonist antibodies to receptors whose signaling is reported to abrogate/diminish suppressor function of resting murine Treg cells, GITR,36 and OX40 (CD134)37 were tested and found to have minimal effects on the cultured suppressor cells. Agonist antibody to OX40 was interesting in that, in some donors more than others, it appeared to impair suppressor function (mean, 32% reversion; n = 6; range, 15% to 75%). However, agonist antibody to OX40 also increased the magnitude of the control MLRs, in approximate correlation with the magnitude of reversion of suppression (Figure 7C). Antibodies to OX40L inhibited the MLRs (with donor variability) but also increased the apparent magnitude of suppression (mean, 96% suppression; n = 5; range, 90% to 98% versus 90% for control cultures) (Figure 7C).
Because of the specific increased cell surface LAP expression on reactivated cultured suppressor cells and the suggested importance of TGF-β to suppressor function,35,38 we sought to evaluate multiple antagonists of the TGF-β pathway. Neutralizing monoclonal and polyclonal antibodies, soluble receptors, recombinant LAP, and antibody to LAP were all tested in the MLR system. All reagents alone or in multiple combinations failed to reverse suppression mediated by the cultured Treg cell lines. Experiments were conducted with a lower number of suppressor cells, 1:10 responder stimulator ratio, to minimize the potency of the suppression, resulting in approximately 50% inhibition in control MLRs. Even under these conditions, minimal effects were noted (Figure 7D).
Discussion
These studies demonstrate that cord blood as compared with adult blood is an improved source for Treg-cell isolation and culture. Potent suppressive cell lines were isolated from virtually every donor, and these results were obtained with a straightforward direct MACS-based purification. Flow cytometric profiles of antigen expression on cord blood Treg cell lines were surprisingly uniform. We then used this system to further characterize suppressor cell phenotype and function.
Purification of Treg cells from adult blood is possible but more difficult. We have previously experienced variability in isolating Treg cells from adult blood by MACS purification that are sufficiently pure for consistent suppressor cell line generation. We believe this is largely due to the presence of CD25dim memory cells, which overlap with Treg cells. Use of the cell sorter has facilitated the isolation of Treg cells22 and enabled the generation of suppressor cell lines from adult blood.24 However, even sorted populations of adult blood–derived CD25+ cells (top 2.9%) were found in one report to contain a mix of conventional and regulatory T cells on cloning and functional analysis.39
To date, all our assays of suppressor cell phenotype and function reveal that the cord blood–derived and the purest of the adult-derived cells have similar profiles. Flow cytometric analysis, cytokine production potential, and functional profiling have all been essentially the same. Thus, we favor the hypothesis that both types of lines, when pure, express equivalent suppressor mechanisms and potency, and the advantage of cord blood primarily relates to ease of purification and culture.
The critical role of Treg cells in cord blood immunology and transplantation has not been generally appreciated.40 Many laboratories use total CD4+-selected cord blood populations as a representative model of truly naive T cells for purposes of immunologic characterization.41 However, in light of our findings, some prior studies may need to be reevaluated with CD25-depleted CD4+ cells.42 The relevance of these Treg cells for cord blood transplantation (CBT) has so far been unexplored. These cells may be a contributing factor to the low rate of GVHD experienced in CBT.43
Our studies of these suppressor cell lines revealed several interesting facets of FoxP3 mRNA message and protein regulation. Most importantly, FoxP3 protein was found to be relatively specific for CD25+-derived suppressor cells and was minimally present in CD25--derived cell lines. We also determined that even exhaustively CD25-depleted CD4+ T-cell–derived lines could make significant amounts of FoxP3 message upon culture activation, about 20- to 30-fold more versus resting CD25- cells. The discordance between FoxP3 mRNA and protein expression suggests that caution must be used in assuming that FoxP3 message levels identify or quantify suppressor cells. In addition, we found that restimulated CD25+ cells expressed much more FoxP3 protein despite decreased message levels. These findings indicate FoxP3 expression is augmented with activation and suggest that posttranscriptional mechanisms may contribute to regulation of FoxP3 protein expression.
Suppressor cells were found (on restimulation) to have limited potential for cytokine production. There was a profound lack of IL-2, IFN-γ, and IL-10 production and a markedly reduced ability to produce GM-CSF, TNF, IL-5, and IL-8. In contrast, activation was assessed by up-regulated expression of CD69, OX40, and GITR, and production of MIP-1α and RANTES was generally intact. Interestingly, cytokine production ability was restored with PMA/ionomycin stimulation. This suggests a partial proximal TCR signaling block in the suppressor cells and is consistent with the anergic response characteristics of the suppressor cells to TCR stimulation.
Studies of both human and mouse CD4+CD25+ Treg cells have yet to reveal a clear mechanism of action of in vitro suppression.10 Consistent with previous studies, the cord blood–derived cultured suppressor cells required cell contact for function (did not suppress across a semipermeable membrane) and were not cytotoxic (not shown). Because TGF-β is variably reported to be a primary factor in Treg cell–mediated suppression38 and recombinant LAP (rLAP) was reported to impair suppressor function,35 we carefully evaluated the TGF-β pathway with multiple neutralizing reagents, including rLAP. We found that multiple antagonists of TGF-β, even in various combinations, minimally affected suppression. Neutralizing antibodies to immunosuppressive factor IL-10 or its receptor had no effect on suppression. This is consistent with the lack of IL-10 production by the cord blood suppressor cells even with very strong activating stimuli. Thus, in our in vitro MLR system, neither TGF-β nor IL-10 appear to be the primary mediators of suppression, and the molecules mediating suppression remain uncharacterized.10
The availability of large numbers of suppressor cells has enabled the beginning of the biochemical and molecular characterization of these special cells. We can now routinely generate more than 300 × 106 uniform and potent suppressor cells from one average-sized research grade cord blood unit (about 300 × 106 cells) without a cell sorter. These results reflect a 1% CD4+CD25+ cell recovery and a 100-fold expansion in culture. This number of cells will enable further characterization of TCR signaling alterations, FoxP3 regulation and function, and suppressor effector mechanisms. An important utility of these cells may be to enable clinical testing of a new form of immunotherapy. Suppressor cell lines may be useful for enhancing allograft tolerance induction or down-modulating autoimmune diseases.44
Prepublished online as Blood First Edition Paper, September 16, 2004; DOI 10.1182/blood-2004-06-2467.
Supported in part by grants from the Vikings Children's Fund, American Cancer Society, Children's Cancer Research Fund, Leukemia Research Fund, Leukemia and Lymphoma Society Translational Award no. 6220-04 (W.R.G.), and National Institutes of Health (NIH) grants R01 AI34495 and R37 HL56067 (B.R.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Jeffrey S. Miller, Catherine Verfaillie, Craig Eckfeldt, and Valarie McCullar for provision of CD34-negative cord blood cells.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal