Abstract
Attempts at inducing allograft immune privilege by enforced Fas ligand expression have shown accelerated rejection mediated by neutrophils. While it has been proposed that Fas ligand was directly chemotactic toward neutrophils, several lines of evidence argue for an indirect recruitment mechanism. This question was addressed by using in vitro migration assays that used highly purified human leukocyte subsets. Granulocytes did not migrate in response to Fas engagement and required the presence of T cells expressing several natural killer (NK) cell markers. These rare CD8 memory T cells expressed T and NK cell markers and were not restricted to CD1d, showing that they are distinct from conventional natural killer T (NKT) cells. These cells were able to kill both NK-sensitive and -insensitive targets and secreted several CC and CXC chemokines active toward granulocytes, monocytes, and NK cells upon Fas engagement. Chemotactic factor release depended on caspase activity, in the absence of NKT cell apoptosis. The ability of CD1d-unrestricted NKT cells to recruit innate immune system cells might play a role in cancer cell eradication and contribute to inflammatory diseases.
Introduction
Apoptosis plays a crucial role in development, tissue homeostasis, and immune system function. Fas-mediated apoptosis occurs through receptor oligomerization by Fas ligand (FasL),1 leading to the recruitment of caspase-8. The auto-proteolytic activation of caspase-8 leads to executor caspase processing that, in turn, cleaves proteins essential for cell survival. Apoptotic cells are then engulfed by neighboring cells, limiting inflammation.1 FasL expression by activated T cells is crucial to the resolution of immune responses through activation-induced cell death.1 Besides activated T cells, FasL is expressed in immune-privileged sites, inducing apoptosis of self-reactive T cells infiltrating the site.2 FasL expression by cancer cells has also been proposed to participate in tumor immune privilege.3 Given the sensitivity of T cells to FasL-mediated apoptosis, it was hypothesized that enforced expression of FasL in allografts would create artificial immune privileged sites, allowing organ acceptance without having to resort to immunosuppressive drugs.2 While enforced FasL expression prolonged allograft survival in certain models, several reports showed massive granulocyte infiltration leading to acute rejection.4-8 The outcome of graft acceptance might depend on microenvironmental factors such as FasL expression levels, local cytokine expression, and cell types present near the graft site. In vitro migration assays have shown that FasL could behave as a neutrophil chemokine,9,10 a claim disputed by others.11,12
It is becoming increasingly clear that Fas engagement can mediate nonapoptotic signaling events, leading to cytokine and chemokine expression in different cell types.13 For instance, serum-starved fibroblasts release interleukin 6 (IL-6) and IL-8 upon Fas engagement through transcription factor activation.14 Caspase inhibition in Fas-treated T lymphocytes blocks apoptosis and unmasks proinflammatory cytokine release, also mediated through transcription factor activation.15 Caspase activity has been reported to contribute to T-cell activation through selective caspase substrate cleavage in T cells without apoptosis.16,17 Finally, naturally occurring caspase-8 mutations in humans not only prevents apoptosis but also leads to decreased T-lymphocyte activation.18 Thus, Fas signaling and caspase activity can participate in T-cell activation and inflammatory processes.
To better understand the contribution of FasL in neutrophil recruitment, in vitro migration assays were performed using cross-linked soluble FasL, since anti-Fas antibodies do not behave like the natural ligand.19 Highly purified granulocytes did not migrate in response to Fas engagement, arguing for an indirect chemotactic mechanism. Using different highly purified leukocyte subsets, we found that Fas engagement in CD8+ memory T cells expressing several natural killer (NK) cell markers leads to the release of several CC and CXC chemokines that induce not only neutrophil recruitment but also monocyte and NK cell migration. These leukocytes are distinct from conventional natural killer T (NKT) cells since they are not restricted to CD1d and do not express the NKT invariant T-cell receptor (TCR). This is a novel example of nonapoptotic Fas signaling since chemotactic factor release depended on caspase activity, yet occurred in the absence of NKT cell apoptosis. The ability of CD1d-unrestricted NKT cells to recruit other leukocytes upon Fas cross-linking represents a novel cross talk between cells of the acquired and innate immune system. This unique cell subset may contribute to the destruction of tumor cells expressing FasL for immune evasion.
Materials and methods
Cells and reagents
U937 and Raji cells from ATCC (American Type Culture Collection [Manassas, VA]; CCL-243 and CCL-86) were grown at 37°C with 5% CO2 in RPMI-1640 with 10% fetal bovine serum (FBS) and penicillin-streptomycin. CD3, CD4, CD8, CD14, CD15, CD16, CD45RA, CD8 multisort; CD56 multisort; and streptavidin magnetic beads were from Miltenyi Biotec (Auburn, CA). FLAG-tagged recombinant soluble human FasL (sFasL) and anti-FLAG enhancer antibody were from Alexis Biochemicals (Carlsbad, CA). The isotypic control for the enhancer antibody was purified mouse immunoglobulin G1κ (IgG1κ; MOPC-21) from BD Biosciences (Mississauga, Canada). Streptavidins conjugated with phycoerythrin (PE), fluorescein isothiocyanate (FITC), or CyChrome were from BD Biosciences. Antibodies used were Vα24-Biotin (C15) from BioDesign (Saco, ME); CD3-Quantum Red (UCHT-1), CD14-FITC (UCHM-1), CD15-Biotin (DU-HL30-3) from Sigma (St Louis, MO); CD19-Biotin (HIB19), CD28 (CD28.2) from eBioscience (San Diego, CA); γ/δ TCR-Biotin (B1.1), CD4-PE (RPA-T4), CD8α-FITC (RPA-T8), CD16-Biotin (3G8), CD45RO-FITC (UCHL-1), CD56-Biotin (B159), CD161-PE (DX12), FasL (NOK-1) from BD Biosciences. PE-conjugated CD1d tetramers loaded or not with α-galactosyl ceramide (α-galcer) were provided by Mitchell Kronenberg (La Jolla Institute for Allergy and Immunology, La Jolla, CA). HEMA-3 stain was from Fisher Scientific (Nepean, Canada); the apoptosis detection kit and GolgiPlug were from BD Biosciences; polymyxin B, indomethacin, nordihydroguaiaretic acid (NDGA), actinomycin D, cycloheximide, caspase-1 and caspase-8 inhibitors, and formyl-Methionine-Leucine-Phenylalanine (fMLP) were from Calbiochem (La Jolla, CA).
Cell purification
Leukocytes were isolated from heparinized peripheral blood of healthy volunteers who had signed a consent form. The study protocol was approved by the Comité d'Éthique en Recherche (CÉR) of the Institut National de la Recherche Scientifique at Université du Québec. Granulocytes were purified by Dextran sedimentation and Ficoll-Hypaque centrifugation (Amersham Biosciences, Baie d'Urfé, Canada), or from the buffy coat by magnetic cell sorting (MACS) positive selection using CD15 microbeads or MACS depletion using a combination of CD3, CD14, CD19, and CD56 microbeads. Two rounds of MACS-positive or -negative selection were performed in all instances. Other cell types were obtained from the buffy coat by MACS-positive selection using microbeads specific for CD3, CD14, CD19, CD56, or Vα24-Biotin together with Streptavidin microbeads. CD56+CD3+ cells were isolated by MACS-positive selection using CD56-multisort and CD3 microbeads. CD56+CD3+ cells obtained were sorted on a fluorescence-activated cell sorter (FACS) Vantage (Beckman Coulter, Fullerton, CA) flow cytometer using a CD8-FITC antibody. Alternatively, NKT cells were isolated by MACS-positive selection with CD56 and CD8 multisort microbeads followed by negative selection with CD45RA microbeads. For all magnetic separations, Fc receptors were blocked with Fc Block (Miltenyi Biotec). The cell populations were analyzed on a FACSCalibur (BD Biosciences) flow cytometer.
Chemotaxis assays
Migration assays were performed in triplicate in a MBA96-modified Boyden Chamber (NeuroProbe, Gaithersburg, MD) fitted with 5-μM (for monocytes) or 3-μM (for other cell types) polycarbonate filters. All reagents were endotoxin-free (< 5 EU) as determined by the end point chromogenic Limulus Amebocyte Lysate (LAL) assay (Endosafe, Wilmington, MA). FasL (30 ng/mL) with enhancer (1 μg/mL) or control mouse IgG1κ antibody (1 μg/mL) were diluted in RPMI-1640 supplemented with 1 mg/mL bovine serum albumin and 5 μg/mL polymyxin B. fMLP (1 nM) was used as a positive control. Cells (106/mL) were placed in the upper wells, and 2.5 × 103 other cell types were placed in the lower chamber. Determination of the number of migrated cells was performed as previously described.20 In brief, following a 90-minute incubation at 37°C, the upper wells were washed with phosphate-buffered saline (PBS) and incubated 10 minutes in PBS supplemented with 1 mM ethylenediaminetetraacetic acid. Cells having migrated in the lower wells were harvested by a 5-minute centrifugation at 500g and taken up in 350 μL PBS. The number of migrated cells was determined by flow cytometric cell counting, and the migration index was obtained by dividing the number of cells in the test sample by the number of cells in the negative control.
Apoptosis and cytotoxicity assays
The sensitivity of CD56+ and CD56- T cells to FasL-mediated apoptosis was evaluated using different concentrations of FasL with or without 1 μg/mL cycloheximide in a 4-hour assay. Cells were washed twice with Annexin V binding buffer and stained with Annexin V and 7-Amino-Actinomycin D (7-AAD) according to the manufacturer's instructions. Cells positive for Annexin V and negative for 7-AAD were scored as apoptotic.21 The cytotoxic ability of lymphocytes toward NK-sensitive U937 and NK-resistant Raji targets22 was performed at effector-to-target (E/T) ratios ranging from 5:1 to 40:1. Targets were incubated with lymphocytes in RPMI-1640 for 4 hours at 37°C, and apoptotic cells were identified by annexin-V and 7-AAD staining.
Inhibitor studies
Different inhibitors were used to block indirect neutrophil chemotactic recruitment. The cell permeable caspase inhibitors YVAD-CHO and IETD-CHO were used at 100 μM, actinomycin D was used at 10 μg/mL, GolgiPlug (brefeldin A) was used at 1 μl/mL, lipid biosynthesis inhibitors NDGA and indomethacin were used at 200 μM and 300 μM, respectively. CD56+CD3+ cells were pretreated for 1 hour with the inhibitors, washed with PBS, and used in chemotaxis assays.
Supernatant transfer experiments
To assess soluble factor release, 2500 CD56+CD3+ cells/mL were treated with 30 ng/mL sFasL with 1 μg/mL enhancer or control antibody for 90 minutes at 37°C. Cells were removed by a 5-minute centrifugation at 500g, and supernatants were filtered through a 0.22-μM polycarbonate filter. Supernatants were clarified on an Amicon 8010 stirred cell (Millipore, Nepean, Canada) with a YM-100 membrane (100-kDa cutoff) and concentrated 5-fold on a stirred cell with a YM-1 membrane (1-kDa cutoff). Dilutions of concentrated supernatants were placed in the lower wells of the Boyden chamber, and migration assays were performed as described in “Chemotaxis assays.” The NOK-1 neutralizing anti-FasL antibody was added at a concentration of 200 ng/mL. Heat inactivation of supernatants was performed by a 30-minute incubation at 95°C.
Detection of chemokine release
To evaluate chemokine release, 2.5 × 105 CD56+CD3+ cells were treated for 2 hours at 37°C with 100 ng/mL sFasL with 3 μg/mL enhancer or control antibody in 3 mL. The supernatants were analyzed with the RayBio Human Cytokine Antibody Array C Series 1000 (RayBiotech, Norcross, GA) that allows us to detect 34 different chemokines. Membranes with prespotted capture antibodies were incubated with supernatants for 2 hours at 25°C, and chemokines were detected by incubation with biotinylated detection antibodies for 2 hours at 25°C. Following addition of horseradish peroxidase-conjugated streptavidin and overnight incubation at 4°C, signals were revealed by enhanced chemiluminescence (Amersham Biosciences). Signals were captured by a 10-minute exposure on a Chemigenius2 imaging system with GeneSnap version 6.03 (Synoptics, Cambridge, United Kingdom). Signal integration was performed with GeneTool version 3.04 (Synoptics), membrane negative control values were subtracted, and signal intensities were normalized against the membrane positive controls.
Results
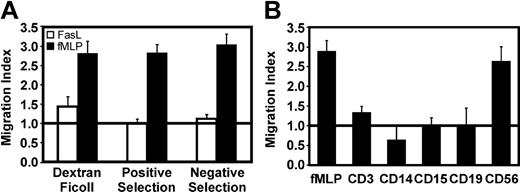
Reports have suggested that FasL was directly chemotactic toward neutrophils,9,10 while other studies could not corroborate these findings.11,12 To resolve this controversy we performed migration assays using granulocytes isolated by differential centrifugation or MACS selection. It has been reported that neutrophils respond maximally to 30 ng/mL recombinant sFasL.9,10 Using the same source of sFasL used by Ottonello et al,10 granulocytes did not migrate toward cross-linked sFasL at concentrations ranging from 5 ng/mL to 1 μg/mL (data not shown). Figure 1A clearly shows that 30 ng/mL sFasL did not induce granulocyte migration, regardless of the cell purification method used. MACS-positive selection using CD15 microbeads gave the highest cell purity (> 98%) and did not lead to unresponsiveness, since the response to fMLP was similar to cells obtained with other methods (Figure 1A). The slightly higher migration index observed with cells purified by Dextran-Ficoll might be linked to shear-stress activation compared with magnetic separation, as previously reported.23 With positive selection, viability was consistently more than 98% as assessed by dye exclusion, and the granulocyte population was composed on average of 95% neutrophils and 5% eosinophils, as assessed by HEMA-3 staining. Given that no significant differences in migration were observed between negatively or positively selected granulocytes, positive selection was used in all other experiments.
CD56+ cells are involved in FasL-mediated granulocyte migration. (A) Granulocyte migration was evaluated using fMLP (▪) or cross-linked FasL (□). Granulocytes were isolated by Dextran-Ficoll (> 90% pure), CD15+ selection (> 98% pure), or MACS depletion with CD3, CD14, CD19, and CD56 microbeads (> 95% pure). (B) Ability of other leukocytes to induce indirect FasL-mediated granulocyte migration. The horizontal line at migration index 1.0 indicates lack of chemotaxis. Cell purity was as follows: CD3 (> 98%), CD14 (> 75%), CD15 (> 98%), CD19 (> 95%), CD56 (> 95%). Data shown are the mean ± SEM of 3 different donors.
CD56+ cells are involved in FasL-mediated granulocyte migration. (A) Granulocyte migration was evaluated using fMLP (▪) or cross-linked FasL (□). Granulocytes were isolated by Dextran-Ficoll (> 90% pure), CD15+ selection (> 98% pure), or MACS depletion with CD3, CD14, CD19, and CD56 microbeads (> 95% pure). (B) Ability of other leukocytes to induce indirect FasL-mediated granulocyte migration. The horizontal line at migration index 1.0 indicates lack of chemotaxis. Cell purity was as follows: CD3 (> 98%), CD14 (> 75%), CD15 (> 98%), CD19 (> 95%), CD56 (> 95%). Data shown are the mean ± SEM of 3 different donors.
Since purified granulocytes failed to respond to Fas crosslinking, we investigated whether other leukocytes could restore migration, since other cell types have been shown to release chemokines upon Fas engagement.11,13,14,24 Different leukocyte subsets were obtained by MACS-positive selection and placed in the lower wells together with cross-linked FasL, while granulocytes were placed in the upper wells. Figure 1B shows that CD19+ cells (B cells), CD14+ cells (monocytes), or CD15+ cells (granulocytes) were unable to restore neutrophil migration. While CD3+ cells (T and NKT cells) gave a marginal migration increase, CD56+ cells (mostly NK cells) induced a migration similar to the fMLP control in 1.5-hour migration assays (Figure 1B). Longer migration assays of 3, 6, and 16 hours did not lead to an increase in migration index, arguing that de novo chemotactic factor production may not be involved (data not shown).
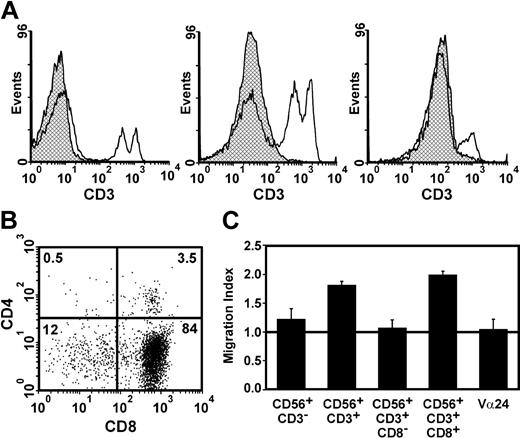
The slight increase in granulocyte migration induced by T cells prompted us to characterize the CD56+ cell population. Figure 2A shows different patterns of CD3 expression within purified CD56+ cells. The percentage of CD3+ cells among CD56+ cells varied between donors, ranging from 44% (Figure A, middle panel) to 7% (Figure A, last panel), and their absolute number varied in same donors at different time points, arguing that environmental factors might contribute to their abundance. The rare CD56+CD3+ cell subset, represented between 0.2% and 8% of circulating leukocytes (average, 2.5%) in 20 donors, a range similar to what has been described.25,26 CD4 and CD8 double staining of CD56+CD3+ cells (Figure 2B) revealed that the majority of cells were CD8+ (range, 55% to 96%; mean, 75% among 10 donors), as described by others.27-29 The ability of different CD56+ subsets to restore neutrophil migration was evaluated, and Figure 2C shows that CD3-CD56+ cells (NK cells) were unable to restore neutrophil migration, while CD3+CD56+ cells (presumably NKT cells) were the most efficient. When the CD56+CD3+ population was subdivided according to CD8 expression, the only subset able to induce migration was CD56+CD3+CD8+ cells. Even though NKT cells are usually CD4+ or CD4-CD8-,30 we investigated whether these cells contributed to granulocyte recruitment. Since conventional NKT cells express an invariant TCR composed of the Vα24 chain,30 Vα24 T cells obtained by MACS selection were used in migration assays. Figure 2C shows that Vα24+ did not induce granulocyte migration, arguing that unconventional NKT cells are involved in indirect chemotaxis.
T cells expressing CD56 mediate indirect granulocyte chemotaxis. (A) CD3 staining of CD56+ cells (> 98% pure) obtained by MACS-positive selection; isotypic control (▩), CD56+ cells (□). The profile of 3 different donors is shown. (B) Coreceptor expression pattern in CD56+CD3+ cells obtained by MACS. (C) Ability of different cell populations to induce indirect FasL-mediated granulocyte migration. The horizontal line at migration index 1.0 indicates lack of chemotaxis. Cell purity was as follows: CD56+CD3- (> 80%), CD56+CD3+ (> 95%), CD56+CD3+CD8- (> 85%), CD56+CD3+CD8+ (> 90%), Vα24+ cells (> 90%). Data shown are the mean ± SEM of 3 different donors.
T cells expressing CD56 mediate indirect granulocyte chemotaxis. (A) CD3 staining of CD56+ cells (> 98% pure) obtained by MACS-positive selection; isotypic control (▩), CD56+ cells (□). The profile of 3 different donors is shown. (B) Coreceptor expression pattern in CD56+CD3+ cells obtained by MACS. (C) Ability of different cell populations to induce indirect FasL-mediated granulocyte migration. The horizontal line at migration index 1.0 indicates lack of chemotaxis. Cell purity was as follows: CD56+CD3- (> 80%), CD56+CD3+ (> 95%), CD56+CD3+CD8- (> 85%), CD56+CD3+CD8+ (> 90%), Vα24+ cells (> 90%). Data shown are the mean ± SEM of 3 different donors.
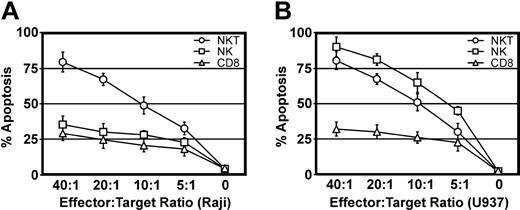
The markers expressed by NKT cells (CD3+CD8+CD56+) restoring neutrophil migration were similar to those expressed by ex vivo-expanded T cells termed cytokine-induced killer cells (CIKs). CIKs are obtained by in vitro culture of human peripheral blood mononuclear cells (PBMCs) with various cytokines.31 Most CIKs express an α/β TCR, CD3, CD8, CD45RO, CD56, CD95, and CD161.31,32 As one salient feature of CIKs is the ability to kill both NK-sensitive and NK-resistant targets,31 the cytotoxicity of CD56+CD3+ cells toward NK-sensitive (U937) and NK-resistant (Raji) targets was determined. Figure 3 shows that CD56+CD3+ cells were able to kill both U937 and Raji cells, while CD8+ T cells were ineffective, using effector-to-target ratios ranging from 40:1 to 5:1. While NK-sensitive cells were efficiently killed by CD56+CD3+ and NK cells, NK-resistant cells could only be killed by CD56+CD3+ cells. Thus, freshly isolated CD3+CD56+ NKT cells have cytotoxic abilities similar to CIKs.
CD56+CD3+ cells can kill NK-sensitive and NK-insensitive targets. The ability of CD8+ (▵), CD56+CD3- (□) and CD56+CD3+ (○) cells to kill NK-resistant Raji cells (A) and NK-sensitive U937 cells (B) was assessed at different E/T ratios. Cells were incubated together for 4 hours, and apoptotic cells were identified by Annexin V-PE and 7-Amino-Actinomycin D staining after gating the targets by forward and side scatter. Data shown are the mean ± SEM of duplicates from 2 different donors.
CD56+CD3+ cells can kill NK-sensitive and NK-insensitive targets. The ability of CD8+ (▵), CD56+CD3- (□) and CD56+CD3+ (○) cells to kill NK-resistant Raji cells (A) and NK-sensitive U937 cells (B) was assessed at different E/T ratios. Cells were incubated together for 4 hours, and apoptotic cells were identified by Annexin V-PE and 7-Amino-Actinomycin D staining after gating the targets by forward and side scatter. Data shown are the mean ± SEM of duplicates from 2 different donors.
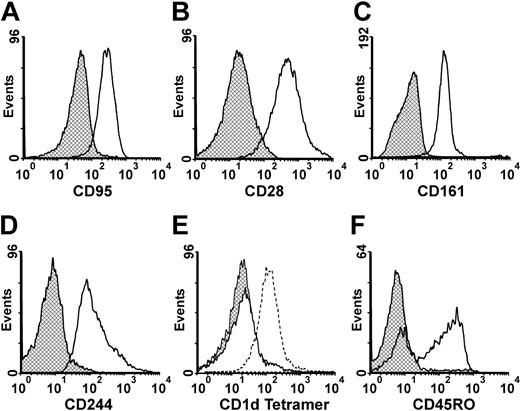
Given the functional and phenotypic similarities between NKT cells restoring granulocyte migration and CIKs, the presence of markers known to be expressed by CIKs was evaluated. CD56+CD3+ cells were all Fas and CD28 positive (Figure 4A-B). The CD28 antibody used for staining is specific to the CD28 T-cell variant not expressed by NK cells,33 arguing that these cells belong to the T-cell lineage. Figure 4C-D show that cells coexpressed the CD161 and CD244 NK markers.34 CD56+CD3+ cells were negative for the γ/δ TCR (data not shown), suggesting that these cells are α/β TCR positive. CD56+CD3+ cells were Vα24 negative (data not shown), as expected given that Vα24 cells were unable to induce indirect granulocyte migration (Figure 2C). Since CD1d-restricted NKT cells that do not express the invariant NKT TCR have been described,35 CD1d tetramer staining was performed. Figure 4E clearly shows that CD56+CD3+ cells did not stain with α-galcer-loaded CD1d tetramers, while Vα24+ cells were readily stained, confirming their lack of CD1d restriction.
Phenotype characterization of cells restoring granulocyte migration. (A) CD95 staining, isotypic control (▩) and CD56+CD3+ (□) cells. (B) CD28 staining, isotypic control (▩) and CD56+CD3+ (□) cells. (C) CD161 staining, cisotypic control (▩) and CD56+CD3+ (□) cells. (D) CD244 staining, isotypic control (▩) and CD56+CD3+ (□) cells. (E) CD1d tetramer staining, Vα24+ cells and unloaded CD1d tetramers (▩), CD56+CD3+ cells and α-galcer-loaded CD1d tetramers (□), and Vα24+ cells and α-galcer-loaded CD1d tetramers (dashed line). (F) CD45RO staining, isotypic control (▩) and CD56+CD3+ (□) cells.
Phenotype characterization of cells restoring granulocyte migration. (A) CD95 staining, isotypic control (▩) and CD56+CD3+ (□) cells. (B) CD28 staining, isotypic control (▩) and CD56+CD3+ (□) cells. (C) CD161 staining, cisotypic control (▩) and CD56+CD3+ (□) cells. (D) CD244 staining, isotypic control (▩) and CD56+CD3+ (□) cells. (E) CD1d tetramer staining, Vα24+ cells and unloaded CD1d tetramers (▩), CD56+CD3+ cells and α-galcer-loaded CD1d tetramers (□), and Vα24+ cells and α-galcer-loaded CD1d tetramers (dashed line). (F) CD45RO staining, isotypic control (▩) and CD56+CD3+ (□) cells.
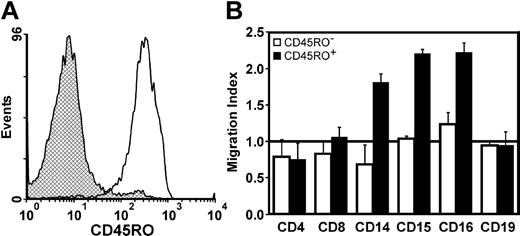
Given that CD56+CD3+ cells were able to kill targets in a manner similar to CIKs (Figure 3), which acquire the CD45RO memory T-cell marker upon in vitro culture in the presence of IL-2,36 the expression of CD45RO by CD56+CD3+ cells was evaluated. CD56+CD3+ cells were on average 65% positive for CD45RO (range, 50% to 90%) (Figure 4F), a percentage much higher than seen in normal circulating T cells.36 Since CD56+CD3+ cells were consistently bimodal for CD45RO expression, both populations were subdivided using CD45RA microbeads and used in migration assays. Figure 5A shows the purity of the 2 subsets by CD45RO staining (98% positive for CD45RO, 95% negative for CD45RA cells). Figure 5B shows that only CD45RO+ cells were able to induce neutrophil migration and were also able to induce NK cell and monocyte recruitment. The ability of CD56+CD3+ memory T cells to attract these different subsets shows that different chemokines are released since neutrophils, monocytes, and NK cells respond to different chemokines.37
CD56+CD8+CD45RO+ cells can recruit different cells of innate immunity. (A) CD56+CD8+ cells were depleted using CD45RA microbeads and stained for CD45RO expression; retained cells (▩) and flow-through (□). (B) Ability of memory CD56+CD3+ cells to recruit different leukocyte subsets in response to cross-linked FasL; CD45RO- cells (□) and CD45RO+ cells (▪). The horizontal line at migration index 1.0 indicates lack of chemotaxis. Cell purity was as follows: CD4+ T cells (> 95%), CD8+ T cells (> 90%), monocytes (> 80%), granulocytes (> 98%), NK cells (> 95%), B cells (> 95%). Data shown are the mean ± SEM of 2 different donors.
CD56+CD8+CD45RO+ cells can recruit different cells of innate immunity. (A) CD56+CD8+ cells were depleted using CD45RA microbeads and stained for CD45RO expression; retained cells (▩) and flow-through (□). (B) Ability of memory CD56+CD3+ cells to recruit different leukocyte subsets in response to cross-linked FasL; CD45RO- cells (□) and CD45RO+ cells (▪). The horizontal line at migration index 1.0 indicates lack of chemotaxis. Cell purity was as follows: CD4+ T cells (> 95%), CD8+ T cells (> 90%), monocytes (> 80%), granulocytes (> 98%), NK cells (> 95%), B cells (> 95%). Data shown are the mean ± SEM of 2 different donors.
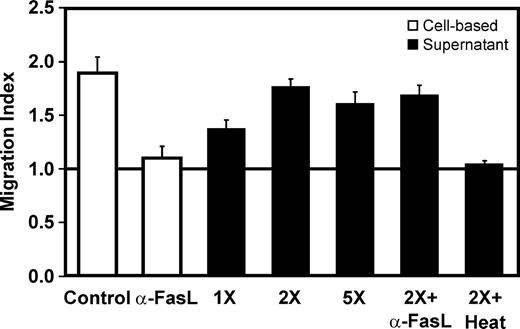
To ascertain whether CD56+CD3+ cells release soluble chemotactic factors, supernatant transfer experiments were performed. Purified CD56+CD3+ cells were treated with FasL and the clarified concentrated supernatants obtained were used in migration assays at different dilutions. Figure 6 shows that in cell-based assays, granulocyte migration was totally abrogated by addition of 200 ng/mL neutralizing FasL antibody, confirming the requirement of Fas-FasL interaction in cell recruitment. When clarified supernatants were placed in the lower chamber, neutrophils migrated with an efficiency similar to what was observed in cell-based assays. Since cross-linked FasL was still present in the clarified supernatant, the neutralizing FasL antibody was added to the amount of supernatant giving the maximal response. Migration was not affected by antibody addition, confirming that granulocytes do not respond to FasL and demonstrating that CD56+CD3+ cells release chemotactic factors upon Fas engagement. Supernatant heat inactivation completely abrogated the chemotactic effect, suggesting that the factors released are polypeptides. Checkerboard analysis was performed10 and revealed that supernatants derived from FasL-treated CD56+CD3+ cells did not increase neutrophil chemokinesis, confirming the presence of chemotactic factors (data not shown). To identify the chemokines secreted by CD56+CD3+ cells, protein array analysis was performed using supernatants obtained from FasL-treated cells. The chemokines tested included 22 CCL, 10 CXCL, 1 XCL, 1 CX3CL listed in Table 1 and show that CD56+CD3+ cells secreted CCL1, CCL5, CCL7, CCL8, CCL17,and CCL27 and CXCL1,2,3, CXCL8, CXCL11, and CXCL12 following Fas engagement. Among these, strong production of CCL5, CXCL1,2,3, and CXCL8 was detected.
Soluble chemotactic factors are released upon Fas engagement. Indirect granulocyte recruitment and efficiency of the neutralizing FasL antibody in cell-based assays (□). Granulocyte migration induced by CD56+CD3+ supernatants (▪); cells were treated with FasL, supernatants were cleared on a 100-kDa filter and concentrated 5-fold on a 1-kDa filter. Supernatant dilutions were used in migration assays alone or with the neutralizing NOK-1 FasL antibody. The horizontal line at migration index 1.0 indicates lack of chemotaxis. The presence of labile factors in the supernatant was confirmed by heat-inactivation of 30 minutes at 95°C. Data shown are the mean ± SEM of 2 independent experiments.
Soluble chemotactic factors are released upon Fas engagement. Indirect granulocyte recruitment and efficiency of the neutralizing FasL antibody in cell-based assays (□). Granulocyte migration induced by CD56+CD3+ supernatants (▪); cells were treated with FasL, supernatants were cleared on a 100-kDa filter and concentrated 5-fold on a 1-kDa filter. Supernatant dilutions were used in migration assays alone or with the neutralizing NOK-1 FasL antibody. The horizontal line at migration index 1.0 indicates lack of chemotaxis. The presence of labile factors in the supernatant was confirmed by heat-inactivation of 30 minutes at 95°C. Data shown are the mean ± SEM of 2 independent experiments.
Chemokine expression pattern by CD1d-unrestricted NKT cells
Chemokine . | IgG . | FasL . | Induction . |
|---|---|---|---|
| CCL1 | - | +/- | 1.8 |
| CCL2 | - | - | NI |
| CCL3 | +++ | +++ | NI |
| CCL4 | ++ | ++ | NI |
| CCL5 | +/- | +++ | 4.7 |
| CCL7 | - | +/- | 2.0 |
| CCL8 | +/- | + | 1.6 |
| CCL11 | - | - | NI |
| CCL13 | +/- | + | NI |
| CCL15 | +/- | +/- | NI |
| CCL16 | - | - | NI |
| CCL17 | - | +/- | 3.2 |
| CCL18 | - | - | NI |
| CCL19 | ++ | ++ | NI |
| CCL20 | +/- | +/- | NI |
| CCL22 | +++ | +++ | NI |
| CCL23 | ++ | ++ | NI |
| CCL24 | +/- | +/- | NI |
| CCL25 | - | - | NI |
| CCL26 | +/- | +/- | NI |
| CCL27 | + | ++ | 1.7 |
| CCL28 | - | - | NI |
| CXCL1 | +/- | + | 1.5 |
| CXCL1,2,3 | - | + | 3.1 |
| CXCL5 | - | - | NI |
| CXCL6 | +/- | +/- | NI |
| CXCL7 | + | +/- | NI |
| CXCL8 | + | ++ | 2.4 |
| CXCL9 | +/- | +/- | NI |
| CXCL11 | +/- | + | 2.1 |
| CXCL12 | - | +/- | 2.0 |
| CXCL13 | + | + | NI |
| XCL1 | + | ++ | NI |
| CX3CL1 | + | + | NI |
Chemokine . | IgG . | FasL . | Induction . |
|---|---|---|---|
| CCL1 | - | +/- | 1.8 |
| CCL2 | - | - | NI |
| CCL3 | +++ | +++ | NI |
| CCL4 | ++ | ++ | NI |
| CCL5 | +/- | +++ | 4.7 |
| CCL7 | - | +/- | 2.0 |
| CCL8 | +/- | + | 1.6 |
| CCL11 | - | - | NI |
| CCL13 | +/- | + | NI |
| CCL15 | +/- | +/- | NI |
| CCL16 | - | - | NI |
| CCL17 | - | +/- | 3.2 |
| CCL18 | - | - | NI |
| CCL19 | ++ | ++ | NI |
| CCL20 | +/- | +/- | NI |
| CCL22 | +++ | +++ | NI |
| CCL23 | ++ | ++ | NI |
| CCL24 | +/- | +/- | NI |
| CCL25 | - | - | NI |
| CCL26 | +/- | +/- | NI |
| CCL27 | + | ++ | 1.7 |
| CCL28 | - | - | NI |
| CXCL1 | +/- | + | 1.5 |
| CXCL1,2,3 | - | + | 3.1 |
| CXCL5 | - | - | NI |
| CXCL6 | +/- | +/- | NI |
| CXCL7 | + | +/- | NI |
| CXCL8 | + | ++ | 2.4 |
| CXCL9 | +/- | +/- | NI |
| CXCL11 | +/- | + | 2.1 |
| CXCL12 | - | +/- | 2.0 |
| CXCL13 | + | + | NI |
| XCL1 | + | ++ | NI |
| CX3CL1 | + | + | NI |
- indicates < 3000 signal; +/-, 3000 to 6000 signal; +, 6000 to 10 000 signal; ++, 10 000 to 15 000 signal; +++, > 15,000 signal; NI, not induced. Chemokines induced 1.5-fold or more were considered to be induced.
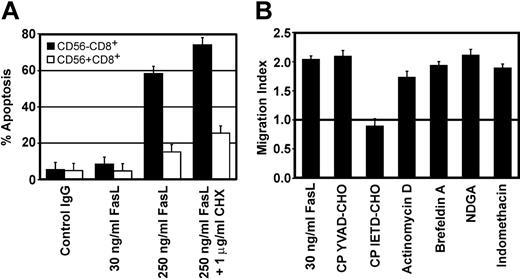
Since cell apoptosis can participate in chemokine release,24,38,39 the sensitivity of CD56+CD3+ cells to FasL-mediated apoptosis was evaluated. Figure 7A clearly shows that CD56+CD3+ cells are much more resistant to apoptosis than CD56-CD3+ lymphocytes and that the dose of FasL used in chemotaxis assays (30 ng/mL) causes negligible cell death. Interestingly, CIKs are also more resistant to FasL-mediated apoptosis.40 Since Fas engagement usually leads to caspase-8 activation, we evaluated the contribution of caspase activity in chemotaxis. While pretreatment of CD56+CD3+ cells with the caspase-1 inhibitor had no effect, the caspase-8 inhibitor completely abrogated neutrophil recruitment (Figure 7B). This concentration of caspase-8 inhibitor was able to completely block NKT cell apoptosis induced by high doses of FasL (data not shown).
Chemokine release depends on caspase-8 activity in the absence of apoptosis. (A) Cells were incubated for 4 hours with different amounts of cross-linked FasL, with or without cycloheximide (CHX) and apoptotic cells were scored by FACS using Annexin V staining; CD56-CD8+ cells (□) and CD56+CD8+ cells (▪). (B) CD56+CD3+ cells were pretreated for 1 hour with different inhibitors, washed, and used in indirect neutrophil migration assays. The horizontal line at migration index 1.0 indicates lack of chemotaxis. Concentrations used were 100 μM for the cell permeable caspase-1 inhibitor (CP YVAD-CHO) and the cell permeable caspase-8 inhibitor (CP IETD-CHO), 10 μg/mL for actinomycin D, 1 μL/mL GolgiPlug (brefeldin A), 200 μM for NDGA, and 300 μM for indomethacin. Data shown are the mean ± SEM of triplicates from 1 donor.
Chemokine release depends on caspase-8 activity in the absence of apoptosis. (A) Cells were incubated for 4 hours with different amounts of cross-linked FasL, with or without cycloheximide (CHX) and apoptotic cells were scored by FACS using Annexin V staining; CD56-CD8+ cells (□) and CD56+CD8+ cells (▪). (B) CD56+CD3+ cells were pretreated for 1 hour with different inhibitors, washed, and used in indirect neutrophil migration assays. The horizontal line at migration index 1.0 indicates lack of chemotaxis. Concentrations used were 100 μM for the cell permeable caspase-1 inhibitor (CP YVAD-CHO) and the cell permeable caspase-8 inhibitor (CP IETD-CHO), 10 μg/mL for actinomycin D, 1 μL/mL GolgiPlug (brefeldin A), 200 μM for NDGA, and 300 μM for indomethacin. Data shown are the mean ± SEM of triplicates from 1 donor.
To understand the mechanisms involved in chemokine release, chemical inhibitor studies were performed. Since lipid mediators such as leukotrienes and prostaglandins can induce neutrophil migration,41 their contribution in indirect neutrophil recruitment was evaluated. NDGA is a selective lipoxygenase inhibitor (inhibitory concentration 50% [IC50], 0.2 μM for 5-lipoxygenase [5-LOX and 30 μM for 12-LOX and 15-LOX) and was used to inhibit leukotriene production.42 Indomethacin, a broad specificity cyclooxygenase inhibitor (IC50, 0.74 μM for cyclooxygenase-1 [COX-1]; 0.97 μM COX-2) was used to inhibit prostaglandin production.43 Figure 7B shows that CD56+CD3+ cells pretreated with doses of inhibitors vastly superior to the IC50 of targeted enzymes did not reduce granulocyte recruitment, confirming that chemokines are the only chemotactic factors released. Actinomycin D, a transcription inhibitor, and brefeldin A, an inhibitor of Golgi transport, were used to pretreat CD56+CD3+ cells before performing chemotaxis assays. Figure 7B shows that these inhibitors did not prevent neutrophil recruitment. Altogether, these results show that preformed chemokines present in post-Golgi compartments are released in a caspase-8-dependent manner but in the absence of cell apoptosis.
Discussion
Given the controversy surrounding the direct chemotactic activity of FasL,9-12 we rigorously evaluated this phenomenon. Our data show that purified granulocytes did not migrate in response to concentrations of recombinant sFasL used by others.9,10 This discrepancy might be due to differences in methods used for granulocyte purification and/or migration analysis. Since in vivo studies have suggested that other cell types might participate in FasL-mediated cell recruitment, we evaluated their in vitro contribution. Among different leukocyte subsets analyzed, only CD56+CD3+CD8+ cells were able to induce rapid granulocyte recruitment following Fas engagement. Not only could these cells recruit neutrophils, they also released CC and CXC chemokines active toward monocytes and NK cells. Phenotypic characterization revealed that CD56+CD3+ cells were positive for CD161 and CD244, NK cell markers expressed by a subset of CD8+ memory T cells.44 Even though conventional NKT cells are usually CD4+ or double negative, in humans some CD8+ NKT cells that coexpress CD56 and CD161 exist.45 Our results show that these NKT cells are distinct from conventional NKT since they were not CD1d restricted and did not express the invariant TCR.
Expression of CD56 by cytotoxic T cells has been shown in a population termed CIKs. CIKs are CD8+ T cells that express CD56 and are obtained by in vitro culture of PBMCs in the presence of cytokines such as interferon-γ (IFN-γ) and IL-2 together with anti-CD3 antibodies.26,46 Since the procedure used to obtain CIKs involves bulk PBMC cultures, it remains unclear whether NK markers become acquired or a preexisting population becomes selectively expanded. Since CIKs are able to kill NK-sensitive and NK-insensitive targets, the cytotoxic ability of CD56+CD3+ cells was evaluated. Freshly isolated CD56+CD3+ T cells were able to kill both targets in a manner similar to CIKs. In the case of CIKs, a correlation between CD56 expression and increased cytotoxicity has been suggested.26 It remains unclear whether CD56 plays a role in cell killing since this adhesion molecule does not induce cell signaling.47 The ability of CD56+CD3+ cells to kill NK-sensitive targets might be linked to the acquisition of NK markers such as CD161 and CD244. CD161 is an activating NK receptor that increases cytotoxicity and induces IFN-γ secretion.48 CD244 is a member of the CD2 family that can provide costimulation and enhance major histocompatibility complex (MHC)-independent cytotoxicity through its ability to associate with linker for activation of T cells (LAT) and transduce activation signals. The coordinate expression of activating NK receptors by CD56+CD3+ cells might contribute to their ability to kill targets in a MHC-independent manner. Such a developmental program would allow cytotoxic T cells to eradicate transformed or virus-infected cells with reduced MHC-I levels.
The NKT cells described here represent a minor subset of total circulating lymphocytes (2.5% on average). Given that the absolute number of CD56+CD3+ T cells varied between donors and time points, environmental factors appear to participate in expansion of this subset. The acquisition of CD56 and CD161 NK markers in CD8+ T cells has been proposed to be indicative of antigen-experienced T cells, as shown by the expansion of CD56+CD3+ T cells in mice and humans following viral infections.49,50 It has also been shown that the absolute number of CD56+CD3+ cells gradually increases in older individuals while this subset is absent in cord blood material,51 suggesting that this accumulation is due to repeated antigen exposure. In mice, a subset of CD8+ memory T cells undergoes extensive proliferation in the presence of IL-2 or IL-15, independently of TCR stimulation.27 Upon cytokine exposure, these cells acquire NK markers such as CD244 and can kill syngeneic tumors in a CD48-dependent manner.52
Since CD45RO was expressed by about 65% of CD56+CD3+ cells, this argues that NK markers were acquired prior to becoming positive for this memory marker. The expression of CD45RO by CD56+CD3+ cells suggests that these cells might be mature CD8+ T cells having acquired NK markers upon repeated antigenic stimulation, as described by others.27 Our results show that only memory CD56+CD3+ T cells have the ability to rapidly release chemokines active toward different cells of the innate immune system. Given that CD56+CD3+ cells can kill NK-sensitive and insensitive targets, this additional specialization might contribute to the destruction of tumors or virus-infected cells attempting to exploit FasL-mediated immune privilege.
FasL-expressing tumors or grafts have been shown to be rejected by neutrophil infiltration in nude mice, arguing that T cells do not play a significant role in this phenomenon.53,54 However, it has been reported that nude mice harbor a population of cells that coexpress CD3 and CD56, and it was suggested that these might be of thymus-independent origin.55 Thymus-independent T cells are likely to be self-specific, as illustrated by the fact that thymectomized female mice do not develop T cells reactive against the male H-Y antigen, while thymectomized males do.56 If CD56+CD3+ cells present in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) and nude mice release chemokines following Fas engagement like the human cells described in this manuscript, this might explain the neutrophil-mediated rejection of FasL-expressing cells observed in these models.53,54
As the Fas-FasL pathway plays a major role in the resolution of immune response through activation-induced cell death, we investigated the sensitivity of CD56+CD3+ cells to FasL-mediated apoptosis. The low doses of FasL required for maximal neutrophil recruitment did not induce CD56+CD3+ cell apoptosis. Furthermore, these memory T cells were much more resistant to FasL-mediated apoptosis than naive CD8+ T cells. It has been shown that memory T cells have increased levels of survivin and Bcl-2, rendering them more resistant to FasL-mediated apoptosis,57,58 and this might explain the apoptotic resistance of CD56+CD3+ cells. While apoptosis has been shown to participate in chemokine release,24,38,39 this is clearly not the case in this instance. Surprisingly, caspase inhibition revealed that chemotactic factor release required effector caspase-8 activity, but not caspase-1, which is involved in inflammasome formation and IL-1β processing.59 Thus, chemokine release by unconventional NKT cells is a novel example of nonapoptotic Fas signaling.
Fas engagement can mediate nonapoptotic signaling through transcription factor activation leading to cytokine or chemokine expression in different cell types.13 Serum-starved fibroblasts up-regulate IL-6 and IL-8 levels upon Fas engagement through an extracellular signal-regulated kinase (ERK)- and nuclear factor-κB (NF-κB)-dependent pathway.14 Caspase inhibition in FasL-treated T cells blocks apoptosis and unmasks proinflammatory cytokine production mediated through ERK and NF-κB activation.15 Since blocking de novo transcription with actinomycin D did not abrogate chemokine release by CD56+CD3+ cells, it is unlikely that transcription factor activation plays a role in this phenomenon. Caspase activity has been shown to participate in T-cell activation, and selective caspase substrate cleavage occurs in activated T cells in the absence of apoptosis.16,17 Given that CD56+CD3+ cells did not undergo apoptosis, yet depended on caspase-8 activity for chemokine release, it is possible that selective caspase substrate cleavage contributes to chemokine secretion.
The chemotactic factors released were not lipid mediators since inhibition of the COX and LOX pathways did not abrogate neutrophil recruitment. Protein array analysis identified CCL1, CCL5, CCL7, CCL8, CCL17, and CCL27 and CXCL1,2,3, CXCL8, CXCL11, and CXCL12 as being secreted by CD56+CD3+ cells following Fas engagement. The chemokines produced correspond to factors known to recruit innate immune cells since neutrophils respond to CXCL1,2,3, CXCL8, and CCL5; NK cells respond to CCL1, CCL5, CCL7, CCL8 and CXCL1,2,3, CXCL8, CXCL11, CXCL12, while monocytes are attracted by CCL5, CCL7, CCL8, and CXCL12.60-62 Among secreted chemokines, CCL5, which can attract all 3 cell subsets, and CXCL8, which is chemotactic toward NK cells and granulocytes, showed major increases in their concentrations. Human CD8+ memory T cells were shown to possess unique CCL5-containing granules that can be rapidly released in a brefeldin A-insensitive manner following TCR stimulation,63 and such granules might also exist in this NKT cell subset. Indeed we found that chemokine release was not impaired by actinomycin D or brefeldin A treatment, arguing that preformed chemokines are present in post-Golgi compartments.
Although CD56+CD3+CD45RO+ cytotoxic T cells represent a small fraction of circulating lymphocytes, they appear to be a specialized subset that could play a role in cancer cell destruction by direct cell killing combined to their unique ability to induce indirect innate immune cell recruitment. The ability of neutrophils to destroy tissues has been abundantly described in attempts to induce artificial immune privilege.7,64,65 Given that cells described in this manuscript can induce the recruitment of different cells of innate immunity, their inappropriate activation could have disastrous inflammatory consequences if they are self-restricted. It would be of interest to assess whether individuals suffering from autoimmune diseases show functional or numerical defects in this specialized cell subset. In summary, we have identified a novel T-cell subset that has the ability to recruit different leukocytes of the innate immune system upon Fas engagement. These cells possess activating NK receptors that might contribute to their cytotoxicity and can secrete CC and CXC chemokines in a caspase-dependent manner without undergoing apoptosis. Further characterization of signaling mechanisms involved in chemokine secretion might allow identifying novel therapeutic targets for controlling inflammatory diseases.
Prepublished online as Blood First Edition Paper, September 2, 2004; DOI 10.1182/blood-2004-04-1537.
Supported by grants from Roche Organ Transplantation Research Foundation (ROTRF) (grant 472805283) and Canadian Institutes of Health Research (CIHR) (grant MOP-55848) (F.D.) and by a Fonds de Recherche en Santé du Québec (FRSQ) PhD Fellowship (M.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mitchell Kronenberg (La Jolla Institute for Allergy and Immunology, La Jolla, CA) for providing PE-conjugated CD1d tetramers loaded or not with α-galactosyl ceramide.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal