Abstract
Activating mutations of the Fms-like tyrosine kinase 3 (FLT3) receptor are the most common genetic alteration in acute myeloid leukemia (AML). Two distinct groups of FLT3 mutations are found: internal tandem duplications (ITDs) of the juxtamembrane region and point mutations within the tyrosine kinase domain (TKD). Recently, point mutations within the activation loop of FLT3 have also been described in childhood acute lymphoblastic leukemia (ALL). FLT3-ITD has been shown to induce a myeloproliferative syndrome in a murine bone marrow transplantation model. The phenotype of FLT3-TKD in mice has not yet been investigated. We transduced murine bone marrow with retrovirus-expressing FLT3-TKD mutants or FLT3-ITD and transplanted these cells into lethally irradiated mice. Mice that received a transplant of FLT3-ITD developed an oligoclonal myeloproliferative disease as previously described. In contrast, FLT3-TKD mutants induced an oligoclonal lymphoid disorder with longer latency and distinct hematologic manifestations: importantly, induction of the lymphoid phenotype was not due to a low number of transplanted cells. The lymphoid manifestation and longer latency of FLT3-TKD compared with FLT3-ITD mutants together with the lack of influence of FLT3-TKD mutations on the clinical outcome of patients with AML suggest differences in cell signaling between FLT3-TKD mutants and FLT3-ITDs. Indeed strong signal transducers and activators of transcription 5 (STAT5) activation could only be demonstrated for FLT3-ITDs. (Blood. 2005;105:4792-4799)
Introduction
The receptor tyrosine kinase (RTK) Fms-like tyrosine kinase 3 (FLT3) belongs to the class III RTK subfamily that also includes c-kit receptor tyrosine kinase (KIT), FMS, and platelet-derived growth factor receptor.1,2 These class III RTK members are characterized by an extracellular domain consisting of 5 immunoglobulin-like domains, a juxtamembrane domain, and 2 kinase domains (KDs) interrupted by a kinase insert.3 Ligand binding to the extracellular domain results in dimerization of the receptor followed by autophosphorylation on specific intracellular tyrosine residues. Subsequently, multiple downstream signaling pathways are activated.4-6 Activation of the FLT3 receptor with its ligand (FL) plays an important role in proliferation and differentiation of early hematopoietic progenitors.7-10
The FLT3 receptor tyrosine kinase is expressed on blast cells in most patients with acute myeloid leukemia (AML), and activating mutations of FLT3 have been detected in approximately 30% of these patients.11 Two distinct groups of FLT3 mutations are most common: internal tandem duplications (ITDs) of the juxtamembrane coding sequence in 20% to 27% of patients with AML12-14 and point mutations within the activation loop (A-loop) of the second tyrosine kinase domain (TKD) in about 7% of patients with AML.15,16 Both types of mutations constitutively activate the FLT3 receptor, leading to activation of downstream signaling proteins, including signal transducers and activators of transcription 5 (STAT5) and mitogen-activated protein (MAP) kinase, and result in factor-independent proliferation of growth factor-dependent murine lymphoid and myeloid cells.16-20
FLT3-ITD is recognized as a significant prognostic factor in AML,13,21-24 whereas FLT3-TKD mutations do not seem to have an impact on the prognosis of patients with AML.13,25-27 Recently, point mutations of codons 835/836 within the tyrosine kinase domain of FLT3 were also found in infant and childhood acute lymphoblastic leukemia (ALL), especially in the setting of mixed-lineage leukemia (MLL) gene rearrangement28,29 and T-ALL.30 In contrast, FLT3-ITD mutations are rarely found in pediatric and adult ALL.12,31-34
In transgenic mice and in a murine bone marrow transplantation (BMT) model, FLT3-ITD induces a myeloproliferative disease (MPD).35,36 However, the effect of FLT3-TKD mutations in vivo has not yet been defined.
In this study, we investigated the role of FLT3-TKD mutants in vivo using a murine retroviral infection/BMT model. For this purpose, we compared FLT3-ITD, FLT3 D835Y (the most common kinase domain mutant in AML11 ), and FLT3 I836M+R (a rarely found kinase domain mutant13 ). We report that mice that received a transplant of FLT3-ITD develop an oligoclonal myeloproliferative disorder, as previously described.35 In contrast, mice that received a transplant of FLT3-TKD mutants develop a lymphoid disease with distinct hematologic manifestations.
Materials and methods
Growth factors and antibodies
Recombinant mouse FLT3 ligand (FL), interleukin-3 (IL-3), interleukin-6 (IL-6), and recombinant murine stem cell factor (SCF) were purchased from R&D Systems (Wiesbaden, Germany). Rabbit polyclonal anti-mouse FLT3 antibody and rabbit anti-STAT5 A/B antiserum were obtained from Upstate Biotechnology (Lake Placid, NY). Monoclonal phosphospecific STAT5 A/B antibody was a generous gift from Tom Wheeler (Hamilton, New Zealand).37 Antiphosphotyrosine antibodies were purchased from Upstate Biotechnology (4G10) and PharMingen (Heidelberg, Germany; pY20). Phosphospecific AKT antibody (Ser473), rabbit polyclonal p44/42 MAP kinase (extracellular signal-related kinase 1 [ERK1]/ERK2) antibody, and phosphospecific ERK1/ERK2 antibody were obtained from Cell Signaling (Frankfurt/Main, Germany). Goat polyclonal AKT1/2 antibody was purchased from Santa Cruz Biotechnology (Heidelberg, Germany).
DNA constructs
The cDNA of murine wild-type FLT3 and FLT3-ITD was kindly provided by Hubert Serve (Münster, Germany).20 Site-directed mutagenesis of D835 and I836 (numbering is based on the human FLT3) was performed with a QuikChange Site-Directed Mutagenesis Kit (Stratagene, Heidelberg, Germany) according to the manufacturer's instructions. All constructs were confirmed by sequencing. The retroviral vector was constructed by cloning the FLT3 cDNA into the MigRI retroviral vector coexpressing the enhanced green fluorescent protein (EGFP; a kind gift from W. Pear, Philadelphia, PA).38 In the higher-expressing FLT3-ITD construct the FLT3 cDNA is cloned into the multiple cloning site (MCS) of the vector starting directly with the start codon ATG, whereas the low-expressing construct contains 80 bp of the untranslated region upstream of the ATG.
Cell culture, retrovirus preparation, and helper virus assay
Phoenix E helper virus-free ecotropic packaging cells (G. Nolan, Stanford, CA) and NIH3T3 cells were maintained in Dulbecco modified Eagle medium (DMEM; GIBCO-BRL, Karlsruhe, Germany) supplemented with 10% fetal calf serum (FCS).
Phoenix E cells were transiently transfected using Lipofectamine 2000 (Invitrogen, Karlsruhe, Germany) and retroviral stocks were collected twice at 12-hour intervals beginning 24 hours after transfection. Retrovirus was titered by transduction of 5 × 104 NIH3T3 cells with serial dilutions of retrovirus in the presence of 4 μg/mL polybrene (Sigma, Deisenhofen, Germany). Forty-eight-hour posttransduction percentage of infected cells was determined by flow cytometric analysis of EGFP expression. The titer was calculated by multiplication of the total number of EGFP-positive cells with the dilution factor of the retroviral supernatant.
We tested for the presence of replication-competent viral particles by transferring supernatant, in which leukemic cells derived from spleens of diseased mice had been grown for more than 24 hours, onto NIH3T3 cells. The NIH3T3 cells were subsequently analyzed for EGFP expression by fluorescence-activated cell sorter (FACS) analysis.
Transduction and transplantation of murine bone marrow
Murine bone marrow was collected and transduced as described previously.39 Briefly, bone marrow was harvested from male Balb/C donor mice 4 days after injection of 150 mg/kg 5-fluorouracil (5-FU; Ribosepharm, Munich, Germany) and prestimulated overnight in Iscove modified Dulbecco medium (IMDM)/20% FCS supplemented with growth factors (10 ng/mL murine IL-3 [mIL-3], 10 ng/mL mIL-6, 50 ng/mL mSCF). Cells were transduced by 4 rounds spin infection (1200g, 32°C, 90 minutes) every 12 hours in retroviral supernatant supplemented with growth factors and 4 μg/mL polybrene (Sigma). Subsequently, cells were resuspended in Hanks balanced salt solution (Sigma-Aldrich, Irvine, United Kingdom) and injected into the tail vein of lethally irradiated (8 Gy [800 rad]) female Balb/C recipient mice. Animals that received a transplant were monitored for signs of disease development by serial measurement of peripheral blood (PB) counts. All animals were caged in a special caging system (Thoren Caging Systems, Hazleton, PA) with autoclaved food and acidified water. All procedures were reviewed and approved by the university's supervisory animal care committee.
Flow cytometric immunophenotyping (FACS analysis)
Single-cell suspension of indicated tissue samples were prepared and red blood cells of peripheral blood were lysed prior to analysis. Cells were preincubated with Fc-block and CD45-CyChrome 5 (Cy5) and subsequently stained with either phycoerythrin (PE)-conjugated anti-CD11b (Mac-1), anti-CD45R/B220, anti-CD90.2 (Thy1.2), anti-CD4, or anti-CD8a antibodies. Fc-block and all antibodies were purchased from BD Pharmingen (Heidelberg, Germany). Dead cells were excluded by propidium iodide staining.
Southern blot analysis
Genomic DNA was prepared from spleen cells of diseased mice by proteinase K digestion and phenol/chloroform precipitation. After digestion of 10 μg total DNA with EcoRI and electrophoretic separation, the DNA was transferred to a GeneScreenPlus nylon membrane (NEN Life Science Products, Boston, MA) and hybridized with a P32-labeled 0.7-kilobase (kb) EGFP fragment to identify retroviral integration sites by autoradiography.
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting were performed as described previously.17 Briefly, cells were lysed in lysis buffer containing 10 mM tris(hydroxymethyl)aminomethane-HCl (Tris-HCl) (pH 7.4), 5 mM EDTA (ethylenediaminetetraacetic acid), 130 mM NaCl, 1% Triton X-100, 20 mM sodium phosphate (pH 7.5), 10 mM sodium pyrophosphate (pH 7.0), 50 mM NaF, 1 mM sodium orthovanadate, 1 mM glycerolphosphate, and protease inhibitors (Roche Diagnostics, Mannheim, Germany). After clarification by centrifugation and preclearing with protein A-Sepharose (Amersham/Pharmacia Biotech, Freiburg, Germany), 2 μg anti-FLT3 antibody was added. Antibody-protein complexes were precipitated with protein A-Sepharose. For immunoblotting and immunoprecipitation, whole-cell lysates and bound fractions, respectively, were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotting was performed on polyvinylidene fluoride (PVDF) membranes (Immobilon-P; Millipore GmbH, Eschborn, Germany). Detection of phosphotyrosine was performed using a mixture of the antiphosphotyrosine antibodies 4G10 and pY20. For detection of FLT3, phosphorylated AKT1/2 (p-AKT1/2), AKT1/2, p-ERK1/2, ERK1/2, p-STAT5, and STAT5, the indicated antibodies were used. After incubating the blots with horseradish peroxidase-conjugated secondary antibody, they were developed using SuperSignal chemiluminescent substrates from Pierce (Perbio Science GmbH, Bonn, Germany).
Results
Similar transduction efficacy results in equal FLT3 protein expression in murine bone marrow cells
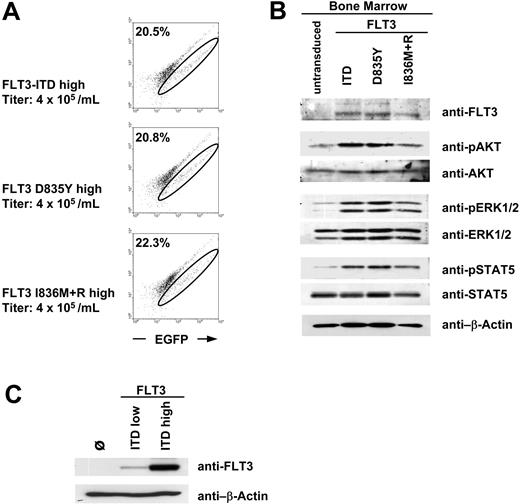
The comparative investigation of the transforming capability of different FLT3 mutants in vivo requires a similar transduction efficacy and comparable protein expression of these mutants in the transduced murine bone marrow. We generated murine ecotropic retrovirus expressing either FLT3 D835Y, FLT3 I836M+R, or FLT3-ITD using a bicistronic retroviral vector coexpressing the FLT3 mutants together with the enhanced green fluorescent protein (EGFP) via an internal ribosomal entry site (IRES). Bone marrow of 5-FU-treated donor mice was transduced with each retrovirus at a titer of 4 × 105 retroviral particles per milliliter and analyzed by flow cytometry for EGFP expression. Transduction of bone marrow with equivalent titers of each FLT3 mutant resulted in a comparable fraction of EGFP-positive cells (Figure 1A). Western blot analysis confirmed that the transduced bone marrow cells expressed comparable protein levels of each FLT3 mutant (Figure 1B). Next, we investigated whether kinase activity of the mutants leads to constitutive activation of downstream signaling cascades and examined the phosphorylation status of AKT, ERK, and STAT5. As shown in Figure 1B, all BM cells expressing mutant FLT3 receptors showed constitutive phosphorylation of AKT, ERK, and STAT5. These results suggested that the FLT3-TKD mutants are also able to activate these signaling pathways in primary hematopoietic cells. However, since the bone marrow cells were not starved before lysis, part of the activation of these proteins is due to the residual cytokines of the infection procedure.
Expression analysis of retroviral FLT3 constructs. (A) Flow cytometric analysis of murine bone marrow retrovirally transduced with FLT3 high-expressing constructs. A fluorescein isothiocyanate (FITC)-versus-PE channel dot plot is shown without compensation to exclude autofluorescent cells. The EGFP-positive population shifts on a diagonal to the right. The circle indicates the EGFP-positive (FLT3) cells. Numbers in top left corners indicate the percentage of EGFP+ cells. (B) Western blot analysis of bone marrow shown in panel A. Bone marrow cells were not starved from the cytokines of the infection procedure before preparation of total cell lysates. Similar transduction efficacy for different FLT3 mutants results in equal FLT3 protein expression and activation of downstream targets in murine bone marrow. The cells loaded in the first lane (“untransduced”) were treated like the FLT3-transduced cells but without retrovirus during the infection procedure. (C) Comparison of FLT3 protein expression of the high- and low-expressing FLT3-ITD constructs in Phoenix E cells.
Expression analysis of retroviral FLT3 constructs. (A) Flow cytometric analysis of murine bone marrow retrovirally transduced with FLT3 high-expressing constructs. A fluorescein isothiocyanate (FITC)-versus-PE channel dot plot is shown without compensation to exclude autofluorescent cells. The EGFP-positive population shifts on a diagonal to the right. The circle indicates the EGFP-positive (FLT3) cells. Numbers in top left corners indicate the percentage of EGFP+ cells. (B) Western blot analysis of bone marrow shown in panel A. Bone marrow cells were not starved from the cytokines of the infection procedure before preparation of total cell lysates. Similar transduction efficacy for different FLT3 mutants results in equal FLT3 protein expression and activation of downstream targets in murine bone marrow. The cells loaded in the first lane (“untransduced”) were treated like the FLT3-transduced cells but without retrovirus during the infection procedure. (C) Comparison of FLT3 protein expression of the high- and low-expressing FLT3-ITD constructs in Phoenix E cells.
In addition to the FLT3-ITD high-expressing construct (designated as FLT3-ITDhigh) we used another FLT3-ITD construct with lower protein expression (designated as FLT3-ITDlow) (Figure 1C) for the bone marrow transplantation model. Higher expression of the FLT3-ITDhigh construct is due to the deletion of the 5-prime untranslated region of the FLT3 cDNA. For transplantation experiments with FLT3-TKD mutants we exclusively used high-expressing constructs.
Latency of FLT3-ITD-induced MPD correlates with the number of transplanted cells and the expression intensity of the FLT3-ITD protein
To investigate the influence of total number of transplanted EGFP-positive cells on disease phenotype, we tested different retroviral titers in 4 independent experiments for each FLT3-ITD and FLT3-TKD mutant. Transduced bone marrow was analyzed by FACS for EGFP expression and the total cell number was determined. Lethally irradiated syngeneic mice received a transplant of the indicated number of EGFP-positive cells (Table 1).
Analysis of mice that received a transplant of FLT3-WT- or FLT3 mutant-transduced bone marrow
FLT3 and phenotype . | No. mice . | Total EGFP+ cells of transplanted BM . | Survival time, d (median) . | WBC count, × 103/μL (median) . | Spleen weight, mg (median)* . | Disease manifestation . |
|---|---|---|---|---|---|---|
| WT, none | 4 | 95 000 | > 365 | 4.9-17.6 (12.4) | 60-100 (80) | None |
| ITDlow, MPD | 4 | 110 000 | 53-70 (58) | 97.2-188 (141) | 510-690 (580) | Spleen, BM, PB |
| ITDhigh, MPD | 3 | 146 000 | 14-16 (15) | 9.5-38.8 (28.4) | 290-360 (300) | Spleen, BM, PB |
| ITDhigh, MPD | 3 | 104 000 | 19-20 (20) | 17.2-22.4 (19.1) | ND | Spleen, BM, PB |
| ITDlow, MPD | 3 | 25 500 | > 365 | 4.2-11.8 (7.8) | ND | None |
| D835Yhigh | ||||||
| T lymphoma | 2 | 318 000 | 56-85 | 5.8-10.5 | 390-910 | Thymus, spleen, BM, LN, intestine |
| B-ALL | 2 | 318 000 | 53-89 | 11.6-25.7 | 930 | LN, spleen, BM, PB |
| D835Yhigh, T lymphoma | 3 | 30 000 | 104-183 (122) | 10.2-20.1 (16.9) | 130-240 | Thymus, spleen, BM, intestine |
| I836M + Rhigh, T lymphoma | 3 | 240 000 | 86-177 (168) | 6.8-12.8 (12.4) | 120-610 | Thymus |
| I836M + Rhigh, none | 3 | 38 000 | > 270 | 3.5-15.8 (4.9) | 60-90 (81) | None |
FLT3 and phenotype . | No. mice . | Total EGFP+ cells of transplanted BM . | Survival time, d (median) . | WBC count, × 103/μL (median) . | Spleen weight, mg (median)* . | Disease manifestation . |
|---|---|---|---|---|---|---|
| WT, none | 4 | 95 000 | > 365 | 4.9-17.6 (12.4) | 60-100 (80) | None |
| ITDlow, MPD | 4 | 110 000 | 53-70 (58) | 97.2-188 (141) | 510-690 (580) | Spleen, BM, PB |
| ITDhigh, MPD | 3 | 146 000 | 14-16 (15) | 9.5-38.8 (28.4) | 290-360 (300) | Spleen, BM, PB |
| ITDhigh, MPD | 3 | 104 000 | 19-20 (20) | 17.2-22.4 (19.1) | ND | Spleen, BM, PB |
| ITDlow, MPD | 3 | 25 500 | > 365 | 4.2-11.8 (7.8) | ND | None |
| D835Yhigh | ||||||
| T lymphoma | 2 | 318 000 | 56-85 | 5.8-10.5 | 390-910 | Thymus, spleen, BM, LN, intestine |
| B-ALL | 2 | 318 000 | 53-89 | 11.6-25.7 | 930 | LN, spleen, BM, PB |
| D835Yhigh, T lymphoma | 3 | 30 000 | 104-183 (122) | 10.2-20.1 (16.9) | 130-240 | Thymus, spleen, BM, intestine |
| I836M + Rhigh, T lymphoma | 3 | 240 000 | 86-177 (168) | 6.8-12.8 (12.4) | 120-610 | Thymus |
| I836M + Rhigh, none | 3 | 38 000 | > 270 | 3.5-15.8 (4.9) | 60-90 (81) | None |
The construct used for transplantation with FLT3-ITD-transduced bone marrow is indicated by “high” and “low” for the high- and low-expressing construct, respectively. EGFP indicates enhanced green fluorescent protein; BM, bone marrow; WBC, peripheral blood leukocyte count; WT, wild type; ITD, internal tandem duplication; MPD, myeloproliferative disease; PB, peripheral blood; ND, not determined; LN, lymph nodes; and ALL, acute lymphoblastic leukemia.
For spleen weight, 2 WT mice, 3 ITDlow mice, 3 ITDhigh mice, 2 D835Yhigh mice with T lymphoma, 2 D835Yhigh mice with B-ALL, 2 I836M + Rhigh mice with T lymphoma, and 3 I836M + Rhigh mice with negative phenotype were analyzed.
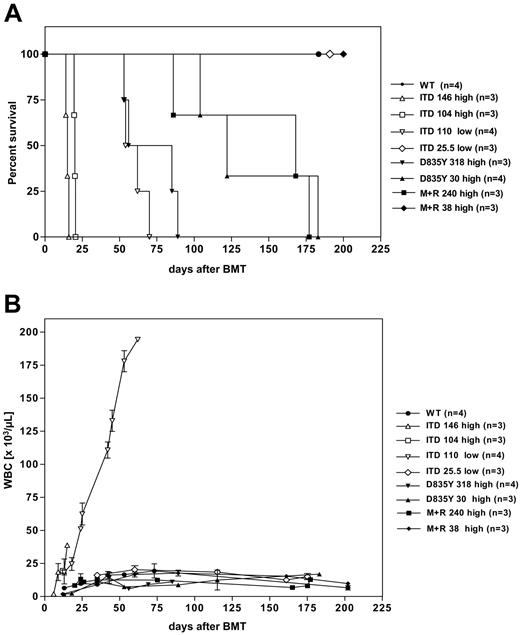
All recipients (n = 4) of 110 000 EGFP-positive bone marrow cells transduced with FLT3-ITDlow (designated as ITD 110low) developed a lethal hematologic disorder with a median latency of 58 days (Table 1; Figure 2A). As described previously,35 they succumbed to an MPD as evidenced by marked leukocytosis (Figure 3B), splenomegaly (Table 1; Figure 3B), and massive expansion of myeloid cells in spleen (Figure 3B), bone marrow, and peripheral blood (Figure 4A). The retroviral integration pattern in the spleen cells of these mice was analyzed by Southern blot analysis. As shown in Figure 5, the MPD induced by FLT3-ITD was oligoclonal, according to the data described previously.35 Mice that received a transplant of 146 000 (n = 3) and 104 000 (n = 3) EGFP-positive bone marrow cells transduced with the high-expressing FLT3-ITD construct (designated as ITD 146high and ITD 104high, respectively) also developed an MPD. However, they succumbed to disease with a much shorter median disease latency of 15 and 20 days, respectively, suggesting that disease latency correlates with total number of the transplanted FLT3-ITD-expressing cells (Figure 2A; Table 1). Additionally, flow cytometric analysis of bone marrow, peripheral blood (Figure 4B), and spleen cells (Figure 3C) from recipients of FLT3-ITDhigh showed a more extensive infiltration with EGFP-positive myeloid cells. Macroscopic analysis of the lungs showed massive pulmonary hemorrhages that were the likely cause of the early death (data not shown). Finally we show, that mice that received a transplant of 25 500 EGFP-positive cells transduced with FLT3-ITDlow had a normal survival like recipients of FLT3 wild-type (FLT3-WT) and did not show any signs of disease after more than 365 days (Figure 2A; Table 1), suggesting that a special threshold level of FLT3-ITD protein expression and infected cell number is needed to induce a MPD. Thus, transplantation of a low number of FLT3-ITD-expressing bone marrow cells does not lead to a different phenotype such as a lymphoid disease. On the basis of these observations, we used high-expressing constructs for the transplantation experiments with FLT3-TKD mutants.
Survival and peripheral blood leukocyte count of mice that received a transplant of FLT3-transduced bone marrow. (A) Kaplan-Meier survival curve and (B) peripheral blood leukocyte count (WBC) for recipients of bone marrow transduced with FLT3-WT, FLT3-ITD, FLT3 D835Y, and FLT3 I836M+R. All mice died or were killed because of disease conditions. The numbers indicate the total number of transplanted EGFP-positive cells. The construct used for transplantation with FLT3-ITD-transduced bone marrow is indicated by “high” and “low” for the high- and the low-expressing construct, respectively. Error bars indicate standard deviation (SD).
Survival and peripheral blood leukocyte count of mice that received a transplant of FLT3-transduced bone marrow. (A) Kaplan-Meier survival curve and (B) peripheral blood leukocyte count (WBC) for recipients of bone marrow transduced with FLT3-WT, FLT3-ITD, FLT3 D835Y, and FLT3 I836M+R. All mice died or were killed because of disease conditions. The numbers indicate the total number of transplanted EGFP-positive cells. The construct used for transplantation with FLT3-ITD-transduced bone marrow is indicated by “high” and “low” for the high- and the low-expressing construct, respectively. Error bars indicate standard deviation (SD).
FLT3-TKD mutants induce lymphoid disease in a murine bone marrow transplantation model
To investigate the transforming capability of FLT3-TKD mutants in vivo, we performed 4 independent transplantation experiments. First, lethally irradiated mice received a transplant of 318 000 (n = 4) and 30 000 (n = 3) FLT3 D835Y-positive bone marrow cells. In contrast to mice that received a FLT3-ITD transplant, all recipients of FLT3 D835Y-transduced bone marrow developed a lymphoid disease with distinct hematologic manifestations.
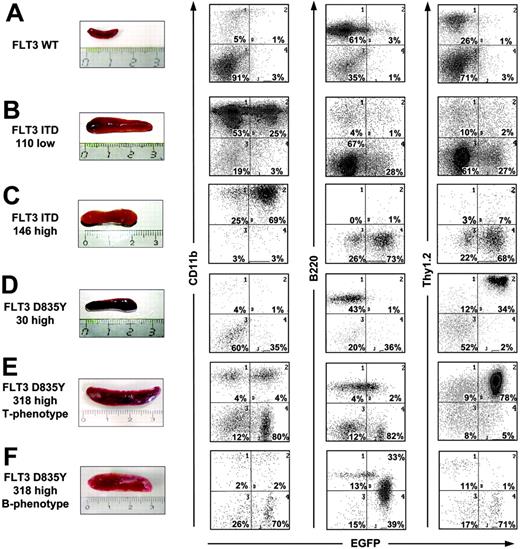
Recipients of 30 000 cells (designated as D835Y 30high) had a median disease latency of 122 days (Figure 2A; Table 1) and all mice succumbed to a disorder resembling T-cell lymphoma with infiltration of thymus, spleen, bone marrow, and intestine (Figures 3D and 6A). Thymus cells were Thy1.2-EGFP-positive with strong expression of CD4 and weak expression of CD8 but negative for myeloid and B-cell markers (Figure 6A).
Each FLT3 mutant induces splenomegaly. Comparison of spleen size (left) and immunophenotype of spleen cells from mice receiving bone marrow transduced with distinct FLT3 mutants. Two-parameter dot plots (right) show expression of lineage-specific antigens (CD11b, B-220, Thy1.2, CD4, CD8) versus EGFP. Numbers indicate the percentage of cells in each quadrant.
Each FLT3 mutant induces splenomegaly. Comparison of spleen size (left) and immunophenotype of spleen cells from mice receiving bone marrow transduced with distinct FLT3 mutants. Two-parameter dot plots (right) show expression of lineage-specific antigens (CD11b, B-220, Thy1.2, CD4, CD8) versus EGFP. Numbers indicate the percentage of cells in each quadrant.
Immunophenotype of cells from the bone marrow and peripheral blood of FLT3-ITD mice. Flow cytometric analysis of bone marrow (BM) and peripheral blood (PB) cells from mice that received a transplant of bone marrow transduced with (A) low- or (B) high-expressed FLT3-ITD. Two-parameter dot plots show expression of lineage-specific antigens (CD11b, Gr-1, B-220, Thy1.2) versus EGFP. Numbers indicate the percentage of cells in each quadrant.
Immunophenotype of cells from the bone marrow and peripheral blood of FLT3-ITD mice. Flow cytometric analysis of bone marrow (BM) and peripheral blood (PB) cells from mice that received a transplant of bone marrow transduced with (A) low- or (B) high-expressed FLT3-ITD. Two-parameter dot plots show expression of lineage-specific antigens (CD11b, Gr-1, B-220, Thy1.2) versus EGFP. Numbers indicate the percentage of cells in each quadrant.
Accordingly, half of the recipients of 318 000 FLT3 D835Y-positive cells (designated as D835Y 318high) also developed T-cell lymphoma (Table 1). Disease latency was shorter, probably due to the higher amount of FLT3 mutant-positive cells transplanted, as also observed in FLT3-ITD mice before. T lymphoma of these mice was characterized by massive enlargement of thymus, lymph nodes, and spleen. The cells of thymus, lymph nodes, spleen, and bone marrow were Thy1.2-EGFP-positive with variable expression of CD4 and CD8 (Figures 3E and 6B), comparable to the mice that received FLT3 D835Y 30high transplants. Interestingly, although the peripheral blood (PB) of the mice that received a FLT3 D835Y transplant showed a high percentage of CD11b-EGFP-positive cells at the end stage of their disease (Figure 6B), indicating that the myeloid lineage was also targeted by the FLT3-TKD mutant, these mice did not develop a myeloproliferative syndrome. The second half of the recipients of 318 000 FLT3 D835Y-positive cells developed a syndrome resembling B-ALL within 53 to 89 days, a latency similar to the mice with T lymphoma (Table 1). The B-cell phenotype was characterized by splenomegaly, enlarged lymph nodes, and infiltration of the bone marrow (Figures 3F and 6C). In contrast to the mice with a T-cell phenotype, malignant cells were also observed in the peripheral blood. The tumor cells stained weakly positive for B220 differing from the EGFP-negative, mature B-cell population with higher B220 surface expression (Figure 6C). Southern blot analysis demonstrated both disease phenotypes to be oligoclonal (Figure 5).
Proviral integrations in cells isolated from spleen cells of diseased mice. DNA isolated from spleen cells of a control mouse (lane 1), FLT3-ITD mice (lanes 2-4), and FLT3 D835Y mice with T-phenotype (lane 5) and B-phenotype (lane 6) was digested with EcoRI, which cuts within the proviral sequence and in the flanking genomic sequence. After blotting onto a nylon membrane DNA was probed with a P32-labeled probe corresponding to the EGFP cDNA to determine the clonality of the disease.
Proviral integrations in cells isolated from spleen cells of diseased mice. DNA isolated from spleen cells of a control mouse (lane 1), FLT3-ITD mice (lanes 2-4), and FLT3 D835Y mice with T-phenotype (lane 5) and B-phenotype (lane 6) was digested with EcoRI, which cuts within the proviral sequence and in the flanking genomic sequence. After blotting onto a nylon membrane DNA was probed with a P32-labeled probe corresponding to the EGFP cDNA to determine the clonality of the disease.
Next, mice received a transplant of 240 000 EGFP-positive cells transduced with FLT3 I836M+R (designated as I836M+R 240high) to determine the ability of an infrequently determined FLT3-TKD mutant to induce a hematopoietic disease in mice. Significantly, all recipients succumbed also to a T-cell lymphoma-like disease with a latency of 86 to 177 days (Figure 2A; Table 1). The FLT3 I836M+R-induced disorder was characterized by a massive enlargement of the thymus. Malignant cells were not observed in peripheral blood, bone marrow, or spleen (Figure 6D). Interestingly, the EGFP-positive thymus cells stained positive for Thy1.2 but were negative for CD4 and CD8, indicating an early T-cell phenotype (Figure 6D).
Immunophenotype of malignant cells from recipients of FLT3-TKD mutants. (A) Flow cytometric analysis of bone marrow and thymus cells from mice receiving 30 000 FLT3 D835Y-positive bone marrow cells (FLT3 D835Y 30high). Flow cytometric analysis of (B) bone marrow (BM), peripheral blood (PB), thymus, lymph node (LN) cells, and (C) bone marrow, peripheral blood, and lymph node cells from mice receiving 318 000 FLT3 D835Y-positive bone marrow cells (FLT3 D835Y 318high). (B) The T-cell phenotype. (C) The B-ALL phenotype. (D) Flow cytometric analysis of bone marrow, peripheral blood, and thymus cells from mice receiving 240 000 FLT3 I836M+R-positive bone marrow cells (FLT3 I836M+R 240high). Two-parameter dot plots show expression of lineage-specific antigens (CD11b, B-220, Thy1.2, CD4, CD8) versus EGFP. Numbers indicate the percentage of cells in each quadrant.
Immunophenotype of malignant cells from recipients of FLT3-TKD mutants. (A) Flow cytometric analysis of bone marrow and thymus cells from mice receiving 30 000 FLT3 D835Y-positive bone marrow cells (FLT3 D835Y 30high). Flow cytometric analysis of (B) bone marrow (BM), peripheral blood (PB), thymus, lymph node (LN) cells, and (C) bone marrow, peripheral blood, and lymph node cells from mice receiving 318 000 FLT3 D835Y-positive bone marrow cells (FLT3 D835Y 318high). (B) The T-cell phenotype. (C) The B-ALL phenotype. (D) Flow cytometric analysis of bone marrow, peripheral blood, and thymus cells from mice receiving 240 000 FLT3 I836M+R-positive bone marrow cells (FLT3 I836M+R 240high). Two-parameter dot plots show expression of lineage-specific antigens (CD11b, B-220, Thy1.2, CD4, CD8) versus EGFP. Numbers indicate the percentage of cells in each quadrant.
Remarkably, in contrast to the FLT3-ITD mice, none of the FLT3-TKD mice developed a leukocytosis in the course of their disease (Figure 1B). Similar to FLT3-ITD, the FLT3 I836M+R construct was not able to induce a malignant disease when only low amounts of EGFP-positive cells were transplanted. Thus, mice that received a transplant of 38 000 FLT3 I836M+R-positive cells remained disease-free with a follow-up of more than 270 days (Table 1; Figure 2A).
Differences of downstream signaling between FLT3-ITD and -TKD mutants
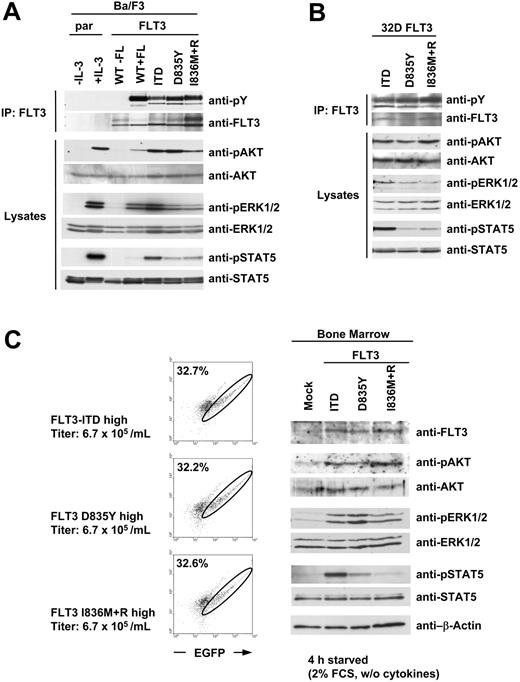
To investigate the potential underlying mechanisms for the induction of different phenotypes, we compared diverse signaling pathways activated by the FLT3 mutants. Therefore we stably transfected the pre-B-cell line Ba/F3 with FLT3-ITD and the FLT3-TKD mutants. As a control, we established the same cell line expressing the wild-type FLT3 receptor. All cells were starved from serum and cytokines for 4 hours. Parental cells and FLT3-WT-expressing cells were either untreated or stimulated with IL-3 or FL, respectively, as a control. Immunoblotting analysis using an antiphosphotyrosine antibody after immunoprecipitation of the FLT3 receptor revealed strong autophosphorylation of the TKD mutants, whereas the autophosphorylation of FLT3-ITD was slightly weaker (Figure 7A). Next, we investigated whether the distinct FLT3 mutants have different signaling properties. As shown in Figure 7A, kinase activity of each FLT3 mutant led to constitutive activation of AKT, ERK1/2, and STAT5 in Ba/F3 cells. Strikingly, the FLT3-ITD-induced activation of STAT5 was much stronger than the STAT5 activation induced by the FLT3-TKD mutants despite stronger autophosphorylation. Furthermore, we were able to confirm these data in the myeloid cell line 32D. As shown in Figure 7B, the presence of FLT3-ITD in 32D cells led to strong phosphorylation of STAT5, whereas STAT5 was only marginally activated by the FLT3-TKD mutants.
Next, we analyzed the signaling properties of the distinct FLT3 mutants in murine bone marrow. Since we could not detect any difference in unstarved bone marrow cells expressing the FLT3 mutants (Figure 1B), we decided to investigate the activation status of the signal transduction intermediates in bone marrow starved from cytokines and serum. Therefore, we transduced bone marrow of 5-FU-treated mice with each FLT3 mutant or vector alone (mock). The viral titers of all constructs used were normalized to the lowest titer of 6.7 × 105 retroviral particles per milliliter. Transduction of bone marrow with equivalent titers resulted in a comparable fraction of EGFP-positive cells (Figure 7C left). The cells were then starved from the cytokines used during the infection procedure for 4 hours in serum-reduced medium. Western blot analysis confirmed that the transduced bone marrow cells expressed comparable protein levels of each FLT3 mutant, and kinase activity of all mutants led to constitutive activation of AKT and ERK1/2. However, strong STAT5 activation was observed only in bone marrow cells transduced with FLT3-ITD (Figure 2C right). These data confirm the difference in STAT5 activation between FLT3-ITD and the FLT3-TKD mutants regardless of the cell type used.
Discussion
Mutations of the FLT3 receptor tyrosine kinase represent the most common genetic alteration in AML. Two distinct groups of activating FLT3 mutations have been detected: internal tandem duplications of the juxtamembrane domain coding sequence and point mutations within the activation loop of the second tyrosine kinase domain (TKD).11 Point mutations within the activation loop were also found in a significant percentage of infant and childhood ALL harboring 11q23 gene rearrangements28,29 and T-ALL.30 In contrast, FLT3-ITD mutations are rarely found in pediatric and adult ALL.12,31-34 Previous studies demonstrate that mice receiving transplants of bone marrow retrovirally infected with FLT3-ITD develop a myeloproliferative disease (MPD).35 In this study, we investigated the transforming properties of FLT3-TKD mutants in primary murine bone marrow cells. When we analyzed FLT3-TKD mutants in comparison with FLT3-ITDs in a murine bone marrow transplantation model, we found major differences in disease phenotype. Interestingly, the expression of FLT3-TKD mutants in murine bone marrow caused a lymphoid disease with distinct hematologic manifestations, whereas FLT3-ITD induced a myeloproliferative phenotype, as described previously.35
Different activation of STAT5 by FLT3-ITD and FLT3-TKD mutants. (A) Ba/F3 and (B) 32D cells were stably transfected with WT or mutated FLT3 constructs as indicated. All cells were serum starved for 4 hours. Parental cells (par) and FLT3-WT-expressing cells were either untreated or stimulated with IL-3 or FLT3 ligand (FL), respectively. FLT3 was immunoprecipitated (IP) from whole-cell lysates and immunoblotted with antiphosphotyrosine antibody and reblotted with anti-FLT3 antibody. Activation of downstream targets is demonstrated by immunoblotting the total cell lysates with the indicated phosphospecific antibodies. After stripping the membrane was reprobed with indicated total antibodies to demonstrate equal loading. (C) Bone marrow of 5-FU-pretreated mice was transduced with equivalent retroviral titers of the indicated constructs. After the infection procedure the cells were washed twice with phosphate-buffered saline (PBS) and subsequently starved for 4 hours in medium containing 2% FCS and no cytokines. After FACS analysis of the cells (left), total cell lysates were prepared and separated by SDS-PAGE. Expression of FLT3 protein and activation of downstream targets is demonstrated by immunoblotting the total cell lysates with the indicated phosphospecific antibodies. After stripping the membrane was reprobed with indicated total antibodies to demonstrate equal loading. In left panels, circles indicate EGFP+ cells; numbers, the percentage of EGFP+ cells.
Different activation of STAT5 by FLT3-ITD and FLT3-TKD mutants. (A) Ba/F3 and (B) 32D cells were stably transfected with WT or mutated FLT3 constructs as indicated. All cells were serum starved for 4 hours. Parental cells (par) and FLT3-WT-expressing cells were either untreated or stimulated with IL-3 or FLT3 ligand (FL), respectively. FLT3 was immunoprecipitated (IP) from whole-cell lysates and immunoblotted with antiphosphotyrosine antibody and reblotted with anti-FLT3 antibody. Activation of downstream targets is demonstrated by immunoblotting the total cell lysates with the indicated phosphospecific antibodies. After stripping the membrane was reprobed with indicated total antibodies to demonstrate equal loading. (C) Bone marrow of 5-FU-pretreated mice was transduced with equivalent retroviral titers of the indicated constructs. After the infection procedure the cells were washed twice with phosphate-buffered saline (PBS) and subsequently starved for 4 hours in medium containing 2% FCS and no cytokines. After FACS analysis of the cells (left), total cell lysates were prepared and separated by SDS-PAGE. Expression of FLT3 protein and activation of downstream targets is demonstrated by immunoblotting the total cell lysates with the indicated phosphospecific antibodies. After stripping the membrane was reprobed with indicated total antibodies to demonstrate equal loading. In left panels, circles indicate EGFP+ cells; numbers, the percentage of EGFP+ cells.
To exclude the possibility that different protein expression levels induced the distinct phenotypes, we compared the levels of FLT3 protein expression in murine bone marrow transduced with FLT3-ITD and FLT3-TKD mutants. We could demonstrate that a similar transduction efficacy, as evidenced by flow cytometry of EGFP, results in equal FLT3 protein expression in the bone marrow (Figure 1).
The expression of FLT3 D835Y, the most common kinase domain mutant in AML,11 caused a lymphoid phenotype with complete penetrance. A comparison of mice that received a transplant of different amounts of FLT3 D835Y-positive cells revealed a direct correlation of disease latency and total number of FLT3 D835Y-expressing cells. This dosage effect was also observed in mice that received a transplant of FLT3-ITD-transduced bone marrow (Figure 3A). Furthermore, weak protein expression by the FLT3-ITD low-expressing construct induced a MPD with a significantly longer latency compared with the high-expressing construct when a comparable total number of EGFP-positive cells was transplanted (Figure 2A). Importantly, mice that received a transplant of a low cell number infected with the FLT3-ITD low-expressing construct neither developed MPD nor acquired a lymphoid disease.
Thus, our results argue for a dual dosage effect. First, the amount of expressed protein seems to influence the latency of disease because the myeloproliferative disease induced by FLT3-ITDlow seems to have evolved more slowly due to the decreased expression of FLT3-ITD. This is important as it shows that a longer disease latency does not automatically imply the need for secondary genetic events. Second, our results imply that there is a limited number of leukemia-initiating cells, which have to be targeted by the retroviral vector in order to induce a hematologic disease. This might explain why mice that received a transplant of a low infected cell number did not develop a malignant disease.
In addition to FLT3 D835Y, we investigated the transforming ability of FLT3 I836M+R, a rarely detected FLT3 kinase domain mutant in AML.13 We found that this mutant also induced a T-lymphoma-like disease when 240 000 EGFP-positive cells were transplanted but failed to induce a hematologic malignancy when mice received only 38 000 positive cells. It could be speculated that FLT3 I836M+R may have a weaker transforming potential in vivo than the more frequently detected FLT3 D835Y. Thus, these data may reflect the lower frequency of FLT3 I836M+R in patients.
The lymphoid manifestation and longer latency of FLT3-TKD in a murine BMT model together with the absent influence of FLT3-TKD mutations on the clinical outcome of patients with AML13,25-27 suggest differences in cell signaling between FLT3-TKD mutants and FLT3-ITDs. To address this point we compared the activation status of some common signaling proteins of FLT318,20,40 in the pre-B-cell line Ba/F3, the myeloid cell line 32D, and in murine bone marrow transduced with each FLT3 mutant. In Ba/F3 and 32D cells we observed similar phosphorylation of AKT and ERK1/2 by both FLT3-ITD and FLT3-TKD mutants, whereas STAT5 was much stronger activated by FLT3-ITD than the FLT3-TKD mutants (Figure 7A-B). Similarly, in cytokine-deprived bone marrow cells we found strong differences between FLT3-ITD and the FLT3-TKD mutants in the activation of STAT5 (Figure 7C). These findings are in accordance with the results in Ba/F3 and 32D cells. These data suggest that strong activation of STAT5 might be necessary for the induction of the myeloproliferative disease but not for induction of a lymphoid disease in mice. Thus, although our experiments do not exclude cell context-specific differences in the downstream signaling of the FLT3 mutants, they argue for a role of this particular signaling pathway in the development of the different phenotypes.
Our results imply that the transforming capability of distinct FLT3 mutants also depends on the specific cellular environment. Although we also noticed an expansion of the myeloid lineage in the FLT3-TKD mutant mice, the FLT3-TKD mutants seem to require a lymphoid cell context for full malignant transformation. In contrast, FLT3-ITD primarily transforms myeloid cells, although it has been shown that FLT3-ITD may also cause lymphoid disease in mice. However, these findings have been observed in mouse strains other than Balb/C.36,41 Furthermore, additional evidence in support of this hypothesis stems from studies demonstrating that FLT3-ITDs are found significantly more frequently in AML12-14 than in pediatric and adult ALL patients,12,31-34 whereas a high incidence of FLT3-TKD mutations has been observed in pediatric and childhood ALL.28,29
In conclusion, our results indicate that FLT3-TKD mutations might contribute to the pathogenesis of ALL and other lymphoid malignancies. Thus, the described mouse model may prove useful in studying the molecular pathogenesis of FLT3-TKD mutant-expressing lymphoid malignancies and should provide a valuable system for testing new treatment options for this type of malignancy.42
Prepublished online as Blood First Edition Paper, February 17, 2005; DOI 10.1182/blood-2004-11- 4430.
Supported by grants from the Wilhelm Sander Stiftung and Mildred-Scheel-Stiftung (J.D.) and Bundesministerium für Bildung und Forschung (BMBF; 01-GS-0105 and 01-GS-0155; J.D. and C.P.). C.M. is supported by a fellowship from the José-Carreras Stiftung.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal