Abstract
Fanconi anemia (FA) is an autosomal recessive disease marked by bone marrow failure, birth defects, and cancer. The FA proteins FANCA, FANCC, FANCE, FANCF, FANCG, and FANCL participate in a core complex. We previously have shown that several members of this complex bind to chromatin until mitosis and that this binding increases after DNA damage. The purpose of the present study was to determine the dynamics of complex movement between cytoplasm and nuclear compartments. Fluorescent-tagged versions of FANCA, FANCC, and FANCG colocalize in cytoplasm and nucleus, chiefly in chromatin. At the G1-S border, the FA core complex exists as foci on chromatin, progressively diffusing and migrating to the nuclear periphery and becoming completely excluded from condensed chromosomes by mitosis. Chromatin fiber analysis shows FA proteins diffusely staining along chromatin fibers during G1-S and S phase. Treatment with the DNA cross-linker mitomycin C results in a diffusion of foci and increased binding of complex proteins to chromatin, as well as diffuse and increased complex binding to chromatin fibers. These data are consistent with the idea that the FA proteins function at the level of chromatin during S phase to regulate and maintain genomic stability.
Introduction
Fanconi anemia (FA) is a genetic disease of cancer susceptibility marked by congenital defects, bone marrow failure, and myeloid leukemia.1-4 To date at least 11 complementation groups have been defined5-7 and 8 genes have been cloned.8-17 However, the gene products resemble no known proteins and have few identifiable functional protein motifs.
Cells derived from patients with the disease exhibit characteristic hypersensitivity to DNA cross-linking agents and generalized decreased survival.18-22 In addition, a well-described G2 phase cell cycle delay has been described that is thought to be secondary to a defective S or G2 checkpoint.23-25 Other studies have implicated cytokine dysregulation, sensitivity to oxidative damage, and defects in DNA repair.23,26-29 However, no defined biochemical mechanism for this hypersensitivity has been elucidated. Patient and cellular phenotypes across all the complementation groups are similar, suggesting an inter-relatedness or cooperativity between the FA proteins.
This cooperativity has been borne out by work we have done in showing binding of FANCA and FANCC in a core protein complex in both nucleus and cytoplasm.30-32 Recent work has found the FANCG, FANCE, FANCF, and FANCL proteins in the complex as well.17,33-36 One group has shown that BRCA2/FANCD1 binds to FANCG.37 A large complex is suggested by our recent work,38 and formation of the core complex does not occur in any of the complementation groups except the FA-D1, FA-D2, FA-I, and FA-J groups.7
Our work has established that FA proteins bind to chromatin.39 Immunoblotting experiments revealed that the increased FA proteins were bound to chromatin after DNA damage and that the complex underwent egress from the nucleus at mitosis. This movement appears to be regulated, as the FANCG protein becomes doubly phosphorylated at mitosis yet remains bound to the core complex.39 Recent work has implicated FANCD2 in chromatin, reinforcing the importance of the FA core complex, since it is required for FANCD2 monoubiquitination.40,41
In this paper we take those observations further in an attempt to better define at the level of chromatin (1) the dynamics of movement of the complex by microscopy during the cell cycle, (2) how the complex responds to DNA damage, and (3) the manner in which the complex localizes to chromatin fibers. In order to look exclusively at chromatin, we strip away soluble nuclear components and cytoplasmic proteins by in situ chromatin preparations. In addition, we demonstrate true localization to chromatin fibers by vertical extraction in a urea buffer, which causes release and elongation of fibers. Our data suggest that the FA protein complex achieves a focal presence on chromatin by G1-S, which is accentuated during S phase, and that the increase in FA complex caused by DNA damage is likely a manifestation of the S-phase concentration of FA complex.
Materials and methods
Cell culture and cell line
Cells were grown at 37°C in a 5% CO2 incubator. FA fibroblast cell lines were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 15% (vol/vol) fetal bovine serum (FBS). HeLa cells were grown in DMEM with 10% FBS. FA-A primary mutant cells (GANO; gift of Hans Joenje, Free University, The Netherlands) were grown in F12 nutrition medium and 15% FBS. HSC536N (FA-C) and EUFA143 (FA-G) mutant lymphoblasts were grown in RPMI 1640 and 15% FBS.
Plasmid constructs
The plasmid pECFP (cyano fluorescent protein)–FANCG was constructed with the FANCG gene fragment from pMMP-FANCG through the BglII and EcoRI site generated by polymerase chain reaction (PCR). The plasmid pEGFP (green fluorescent protein)–FANCA was constructed with the FANCA gene fragment from pcDNA3-Flag-FANCA through the HindIII and EcoRI site generated by PCR. The plasmid pEYFP (yellow fluorescent protein)–FANCC was constructed with the FANCC gene fragment from pLXSN-FANCC through KpnI and BamHI site generated by PCR.
To generate plasmid single-point mutations, PCR was performed with site-directed mutagenesis kit (Quickchange, Stratagene, La Jolla, CA). pEGFP-FANCA (H1110P), pECFP-FANCG (G546R), and pEYFP-FANCC (L554P) were constructed, respectively, by using following pairs of primers: 5′-GCAGTTCTTCCCCTTGGTCA-3′ and 5′-GTTGACCAAGGGGAAGAACT-3′, 5′-GTGCCCACGTAATCGAGACA-3′ and 5′-CTCGATTACGTGGGCACATC-3′, 5′-TTAAAGAGCCGCGAACTCAAG-3′ and 5′-TTGAGTTCGCGGCTCTTTAAG-3′. Resulting constructs were verified by DNA sequencing.
DNA transfection
Transfections were performed by lipofection per manufacturer's protocol (Lipofectamine; Gibco, Carlsbad, CA). For all transient and stable transfection, plasmid DNA was prepared by column purification (Qiagen, Valencia, CA). 5 × 105 cells were placed on 60-mm petri dishes one day prior to transfection and grown until the cells were 60% to 80% confluent. Plasmid DNA was diluted in 100-μL Opti-MEM medium (Gibco) and mixed with plus reagent (Gibco); LipofectAMINE was diluted in 100 μL OPTI-MEM medium (Gibco). These were gently mixed together 15 minutes after incubation at room temperature, and incubated another 15 minutes at room temperature. The final complex was placed on prewashed cells with phosphate-buffered saline (PBS) and incubated for 5 hours.
Cell synchronization and drug treatment
HeLa cells were treated overnight with 2 mM thymidine, washed, released into regular media, and treated again overnight with thymidine, as previously described.39 Cells were then released for variable times or incubated overnight in the presence of 1 μM nocodazole (Sigma, St Louis, MO). Mitotic cells were collected the next morning by shaking the plate and collecting the cells in suspension. Verification of cell cycle state was achieved using FACScan (Becton Dickinson, San Jose, CA) analysis after resuspension of 100 000 cells in 0.3% citrate, 0.01% NP40, 100 μg/mL propidium iodide, and 10 μg/mL RNAse.39
Asynchronous cells or cells synchronized at G1-S border or S phase were incubated with mitomycin (MMC) (0.1 μM) for 4 hours. For the time course, asynchronous cells were incubated with MMC for 0 hours, 6 hours, 12 hours, and 24 hours. For transiently transfected HeLa cells, 48 hours after transfection, cells were treated for 12 hours with MMC.
For sorting, 106 asynchronously growing HeLa cells transfected with pEGFP-FANCA and pECFP-FANCG were collected and pelleted. The cells were resuspended sequentially in 500 μL PBS and 500 μL 2% paraformaldehyde and incubated on ice for 1 hour. After pelleting, the cells were washed and then incubated overnight at 4°C in 70% ethanol. The cells were pelleted again and resuspended in 1 mL of 40 μg/mL propidium iodide and 100 μg/mL RNAse in PBS. This mix was incubated at 37°C for 30 minutes. The cells were then sorted into G1, S, and G2-M groups on the FACS instrument (Becton Dickinson, San Jose, CA).
In situ chromatin preparation
Cells grown on chamber slides were first washed in cold PBS. Soluble proteins were removed by extraction in cytoskeletal buffer [CSK: 10 mM PIPES (piperazine diethanesulfonic acid), pH 6.8/300 mM sucrose/100 mM NaCl/3 mM MgCl2/1 mM EGTA (ethylene glycol tetraacetic acid)/RNAse inhibitor (Gibco)/1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride] containing 0.5% Triton X-100 for 2 minutes at 4°C. The structure remaining was extensively cross-linked by treatment with 4% formaldehyde in CSK for 40 minutes at 4°C. The slides were covered with mounting solution containing 2 ug/mL DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA) after washing with cold PBS.39,42
Chromatin fiber preparation
Chromatin fibers were prepared according to the method of Blower et al.43 Cells were trypsinized 48 hours after transfection, washed twice with cold PBS, then suspended in hypotonic solution (75 mM KCl in PBS) for 10 minutes. Cells adjusted to a density of 1 to 2 × 105 cells/mL were cytospun onto charged slides (double funnels) at 800 rpm (150g) with high acceleration for 4 minutes, 150 μL cells per slide. For attached/monolayer cells, 1 × 105 cells/mL were used, and for suspension cultures, 2 × 105 cells/mL. After cytospin, slides were immersed in a Coplin jar filled with lysis buffer [25 mM Tris (tris(hydroxymethyl)aminomethane) Cl, pH 7.5/0.5 M NaCl/1% Triton X-100/0.2 M urea for 3 to 10 minutes. The slide was slowly lifted vertically from the lysis buffer, and the chromatin was allowed to move down the slide. After fixation in 4% paraformaldehyde in PBS-Tween20 (0.05%) for 20 minutes, the slides were covered with mounting solution containing 2 ug/mL DAPI (4′6-diamidino-2-phenylindole 2HCl) (Vector Laboratories) after wash in PBS. For fluorescent microscopy and analysis, inverted fluorescent microscope (Nikon, Melville, NY) was used, with images captured by digital camera (Hamamatsu, Hamamatsu City, Japan).
Chromatin preparation and immunoblotting
Chromatin extract was prepared and run on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), as previously described, essentially a modification of the above-described in situ preparation.39,42 Immunoblotting was conducted with anti-FANCG(N terminal), anti-FANCA(N terminal), and Ku86 (Santa Cruz Biotechnology, Santa Cruz, CA), respectively.39
Results
Fluorescent-tagged versions of FA proteins are functional
For the purpose of visualizing FANCA, FANCG, and FANCC by direct fluorescent microscopy, we constructed FA cDNAs with fluorescent tags: EGFP-FANCG, EYFP-FANCC, and ECFP-FANCG. In order to determine if the fluorescent versions of the FANCA, FANCC, and FANCG were functional, we transfected the fluorescent and nonfluorescent tagged constructs into FA-A (HSC72), FA-C (HSC536N), and FA-G (EUFA143) mutant cells, respectively. After transfection the cells were analyzed for cytotoxicity against MMC. In Figure 1, each version of the FA genes was able to restore normal MMC resistance in the corresponding mutant line, indicating that the fluorescent tag did not interfere with normal FA function. Thus, use of these constructs is valid in experiments to explore the normal function of the FA proteins.
Fluorescent-tagged versions of FA proteins are functional. Mutant FA-G (EUFA143), FA-A (HSC72), and FA-C (HSC536N) cell lines were transfected with fluorescent-tagged wild-type ECFP-FANCG, EGFP-FANCA, and EYFP-FANCC cDNAs and the nonfluorescent tagged versions, respectively. The cells were treated with the indicated doses of MMC for 5 days and analyzed by XTT (2,3-Bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide) assay. Each tagged version restored MMC resistance to the level of the nontagged version.
Fluorescent-tagged versions of FA proteins are functional. Mutant FA-G (EUFA143), FA-A (HSC72), and FA-C (HSC536N) cell lines were transfected with fluorescent-tagged wild-type ECFP-FANCG, EGFP-FANCA, and EYFP-FANCC cDNAs and the nonfluorescent tagged versions, respectively. The cells were treated with the indicated doses of MMC for 5 days and analyzed by XTT (2,3-Bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide) assay. Each tagged version restored MMC resistance to the level of the nontagged version.
Tagged FANCA, FANCC, and FANCG localize to chromatin
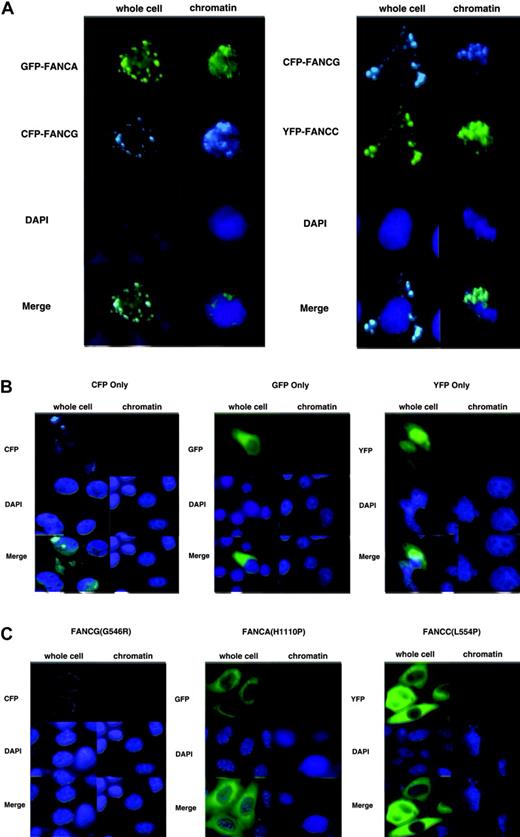
In previous work we showed by immunoblotting that the endogenous FA core complex localized to chromatin. To determine if tagged versions of the FA proteins localized to chromatin, we doubly transfected HeLa cells with either EGFP-FANCA and ECFP-FANCG or ECFP-FANCG and EYFP-FANCC. After 48 hours, we extracted the cells in situ to leave behind what we term a “chromatin prep.” Direct microscopy of either whole cells or chromatin prepared in situ revealed that FANCA, FANCC, and FANCG all localized to the nucleus and chromatin and totally colocalized (Figure 2A). Most expressing cells contained fluorescent protein in both cytoplasm and nucleus. No chromatin binding was observed in control experiments for cells expressing only EGFP, ECFP, or EYFP (Figure 2B). Transfection efficiency was estimated to be at least 50% in all cases, as determined by counting fluorescent cells prior to extraction. In order to show that mutation of the FA proteins could disrupt proper chromatin localization, we prepared similarly tagged versions of FANCA (H1110P), FANCC (L554P), and FANCG (G546R) by PCR-mediated mutagenesis. Each of these mutants exist in nature in FA patients, and the derivative proteins are expressed at near wild-type levels.8,44,45 The FANCA (H1110P), FANCC (L554P), and FANCG (G546R) mutants all were cytoplasmic and were not visible upon chromatin preparation (Figure 2C). Thus, patient-derived point mutations resulted in abrogation of nuclear or chromatin localization, further validating the use of our tagged constructs.
FANCA, FANCC, and FANCG colocalize in chromatin. (A) HeLa cells were transfected with either GFP-FANCA/CFP-FANCG or CFP-FANCG/YFP-FANCC. Whole cells were fixed or chromatin preparations were performed in situ. (B) HeLa cells were transfected with EGFP, EYFP, or ECFP only–containing plasmid, and whole cells and chromatin were prepared. No GFP, YFP, or CFP signal was discernible on chromatin after preparation. Also, CFP was not visible through GFP filters, and YFP and GFP were not visible through CFP filters. (C) Point mutant versions of EGFP-FANCA(H1110P), EYFP-FANCC(L554P), and ECFP-FANCG(G546R) were transfected into HeLa cells. Whole cells contained none of the mutant proteins in the nucleus, and no mutant proteins were visible at all after chromatin preparation.
FANCA, FANCC, and FANCG colocalize in chromatin. (A) HeLa cells were transfected with either GFP-FANCA/CFP-FANCG or CFP-FANCG/YFP-FANCC. Whole cells were fixed or chromatin preparations were performed in situ. (B) HeLa cells were transfected with EGFP, EYFP, or ECFP only–containing plasmid, and whole cells and chromatin were prepared. No GFP, YFP, or CFP signal was discernible on chromatin after preparation. Also, CFP was not visible through GFP filters, and YFP and GFP were not visible through CFP filters. (C) Point mutant versions of EGFP-FANCA(H1110P), EYFP-FANCC(L554P), and ECFP-FANCG(G546R) were transfected into HeLa cells. Whole cells contained none of the mutant proteins in the nucleus, and no mutant proteins were visible at all after chromatin preparation.
The FA proteins form foci at G1-S and during S phase
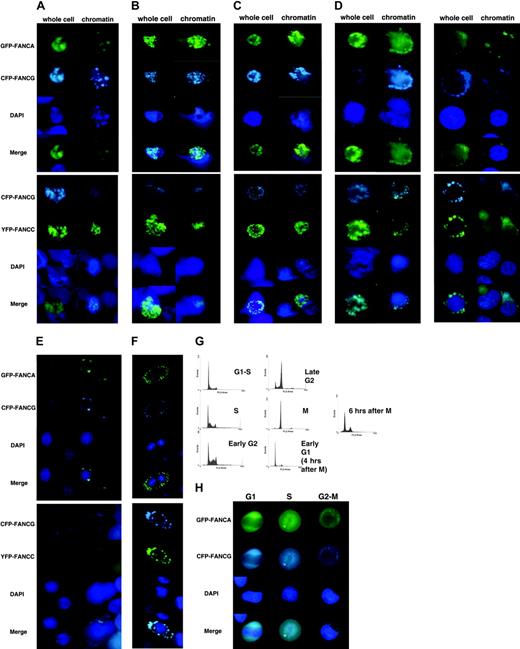
Our previous work did not show any marked change of FA protein amount bound to chromatin during S phase, as compared to the G1-S border.39 In order to show qualitative changes in the FA core complex, we synchronized HeLa cells by double thymidine block, then released into regular media. Chromatin preparations at the G1-S border and during S phase revealed that the FA core complex formed foci, which were not readily apparent in whole cells (Figure 3A). In all cases, FANCA and FANCG as well as FANCG and FANCC colocalized. Interestingly, cells from G1-S to mitosis contained predominately fluorescent FA proteins in the nucleus. The whole cells in the micrographs were underexposed in order to diminish cytoplasmic staining and increase visibility of the nucleus. As the cells continued into G2, the foci appeared to diffuse and migrate to the nuclear periphery by late G2 (Figures 3B-D). This also was apparent in whole cells. Cells that became detached from the plate after nocodazole arrest, assumed to be in mitosis, were collected and cytospun onto slides. Examination of these cells revealed complete exclusion from nuclei (prior to chromosome condensation) in the whole cell as well in chromatin preps (Figure 3E). Once cells are released from G1-S block, approximately 10 to 12 hours later the cells are “shakable” from the surface of the plate and represent mitotic cells. These cells were collected and allowed to reattach to the slide surface for 4 hours. These cells, seen in Figure 3F, had divided, but the FA proteins were still cytoplasmic. These data are consistent with our previous work in which we showed the FA complex is excluded from condensed chromosomes at mitosis. In early G1 it is apparent that the complex is still cytoplasmic. Representative FACS analyses from HeLa cells and EGFP-FANCA/ECFP-FANCG are depicted in Figure 3G; no differences in DNA histograms were seen in any of the transfectants and HeLa cells alone. Cell number at this stage was no different in nontransfected and transfected cells.
The FA proteins form foci at G1-S and during S phase. HeLa cells transfected with either EGFP-FANCA/ECFP-FANCG or ECFP-FANCG/EYFP-FANCC were grown on slides and synchronized by double thymidine block to the G1-S border. Whole cells and chromatin were visualized at the indicated times after release: (A) G1-S, 0 hours; (B) S phase, 4 hours; (C) early G2 phase, 6 hours; (D) late G2 phase, 8 hours; (E) mitosis, after 16 hours in 1 μM nocodazole; and (F) early G1, after collection of floating mitotic cells, followed by 4 hours in regular media to allow for anchorage and cytokinesis. (G) Representative FACScan analysis of transfected, synchronized cells. (H) Asynchronous HeLa cells cotransfected with GFP-FANCA and CFP-FANCG were sorted by FACScan into G1, S, and G2-M groups. Microscopy revealed that FA proteins were cytoplasmic during G1 and G2-M and nuclear during S phase.
The FA proteins form foci at G1-S and during S phase. HeLa cells transfected with either EGFP-FANCA/ECFP-FANCG or ECFP-FANCG/EYFP-FANCC were grown on slides and synchronized by double thymidine block to the G1-S border. Whole cells and chromatin were visualized at the indicated times after release: (A) G1-S, 0 hours; (B) S phase, 4 hours; (C) early G2 phase, 6 hours; (D) late G2 phase, 8 hours; (E) mitosis, after 16 hours in 1 μM nocodazole; and (F) early G1, after collection of floating mitotic cells, followed by 4 hours in regular media to allow for anchorage and cytokinesis. (G) Representative FACScan analysis of transfected, synchronized cells. (H) Asynchronous HeLa cells cotransfected with GFP-FANCA and CFP-FANCG were sorted by FACScan into G1, S, and G2-M groups. Microscopy revealed that FA proteins were cytoplasmic during G1 and G2-M and nuclear during S phase.
In order to verify the phenomenon of protein shuttling in asynchronously growing cells, we sorted by flow HeLa cells cotransfected with GFP-FANCA and CFP-FANCG into G1, S, and G2-M groups. Microscopy revealed that FANCA and FANCG were cytoplasmic and colocalized in G1 and G2-M cells, while in S phase cells they were predominately nuclear (Figure 3H). This is consistent with the idea that FA proteins shuttle between nucleus and cytoplasm during the cell cycle and that synchronization does not affect this event.
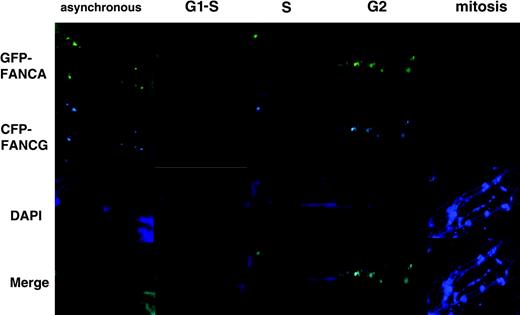
To demonstrate that synchronization does not prevent cells from proceeding to the next cell cycle, we collected HeLa cells transfected with EGFP-FANCA and ECFP-FANCG and arrested at mitosis. These cells were washed and then placed in fresh regular media and observed under time-lapse photography. The film shows that initially FANCA and FANCG are cytoplasmic. However, after 6 hours, FANCA and FANCG can be observed in the nucleus, mostly at the periphery (Supplementary Video 1 [see the Video link at the top of the online article on the Blood website]). FACS (fluorescence-activated cell scans) analysis demonstrates that the cells are at the G1-S border (Figure 3G). Time-lapse photography taken of asynchronous cells demonstrates a similar phenomenon: initially cytoplasmic proteins become nuclear after 2 hours of observation (Supplementary Video 2, also available on the Blood website).
FA core complex localizes to chromatin fibers
Chromatin preparations afford the ability to see structures beyond soluble nuclear material, yet chromatin still represents a densely packed version of the genome. In order to visualize distinct chromatin fibers, we extracted nuclei by vertical immersion in a urea-based extraction buffer after cotransfection with EGFP-FANCA and ECFP-FANCG. In the asynchronous cells, colocalizing FANCA and FANCG were seen in irregularly spaced foci along the length of the fiber (Figure 4). In contrast, upon G1-S and S-phase entry, the proteins could be seen slightly more prominently but more homogeneously spread along the fiber. At G2, the proteins appeared to be more clumped. Strikingly and consistent with our other data, a chromatin fiber prep performed on mitotic cells revealed the complete absence of FA proteins, while the DAPI-stained image showed predictably a heterochromatically staining fiber.
FA core complex localizes to chromatin fibers. HeLa cells transfected with EGFP-FANCA/CFP-FANCG were synchronized and collected as in Figure 3. The mitotic cells were cytospun onto a new slide. Chromatin fibers were then prepared on slides by vertical urea extraction. Micrographs show foci, then diffusion of FA proteins on fibers through S phase, clumping in G2, and absence by mitosis.
FA core complex localizes to chromatin fibers. HeLa cells transfected with EGFP-FANCA/CFP-FANCG were synchronized and collected as in Figure 3. The mitotic cells were cytospun onto a new slide. Chromatin fibers were then prepared on slides by vertical urea extraction. Micrographs show foci, then diffusion of FA proteins on fibers through S phase, clumping in G2, and absence by mitosis.
FA core complex diffuses after MMC treatment
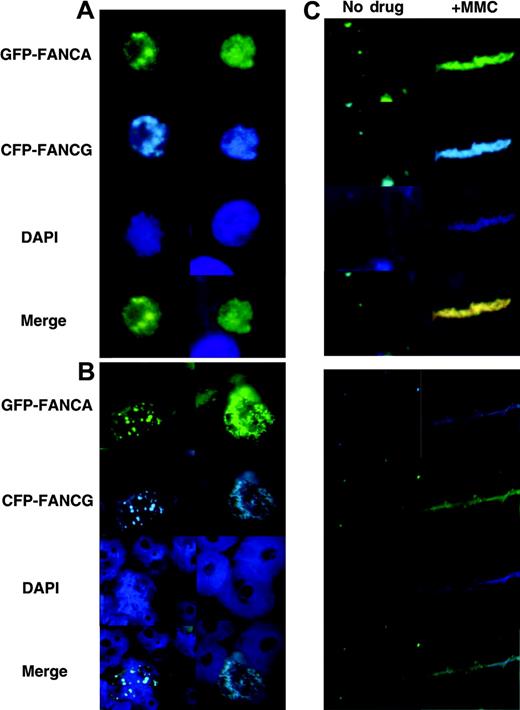
Our previous work showed that increased FA proteins localized to chromatin after MMC treatment. To demonstrate this phenomenon in situ, we treated an asynchronous cell population with 0.1 μM MMC and examined chromatin and whole cells at various time points of MMC treatment. No obvious differences can be seen in whole cells (Figure 5A). However, by 12 hours, foci seen in untreated chromatin had become diffusely stained in MMC-treated chromatin (Figure 5B). We analyzed a slide containing cells similarly treated with MMC and performed chromatin fiber preparation. Increased and more-spread FA proteins stained the fibers (Figure 5C), consistent with the in situ appearance in Figure 5B and the immunoblotting data of our prior work.39
FA protein foci diffuse after MMC treatment. HeLa cells transfected with EFGP-FANCA/CFP-FANCG were treated for 24 hours with 0.1 μM MMC. (A) Whole cells, (B) chromatin, and (C) chromatin fibers were prepared and examined. FA proteins diffused on chromatin after MMC treatment.
FA protein foci diffuse after MMC treatment. HeLa cells transfected with EFGP-FANCA/CFP-FANCG were treated for 24 hours with 0.1 μM MMC. (A) Whole cells, (B) chromatin, and (C) chromatin fibers were prepared and examined. FA proteins diffused on chromatin after MMC treatment.
FA core complex localizes to chromatin in primary cells
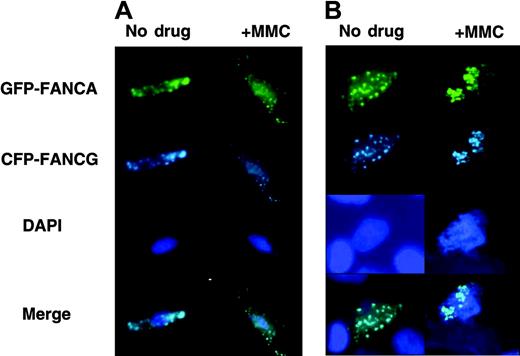
Because HeLa cells are easily synchronized, we have used them for the bulk of the work presented. In order to show that these data are applicable to primary cells as well, we transfected primary FA-A mutant cells with EGFP-FANCA and ECFP-FANCG. In the untreated cells, analysis of both whole cells and chromatin revealed that FANCA and FANCG colocalized to foci in the nucleus of whole cells (Figure 6A) and chromatin (Figure 6B). Cells treated with MMC displayed increased chromatin staining, consistent with the data presented in the HeLa cells. Even in the whole cell, the nucleus can be seen homogeneously stained after MMC treatment. Thus, for FA core complex function, behavior of the complex in immortalized and primary cells appears similar.
FA proteins exist as foci and diffuse on chromatin after MMC treatment in primary cells. Primary FA-A fibroblasts were transfected with EGFP-FANCA/CFP-FANCG and treated with 0.1 μM MMC for 24 hours. (A) Whole cells and (B) chromatin were prepared and visualized.
FA proteins exist as foci and diffuse on chromatin after MMC treatment in primary cells. Primary FA-A fibroblasts were transfected with EGFP-FANCA/CFP-FANCG and treated with 0.1 μM MMC for 24 hours. (A) Whole cells and (B) chromatin were prepared and visualized.
FA proteins localize to chromatin predominately after MMC treatment and during S phase
Chromatin fiber experiments and synchronization experiments suggest similarities in appearance of FA proteins during S phase and after MMC treatment. This suggests that the DNA damage inducibility of the FA protein localization on chromatin may be a function of engagement of an S-phase checkpoint. This has been suggested by work done by Akkari et al,29 who showed that cytotoxicity induced by MMC was dependent on traverse through S phase.
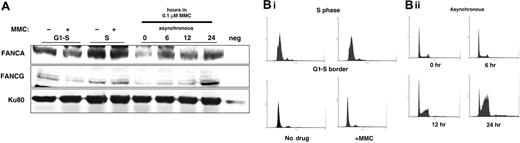
In order to compare the magnitude of MMC effect upon chromatin localization of the FA proteins, we treated HeLa cells synchronized to the G1-S border and asynchronous HeLa cells with MMC. S-phase cells were prepared by release into regular media for 4 hours; cells were treated with MMC during this time. After chromatin extract preparation, we performed FANCA immunoblotting. Unexpectedly, peak levels of FANCA in chromatin were seen during S phase, which were greater than the increased levels induced by MMC in asynchronous cells (Figure 7A). Concomitant treatment with MMC during S phase did not stimulate higher FA protein levels in chromatin. A negative control lane with 100 μg of whole cell extract of EUFA143 FA-G mutant cells was included, as these cells do not express FANCG and express little FANCA. FACS data showed that MMC did not alter the DNA histogram in synchronized cells but, as has been well documented, did induce a marked degree of S (12 hours) and G2-M (24 hours) accumulation (Figure 7B). These data suggest that the MMC inducibility of FA proteins to chromatin is achieved through traverse in S phase and is consistent with the data from Akkari et al.29
FA proteins localize to chromatin predominately after MMC treatment and during S phase. HeLa cells were synchronized by double thymidine block and collected. G1-S cells were not released but were treated with 0.1 μM MMC for 4 hours. S-phase cells were prepared by simultaneous release for 4 hours in regular media and treated with 0.1 μM MMC for 4 hours. Chromatin was prepared and run on SDS-PAGE. After transfer, the membrane was blotted with anti-FANCA, anti-FANCG, and anti–topoisomerase II antisera. Neg indicates negative mutant EUFA143 FA-G cells that express no FANCG and very little FANCA. (B) 100 000 cells from the indicated treatment group were counted on FACScan at each time point.
FA proteins localize to chromatin predominately after MMC treatment and during S phase. HeLa cells were synchronized by double thymidine block and collected. G1-S cells were not released but were treated with 0.1 μM MMC for 4 hours. S-phase cells were prepared by simultaneous release for 4 hours in regular media and treated with 0.1 μM MMC for 4 hours. Chromatin was prepared and run on SDS-PAGE. After transfer, the membrane was blotted with anti-FANCA, anti-FANCG, and anti–topoisomerase II antisera. Neg indicates negative mutant EUFA143 FA-G cells that express no FANCG and very little FANCA. (B) 100 000 cells from the indicated treatment group were counted on FACScan at each time point.
Discussion
We previously have implicated the chromatin-nuclear matrix as a functional unit upon which the FA core complex performs its normal function.39 In this paper, we confirm and extend these findings by showing that this is an S-phase–specific process by (1) demonstrating that the FA core complex forms chromatin foci at the G1-S border and at the beginning of S phase, (2) showing that these foci diffuse in response to DNA damage and during S phase, and (3) showing that the proteins exit the nucleus by the onset of mitosis as a multiprotein complex.
These data suggest a dynamic movement during the cell cycle whereby FA protein foci form on chromatin by G1-S, the foci diffuse, and proteins increase in amount during S phase. This is recapitulated by the chromatin fiber experiments. The MMC treatment appears to induce a similar change in both chromatin foci as well as in fibers, suggesting that the response to DNA damage and during S phase may be similar. This is supported by literature from the Grompe and Rosselli groups suggesting the importance of the FA pathway in an S-phase process as well as from the D'Andrea group showing that FANCD2 is mono-ubiquitinated during S phase and in response to DNA damage.29,46-49
These data have implications for potential function of the FA core complex. First, a specific function for the FAproteins remains obscure in spite of the cloning of 8 genes. Second, the emergence of chromatin regulation as important to genomic stability has become part of the forefront of current research, exemplified by SWI/SNF and by chromatin remodeling activities of cancer related proteins such as core binding protein (CBP) and BRCA1.50 Examples of regulation and involvement of histone modification have been shown to affect transcription, replication, and DNA repair. Indeed, although no associated chromatin remodeling activity could be detected, FANCA has been shown to bind to human SWI/SNF.51 Also, recent work has shown that BRCA1 binds to FANCD2 and that BRCA2 is indeed FANCD1.48,52 Since FANCD2 ubiquitination is dependent upon wild-type FA core complex, and this ubiquitination occurs in response to DNA damage, it is indeed plausible that the genomic surveillance function of the FA pathway occurs at the level of chromatin. Recently, FANCD2 and its importance in chromatin has been supported by work detailing that mono-ubiqutinated FANCD2 localizes to chromatin and is responsible for BRCA2 loading onto chromatin.40,41
Our future studies now will focus on a biochemical function for the FA pathway. These studies suggest a link between replication and repair of DNA damage. Our prior work detailing MMC induction of FA protein to chromatin did not compare directly MMC effect versus S phase. Our direct comparison implies that the 2 are likely inseparable.
Localization to chromatin implies that these biochemical activities may play a role directly in DNA repair, DNA replication, and other genomic surveillance activities. While direct DNA binding of the FA core complex has not been shown, it is plausible that the FA core complex can interact with the functional unit of DNA, namely chromatin. The recent report of Bloom helicase (BLM) binding with the FA core complex suggests work to show how the chromatin interaction occurs.53 We will attempt to show colocalization with BLM and other S-phase proteins in situ, including those involved in recombinatorial repair, replication machinery, and other components of chromatin such as histones.
Prepublished online as Blood First Edition Paper, July 15, 2004; DOI 10.1182/blood-2004-01-0001.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Han Joenje for the GANO cell line and Maureen Hoatlin and members of the Kupfer laboratory for useful discussions.

![Figure 1. Fluorescent-tagged versions of FA proteins are functional. Mutant FA-G (EUFA143), FA-A (HSC72), and FA-C (HSC536N) cell lines were transfected with fluorescent-tagged wild-type ECFP-FANCG, EGFP-FANCA, and EYFP-FANCC cDNAs and the nonfluorescent tagged versions, respectively. The cells were treated with the indicated doses of MMC for 5 days and analyzed by XTT (2,3-Bis[2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide) assay. Each tagged version restored MMC resistance to the level of the nontagged version.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/2/10.1182_blood-2004-01-0001/6/m_zh80020572340001.jpeg?Expires=1765950437&Signature=tGuVqEwSMDj1jdoXkGJdY4nbNM02Jgs5UeOtovISop5czdELgKxh4Sz2wlRXkwGA1hP8m7D-ref26E4QY~~0ClDpHtqy9jHjoRCH-DcVZY8IJQiAUK0Ws23pQEH1WDEe~llugblniU~PdfzRph6eJlaJbzbgLmUtyPfccuWvAdaATV~vVdD4hCdvtovx2PQCfchIjG8JLFKhxsQV1QcWZFbFG-GhGVnw3VKYZiQ4s3vYzo5JJKzvt1jwmlgL5oMP2D5i6mJrpdViiXrvNO3GYxwMYabhz68CgfoeF8nRoiBbxGfB4apdBSWuwbMfoay61A2mxxQ63XYvEAHv57fTvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal