Abstract
Cyclooxygenase 2 (COX-2) is an inflammation-associated enzyme involved in the pathogenesis of many solid tumors, but little is known about its presence and role in hematologic neoplasms. Multiple myeloma (MM) is known to involve a deregulated cytokine network with secretion of inflammatory mediators. We thus decided to investigate the involvement of COX-2 in this neoplasm. Western blotting (WB) was used to evaluate 142 bone marrow (BM) specimens, including MM and monoclonal gammopathy of undetermined significance (MGUS). Selected cases under-went further evaluation by WB on purified CD138+ cells, immunohistochemistry (IC), and real-time polymerase chain reaction (PCR) for mRNA expression. COX-2 was expressed in 11% (2 of 18) of MGUS specimens, 31% (29 of 94) of MM at diagnosis, and 47% (14 of 30) of MM with relapsed/refractory disease. COX-2 positivity was associated with a poor outcome in terms of progression-free (18 vs 36 months; P < .001) and overall survival (28 vs 52 months; P < .05). Real-time PCR showed COX-2 mRNA overexpression. IC and cell separation studies demonstrated COX-2 expression to be restricted to malignant plasma cells. This is the first report of the presence and prognostic role of COX-2 expression in MM. Future studies will assess COX-2 involvement in other hematologic tumors and its potential use as a therapeutic or chemo-preventive target in onco-hematology. (Blood. 2005; 105:4784-4791)
Introduction
Prostaglandins are important mediators implicated in inflammation and angiogenesis, and support the growth of several solid tumors.1-3 Cyclooxygenase 2 (COX-2) is the key enzyme of prostaglandin synthesis in inflamed tissues.4 Its expression has been reported in many human cancers, including colo-rectal, breast, ovarian, uterine cervix, lung, head, and neck.2,5-9 In most of them, COX-2 actively supports cancer growth2,6,7 and its expression acts as an indicator of poor prognosis.5,8 COX-2 is also a useful target for chemopreventive and therapeutic intervention.10-12
Despite considerable data concerning COX-2 expression in solid tumors, its role in hematologic malignancies has been little investigated: some studies have examined chronic myelogenous leukemia and non-Hodgkin lymphoma in this light,13-16 but to our knowledge COX-2 expression has not yet been investigated in multiple myeloma (MM). MM is typically characterized by a deregulated cytokine network with secretion of inflammatory cytokines.17-19 We thus decided to verify whether COX-2 deregulation might play a role in MM pathogenesis by investigating the presence and prognostic role of COX-2 expression in a large panel of bone marrow (BM) specimens obtained from subjects with MM and MM-related disorders.
Patients, materials, and methods
Patients and samples
One hundred forty-two tumor specimens from 132 patients with plasma cell dyscrasias were evaluated for COX-2 protein expression by Western blotting (WB); all contained more than 5% CD138+ cells by flow cytometry. Eighteen samples were from patients with monoclonal gammopathy of undetermined significance (MGUS), and 124 were from patients with MM. Specimens from patients with MM were taken at diagnosis in 94 cases and at relapse/progression in 30 cases. In 10 patients, specimens taken at different disease phases were available. These comprised MGUS and MM in 2 patients and MM at diagnosis and MM at relapse/progression in 8 patients. All patients included in this analysis were treated at Turin over the last 10 years; clinical features at diagnosis and outcome data were available for all patients. Information included age, sex, BM plasma cells, M component, hemoglobin (Hb) and creatinine levels, presence and number of bone lesions, Salmon and Durie clinical stage, first-line treatment, and clinical follow-up until death or most recent examination. β2-microglobulin at diagnosis was available in 77 of the 94 patients and chromosome 13 deletion was assessed by fluorescence in situ hybridization (FISH) in 36 patients. All patients gave written informed consent to the use of BM cells exceeding clinical requirements for research purposes. Patient clinical characteristics are shown in Table 1 (at diagnosis) and Table 2 (refractory/relapse). Additional experiments, including cell selection studies and real-time polymerase chain reaction (PCR), were performed on a number of specimens chiefly selected by material availability. Immunohistochemistry (IC) was performed on 42 specimens: of these, only 11 belonged to the above panel; the other 31 were obtained from subjects treated at Zürich University Hospital. Of these, 5 had extramedullary disease (4 paravertebral, one pleural). These 31 specimens were not evaluated by WB and were not employed for prognosis evaluation. Clinical characteristics of the IC panel are shown in Table 3.
Clinical features of patients at diagnosis, as assessed by WB
. | No. . | % . |
|---|---|---|
| Total | 94 | 100 |
| Mean age, y (range) | 56 (34-77) | NA |
| Female | 38 | 40.4 |
| Mean BM PCs, % (range) | 47 (5-99) | NA |
| M component | ||
| IgG | 53 | 56.4 |
| IgA | 22 | 23.4 |
| Light chain | 17 | 18.1 |
| Nonsecretory | 2 | 2.1 |
| K | 60 | 65.2 |
| Mean Hb level, g/dL (range) | 11 (5.6-16) | NA |
| Bone lytic lesions | ||
| 0 | 26 | 27.6 |
| 1 or more | 68 | 72.3 |
| Salmon and Durie stage | ||
| I | 4 | 4.2 |
| II | 27 | 28.7 |
| III | 63 | 67.0 |
| Mean creatinine level, mg/dL (range) | 2 (0.4-8.4) | NA |
| Del13q* | 18 | 50.0 |
| Mean 2-microglobulin level, g/L (range)† | 5 (1.1-22.8) | NA |
| First-line treatment | ||
| Conventional | 28 | 29.8 |
| Thalidomide plus conventional | 4 | 4.2 |
| Autologous or allogeneic SCT | 62 | 66.0 |
. | No. . | % . |
|---|---|---|
| Total | 94 | 100 |
| Mean age, y (range) | 56 (34-77) | NA |
| Female | 38 | 40.4 |
| Mean BM PCs, % (range) | 47 (5-99) | NA |
| M component | ||
| IgG | 53 | 56.4 |
| IgA | 22 | 23.4 |
| Light chain | 17 | 18.1 |
| Nonsecretory | 2 | 2.1 |
| K | 60 | 65.2 |
| Mean Hb level, g/dL (range) | 11 (5.6-16) | NA |
| Bone lytic lesions | ||
| 0 | 26 | 27.6 |
| 1 or more | 68 | 72.3 |
| Salmon and Durie stage | ||
| I | 4 | 4.2 |
| II | 27 | 28.7 |
| III | 63 | 67.0 |
| Mean creatinine level, mg/dL (range) | 2 (0.4-8.4) | NA |
| Del13q* | 18 | 50.0 |
| Mean 2-microglobulin level, g/L (range)† | 5 (1.1-22.8) | NA |
| First-line treatment | ||
| Conventional | 28 | 29.8 |
| Thalidomide plus conventional | 4 | 4.2 |
| Autologous or allogeneic SCT | 62 | 66.0 |
WB indicates Western blotting; NA, not applicable; BM, bone marrow; PCs, plasma cells; Hb, hemoglobin; SCT, stem cell transplantation.
Assessed by fluorescence in situ hybridization, available in 36 patients.
β2-microglobulin available in 77 patients.
Clinical characteristics of patients with refractory/relapsed disease, as assessed by WB
. | No. . | % . |
|---|---|---|
| Total | 30 | 100 |
| Mean age, y (range) | 59 (42-77) | NA |
| Sex, female | 16 | 53.0 |
| M component | ||
| IgG | 23 | 76.6 |
| IgA | 3 | 10.0 |
| Light chain | 3 | 10.0 |
| Nonsecretory | 1 | 3.4 |
| K | 19 | 65.5 |
| Previous lines of treatment | ||
| Conventional | 14 | 46.7 |
| Thalidomide plus conventional | 8 | 26.7 |
| Autologous transplantation | 8 | 26.6 |
| Treatment after COX-2 assessment | ||
| Thalidomide-containing therapy | 7 | 23.4 |
| Bortezomib-containing therapy | 1 | 3.3 |
| Chemotherapy only | 10 | 33.3 |
| Autologous or allogeneic SCT | 12 | 40.0 |
. | No. . | % . |
|---|---|---|
| Total | 30 | 100 |
| Mean age, y (range) | 59 (42-77) | NA |
| Sex, female | 16 | 53.0 |
| M component | ||
| IgG | 23 | 76.6 |
| IgA | 3 | 10.0 |
| Light chain | 3 | 10.0 |
| Nonsecretory | 1 | 3.4 |
| K | 19 | 65.5 |
| Previous lines of treatment | ||
| Conventional | 14 | 46.7 |
| Thalidomide plus conventional | 8 | 26.7 |
| Autologous transplantation | 8 | 26.6 |
| Treatment after COX-2 assessment | ||
| Thalidomide-containing therapy | 7 | 23.4 |
| Bortezomib-containing therapy | 1 | 3.3 |
| Chemotherapy only | 10 | 33.3 |
| Autologous or allogeneic SCT | 12 | 40.0 |
WB indicates Western blotting; NA, not applicable SCT, stem cell transplantation.
Clinical characteristics of patients assessed by IC
. | No. . | % . |
|---|---|---|
| Total | 42 | 100 |
| Mean age, y (range) | 61 (35-86) | NA |
| Female | 12 | 28.5 |
| M component | ||
| IgG | 36 | 85.7 |
| IgA | 4 | 9.5 |
| Light chain | 2 | 4.8 |
| Nonsecretory | 0 | 0 |
| K | 33 | 78.5 |
| Salmon and Durie stage | ||
| I | 14 | 33.3 |
| II-III | 28 | 66.7 |
| Extramedullary disease | 5 | 12.0 |
. | No. . | % . |
|---|---|---|
| Total | 42 | 100 |
| Mean age, y (range) | 61 (35-86) | NA |
| Female | 12 | 28.5 |
| M component | ||
| IgG | 36 | 85.7 |
| IgA | 4 | 9.5 |
| Light chain | 2 | 4.8 |
| Nonsecretory | 0 | 0 |
| K | 33 | 78.5 |
| Salmon and Durie stage | ||
| I | 14 | 33.3 |
| II-III | 28 | 66.7 |
| Extramedullary disease | 5 | 12.0 |
IC indicates immunohistochemistry; NA, not applicable.
Cell line, controls, protein and RNA extraction, and cDNA preparation
The HT-29 cell line was used as a positive control and BM cells from healthy donors were used as a negative control.20 Protein and RNA were extracted using the Trizol reagent (Invitrogen, Carlsbad, CA) starting from -70°C frozen pellets containing 10 × 106 to 20 × 106 cells. The reagent was used following the manufacturer's recommendations. Total cDNA was prepared following standard procedures, using random hexameres starting from 5 μg total RNA.
Western blotting
The Western blotting assay was performed following the DuBois et al protocol.21 Briefly, cell lysate proteins (100 μg per lane) were separated onto 10% tris-glycine gels and transferred on polyvinylidene difluoride membranes (Invitrogen). Membranes were blocked using fat-free dry milk. Blots were then incubated overnight at 4°C with rabbit anti-human COX-2-specific antiserum (Oxford Biomedical Research, Oxford, MI) diluted 1:1000. This incubation was followed by application of goat antirabbit secondary antibody conjugated to horseradish peroxidase (Amersham Biosciences UK Limited, Little Chalfont, Buckinghamshire, England). Membranes were then developed using an enhanced chemiluminescence-plus detection system (Amersham Biosciences UK Limited). Successful protein transfer and equal loading in all lanes were verified using reversible staining with Ponceau S (Sigma-Aldrich, St Louis, MO) and confirmed by WB of stripped filters using an anti-β-actin antibody (Sigma-Aldrich). The stripping procedure followed standard protocols. Samples in which the β-actin loading control was faint were excluded from the analysis. For each sample, WB was performed at least twice on separate filters. Filters were scored under similar conditions by an investigator (M.D.A.) who was not clinically involved and to whom patient outcome was unknown.
Relative quantification of COX-2 transcripts by real-time PCR
Real-time PCR was employed for relative quantification of RNA transcripts, following standard procedures. Primers and probes for COX-2 amplification were obtained from Applied Biosystems, a company that markets validated quantitative RNA expression assays for numerous human genes (20x Assays-on-Demand Gene Expression Assay Mix; Applied Biosystems, Foster City, CA). Real-time PCR was done on an ABI PRISM 7900 analytical thermal cycler (Applied Biosystems) following the manufacturer's recommendations. The GUS housekeeping gene was used to normalize samples for DNA quality and quantity, as has been reported.22 Relative quantification of COX-2 RNA was done by the ΔΔCt approach, as suggested by Applied Biosystems for the relative quantification of mRNA transcripts.
COX-2 immunohistochemistry
COX-2 IC was performed on BM or extramedullary tissue slides as follows: briefly, after deparaffining, antigens were retrieved by pretreatment with one cycle of incubation with cell conditioning solution CC1-Buffer pH 6.0 (Ventana Medical Systems, Tucson, AZ). Tissue sections were then incubated with the primary COX-2 murine polyclonal affinity-purified antibody (kindly provided by B. Odermatt, Department of Pathology, University Hospital, Zürich) for 32 minutes (dilution 1:60). Immunoreactivity was visualized using IVIEW DAB detection kit secondary antibody (Ventana Medical Systems), containing biotinylated antirabbit and streptavidin horseradish peroxidase, 3′3-diamonobenzidin-tetrahydrochlorid, and Hämalaun for nucleus staining.
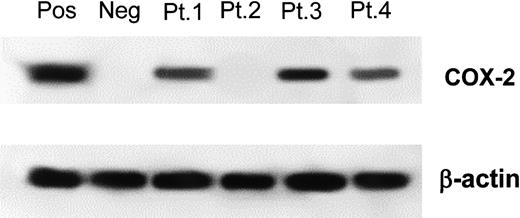
Western blotting for COX-2 expression. A representative example of COX-2 expression analysis on MM samples. Patient 1, patient 3, and patient 4 were scored COX-2 positive. Patient 2 was scored COX-2 negative. β-actin loading control is shown in both experiments. Pos, indicates positive control (HT-29 cell line); Neg, negative control (normal BM).
Western blotting for COX-2 expression. A representative example of COX-2 expression analysis on MM samples. Patient 1, patient 3, and patient 4 were scored COX-2 positive. Patient 2 was scored COX-2 negative. β-actin loading control is shown in both experiments. Pos, indicates positive control (HT-29 cell line); Neg, negative control (normal BM).
Separation of plasma cells
Plasma cells were separated from fresh BM samples using the Miltenyi Minimacs cell separation system (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany) following the manufacturer's indications. Selection was achieved with the CD138 antigen, which is a reliable marker of malignant and nonmalignant plasma cells. CD138+ and CD138- populations were then separately assessed for COX-2 expression by WB.
Survival analysis and statistical considerations
Only the Turin patients who had been assessed by WB were included in the survival analysis. Progression-free survival (PFS) was calculated from the first day of frontline treatment, either to relapse from complete remission, or to evidence of progressive disease, or to last follow-up alive. Relapse and progression were defined by the criteria proposed by Bladé et al.23 Overall survival (OS) was calculated from diagnosis to death or last follow-up alive.
All clinical and demographic information was analyzed by univariate analysis on patient treated at diagnosis in order to determine which parameters had an impact on prognosis. The following parameters were considered: age, sex, Hb level, creatinine level, Salmon and Durie clinical stage, presence of osteolytic lesions, BM plasmocytosis, β2-microglobulin (information available for only 77 patients), and COX-2 positivity. Univariate and multivariate analyses were performed using the Cox model. Colinearity between β2-microglobulin and COX-2 was assessed with the Pearson correlation coefficient. All data were processed with the SAS statistical software package (SAS Institute, Cary, NC).
Results
COX-2 protein expression by WB: assay validation and assessment of MM samples
Our WB assay was validated as follows: 15 BM samples from healthy donors were assessed and all were found with our assay to be COX-2 negative (data not shown), indicating COX-2 expression in the BM of healthy subjects to be below our sensitivity threshold. In contrast, our assay produced a net positive result on the COX-2-positive HT-29 cell line (Figure 1). As previously mentioned, all specimens were assessed twice and concordance between first and second determination was 100%.
COX-2 expression was assessed by WB in the whole panel of 142 specimens; Figure 1 shows a representative experiment. COX-2 expression was detected in 32% of all specimens, in 11% of MGUS specimens, in 31% of MM specimens at diagnosis, and in 47% of MM specimens at relapse. Interestingly, one of the 2 COX-2-positive patients with MGUS evolved from MGUS to MM only 7 months after BM examination. The second COX-2-positive patient with MGUS was lost to follow-up a few months after diagnosis.
In the 10 patients for whom specimens were available at different time-points, concordance was present in 7 (4 COX-2 positive and 3 COX-2 negative). Two patients switched from COX-2 negativity to COX-2 positivity as the disease evolved (MGUS vs MM in one case) or progressed (MM at diagnosis vs MM at relapse in one case). One patient was COX-2 positive at diagnosis and COX-2 negative at first relapse.
Correlation between COX-2 expression and clinical parameters
Correlations were sought between COX-2 expression in patients at diagnosis and the following parameters: age, sex, BM plasmocytosis, Salmon and Durie clinical stage (stage I vs stage II vs stage III), creatinine level, Hb level, presence of bone lesions, β2-microglobulin, and chromosome 13 deletion assessed by FISH. The results are summarized in Table 4; none of the parameters was correlated to COX-2 expression.
Correlation between COX-2 expression and clinical parameters
. | COX-2 positive . | COX-2 negative . |
|---|---|---|
| Mean age, y, ± SD | 56.5 ± 10.5 | 55.4 ± 9.2 |
| Female, % | 51.7 | 35.3 |
| Salmon and Durie stage, % | ||
| Stage I | 3.4 | 4.6 |
| Stage II | 20.7 | 32.5 |
| Stage III | 75.8 | 63.0 |
| Mean β2-microglobulin, μg/L, ± SD* | 5.7 ± 9.2 | 6.0 ± 5.9 |
| Mean creatinine, mg/dL, ± SD | 1.54 ± 1.5 | 1.53 ± 1.4 |
| Mean Hb level, g/dL, ± SD | 10.4 ± 2.2 | 11.0 ± 2.2 |
| Bone lesions at least 1, % | 72.4 | 72.3 |
| Mean BM PCs, %, ± SD | 46.6 ± 24.3 | 47.7 ± 20.8 |
| Del13q, %† | 55.5 | 44.4 |
. | COX-2 positive . | COX-2 negative . |
|---|---|---|
| Mean age, y, ± SD | 56.5 ± 10.5 | 55.4 ± 9.2 |
| Female, % | 51.7 | 35.3 |
| Salmon and Durie stage, % | ||
| Stage I | 3.4 | 4.6 |
| Stage II | 20.7 | 32.5 |
| Stage III | 75.8 | 63.0 |
| Mean β2-microglobulin, μg/L, ± SD* | 5.7 ± 9.2 | 6.0 ± 5.9 |
| Mean creatinine, mg/dL, ± SD | 1.54 ± 1.5 | 1.53 ± 1.4 |
| Mean Hb level, g/dL, ± SD | 10.4 ± 2.2 | 11.0 ± 2.2 |
| Bone lesions at least 1, % | 72.4 | 72.3 |
| Mean BM PCs, %, ± SD | 46.6 ± 24.3 | 47.7 ± 20.8 |
| Del13q, %† | 55.5 | 44.4 |
P values were not significant for any variable.
SD indicates standard deviation; Hb, hemoglobin; BM, bone marrow; PCs, plasma cells.
β2-microglobulin was available in 77 patients.
Assessed by fluorescence in situ hybridization on 36 patients.
Prognostic value of COX-2 expression
An outcome analysis based on PFS and OS was performed in patients assessed at diagnosis. Induction treatment for these 94 patients consisted of autologous or allogeneic stem cell transplantation in 62 (66%), conventional chemotherapy such as melphalan and prednisone in 28 (29.8%), and a thalidomide-containing regimen in 4 (4.2%). There was no significant difference in frontline treatment between COX-2-positive and COX-2-negative patient subgroups (data not shown).
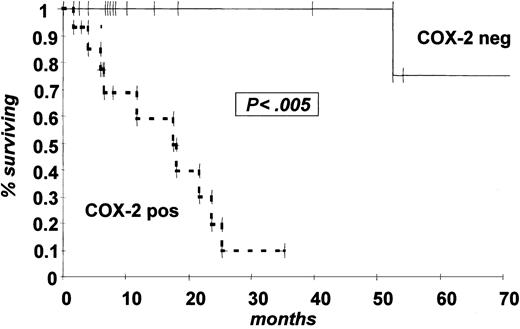
Median OS follow-up of our study population was 48 months (range, 1-138 months). Mean PFS was 18 months for COX-2-positive patients and 36 months for COX-2-negative patients (P < .001; Figure 2A). There was also a statistically significant difference in OS (28 months vs 52 months; P < .05; Figure 2B).
Results of univariate analysis are shown in Table 5. As far as PFS is concerned the following parameters were significant: COX-2 positivity (hazard ratio [HR] 4.04, 95% confidence interval [CI] 2.20-7.40; P < .001) and β2-microglobulin (HR 1.14, 95% CI 1.05-1.24; P < .005). With regard to OS, only COX-2 expression was significant (HR 1.89, 95% CI 1.01-3.60; P < .05), although β2-microglobulin almost achieved statistical significance (HR 1.08, 95% CI 1.00-1.16; P = .06).
Univariate analysis performed on patients at diagnosis
. | OS . | . | . | PFS . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | HR . | 95% CI . | P . | HR . | 95% CI . | P . | ||||
| Age | 0.99 | 0.95-1.03 | NS | 1.00 | 0.97-1.03 | NS | ||||
| Female | 0.77 | 0.41-1.48 | NS | 1.07 | 0.60-1.89 | NS | ||||
| Salmon and Durie stage III | 1.01 | 0.50-2.03 | NS | 1.41 | 0.72-2.76 | NS | ||||
| β2-microglobulin* | 1.08 | 1.00-1.16 | .06 | 1.14 | 1.05-1.24 | < .005 | ||||
| Creatinine level | 0.89 | 0.71-1.11 | NS | 0.99 | 0.83-1.18 | NS | ||||
| Hb level | 0.92 | 0.79-1.08 | NS | 0.83 | 0.72-0.96 | NS | ||||
| Bone lesions, 0 | 0.77 | 0.34-1.74 | NS | 0.93 | 0.46-1.86 | NS | ||||
| BM PCs, % | 1.00 | 0.99-1.02 | NS | 1.01 | 0.99-1.02 | NS | ||||
| COX-2 positivity | 1.89 | 1.01-3.60 | < .05 | 4.04 | 2.20-7.40 | < .001 | ||||
. | OS . | . | . | PFS . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | HR . | 95% CI . | P . | HR . | 95% CI . | P . | ||||
| Age | 0.99 | 0.95-1.03 | NS | 1.00 | 0.97-1.03 | NS | ||||
| Female | 0.77 | 0.41-1.48 | NS | 1.07 | 0.60-1.89 | NS | ||||
| Salmon and Durie stage III | 1.01 | 0.50-2.03 | NS | 1.41 | 0.72-2.76 | NS | ||||
| β2-microglobulin* | 1.08 | 1.00-1.16 | .06 | 1.14 | 1.05-1.24 | < .005 | ||||
| Creatinine level | 0.89 | 0.71-1.11 | NS | 0.99 | 0.83-1.18 | NS | ||||
| Hb level | 0.92 | 0.79-1.08 | NS | 0.83 | 0.72-0.96 | NS | ||||
| Bone lesions, 0 | 0.77 | 0.34-1.74 | NS | 0.93 | 0.46-1.86 | NS | ||||
| BM PCs, % | 1.00 | 0.99-1.02 | NS | 1.01 | 0.99-1.02 | NS | ||||
| COX-2 positivity | 1.89 | 1.01-3.60 | < .05 | 4.04 | 2.20-7.40 | < .001 | ||||
OS indicates overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; Hb, hemoglobin; BM, bone marrow; PCs, plasma cells.
β2-microglobulin was available in 77 patients.
Kaplan Meier estimate of probability of PFS and OS by COX-2 expression status at diagnosis. (A) PFS; (B) OS. Dotted line indicates COX-2-positive patients and solid line COX-2-negative patients by WB expression analysis.
Kaplan Meier estimate of probability of PFS and OS by COX-2 expression status at diagnosis. (A) PFS; (B) OS. Dotted line indicates COX-2-positive patients and solid line COX-2-negative patients by WB expression analysis.
Multivariate analysis was also performed. COX-2 expression was the only significant prognostic factor of PFS (HR 7.11, 95% CI 2.8-17.9; P < .001). As far as OS is concerned, none of the parameters analyzed was statistically significant. However, COX-2 positivity came closest to statistical significance (HR 2.08, 95% CI 0.9-4.8; P = .08). Of note, if β2-microglobulin was removed from the OS analysis, COX-2 positivity achieved statistical significance (HR 2.36, 95% CI 1.16-4.79; P < .05). This effect is probably due to the larger number of evaluable patients (94 vs 77), as no colinearity between COX-2 and β2-microglobulin was found when the 2 parameters were assessed using the Pearson correlation coefficient (r = 0.09, P = .43).
COX-2 expression at progression/relapse was also an indicator of poor prognosis in terms of OS (P < .005; Figure 3). This finding is even more remarkable since in the subgroup of COX-2-negative patients, 6 subjects were already at second or third relapse, whereas all but one COX-2-positive subject were assessed at first treatment failure.
Kaplan Meier estimate of probability of OS by COX-2 expression status at progression relapse. Dotted line indicates COX-2-positive patients and solid line COX-2-negative patients by WB expression analysis.
Kaplan Meier estimate of probability of OS by COX-2 expression status at progression relapse. Dotted line indicates COX-2-positive patients and solid line COX-2-negative patients by WB expression analysis.
COX-2 mRNA expression by real-time PCR: validation of the assay and assessment of MM specimens
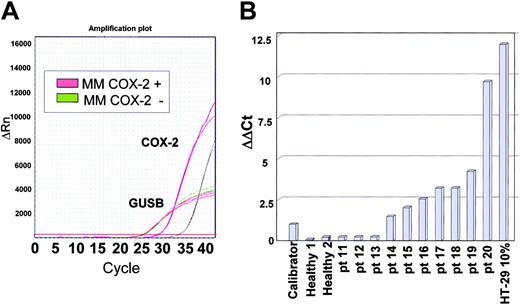
Due to the high sensitivity of real-time PCR, even BM from healthy subjects gave a positive signal and thus our assay for mRNA expression was validated as follows. We compared amplification plots of normal BM to those of HT-29 cell dilutions; the expression level of the specimen containing 0.5% HT-29 cells was in all cases above the values achieved by 10 normal BM samples (data not shown). We thus set this value as a threshold for COX-2 overexpression. The 0.5% HT-29 cell dilution was also used as a calibrator for relative quantification of COX-2 transcripts using the ΔΔCt method. COX-2 mRNA expression was assessed in 10 MM samples, of which 3 had scored COX-2 negative by WB and 7 had scored COX-2 positive. Representative amplification plots for 2 patients are shown in Figure 4A. Results from all patients tested are shown in Figure 4B, and indicate that all patients showing COX-2 overexpression at the protein level also overexpressed COX-2 at the mRNA level. In contrast, patients scoring COX-2 negative by WB had a COX-2 mRNA expression level comparable to that of normal BM.
Immunohistochemistry studies
IC detected COX-2 expression in 24 of 42 tumors (57%; Figure 5A-E). Of note, COX-2 expression was detected in all cases with extramedullary tumor manifestations (4 paravertebral, one pleural; Figure 5A-C). COX-2 positivity was noted in 51% of true BM-derived MM; it was occasionally heterogeneous within a single tumor, and was considered positive where small or large clusters of plasma cells exhibited immunoreactivity (Figure 5A-D). Bright staining of vessel endothelia was not a frequent finding in any of the tumors examined. In no case did we detect any obvious staining of macrophages, megakaryocytes, osteoblasts, or osteoclasts, albeit a faint staining of endothelial cells did rarely occur (Figure 5E). Conversely, tumor-unaffected BM or soft and connective tissue displayed no significant COX-2 protein expression. Figure 5F shows a typical COX-2-negative patient: BM is heavily invaded by malignant plasma cells without COX-2 immunoreactivity. For the 11 patients assessed using both IC and WB, results were concordant in 73% of cases (data not shown). IC was also done on 6 MGUS specimens. COX-2 expression was not observed in the plasma cell or stromal fraction of any of the specimens, confirming the low incidence of COX-2 positivity observed by WB.
Real-time PCR assessment of COX-2 mRNA levels. (A) A representative example of COX-2 real-time PCR. Red lines are derived from a patient scoring COX-2 positive by WB, whereas green lines are from a patient scoring COX-2 negative. Although the housekeeping gene GUSB has identical amplification plots, there is a clear difference in the COX-2 mRNA expression level. (B) Results of 10 patients with MM. The calibrator sample for ΔΔCt analysis is a 0.5% HT-29 cell dilution. Negative controls are normal BM samples whereas the positive control is a 10% HT-29 cell dilution. Concordance with WB results is full, since patients 11 to 13 scored COX-2 negative by WB, whereas patients 14 to 20 scored COX-2 positive.
Real-time PCR assessment of COX-2 mRNA levels. (A) A representative example of COX-2 real-time PCR. Red lines are derived from a patient scoring COX-2 positive by WB, whereas green lines are from a patient scoring COX-2 negative. Although the housekeeping gene GUSB has identical amplification plots, there is a clear difference in the COX-2 mRNA expression level. (B) Results of 10 patients with MM. The calibrator sample for ΔΔCt analysis is a 0.5% HT-29 cell dilution. Negative controls are normal BM samples whereas the positive control is a 10% HT-29 cell dilution. Concordance with WB results is full, since patients 11 to 13 scored COX-2 negative by WB, whereas patients 14 to 20 scored COX-2 positive.
COX-2 expression analysis on selected CD138+ populations
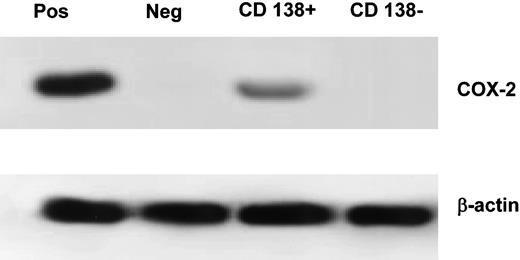
To further investigate whether COX-2 expression is related to malignant plasma cells, BM cells from 4 COX-2-positive subjects were selected for the CD138 antigen. The median percentage of CD138+ cells in the positive fraction was 98% (range, 93%-99%) whereas the percentage in the negative fraction was 0.5% (range, 0.3%-1.2%). In all cases, COX-2 expression was limited to the CD138+ fraction (Figure 6).
In addition, to rule out the possibility of lack of detectable COX-2 expression depending on a dilution effect of COX-2-positive plasma cells within a background of COX-2-negative nonneoplastic cells, COX-2 expression was assessed by WB on selected CD138 cells from 4 COX-2-negative subjects (2 MM and 2 MGUS). The CD138+ cell fraction of these samples was in all cases COX-2 negative. This suggests that plasma cells from these subjects have very little if any COX-2 expression (data not shown).
Discussion
This is the first comprehensive assessment of COX-2 expression in MM. It is also one of the first reports demonstrating that the COX-2 pathway plays a role in hematologic tumors. We demonstrate that a significant proportion of MMs express COX-2. COX-2 expression is progression-related, being uncommon in MGUS but frequent at disease progression/relapse. We also found COX-2 expression to occur in malignant plasma cells, thus it is not merely a microenvironmental response to disease presence. Finally, we show that COX-2 expression is an independent adverse prognostic factor in MM.
Immunohistochemistry in MM tissue sections. (A) Heterogeneous moderate and (B) homogeneous strong COX-2 expression in paravertebral-located extramedullary tumor. (B-C) Little or no COX-2 immunoreactivity in endothelial cells of small venules. (D-E) Larger clusters of plasma cells in BM samples; no significant immunoreactivity of osteoblasts and osteoclasts. (F) An example of COX-2 negativity on malignant plasma cells. This patient had massive bone marrow infiltration by malignant plasma cells that did not show COX-2 immunoreactivity. Original magnification: × 100 for panels A, B, D, E and × 200 for panels C and F. The microscope was an Axioskop (Zeiss, Germany) with Plam-Neofluar objective lens ×20, numerical aperture 10. Immersion oil was not used. The camera was a JVC model 3-CCT, and the acquisition software was Analysis Software (Softimaging Systems, Germany).
Immunohistochemistry in MM tissue sections. (A) Heterogeneous moderate and (B) homogeneous strong COX-2 expression in paravertebral-located extramedullary tumor. (B-C) Little or no COX-2 immunoreactivity in endothelial cells of small venules. (D-E) Larger clusters of plasma cells in BM samples; no significant immunoreactivity of osteoblasts and osteoclasts. (F) An example of COX-2 negativity on malignant plasma cells. This patient had massive bone marrow infiltration by malignant plasma cells that did not show COX-2 immunoreactivity. Original magnification: × 100 for panels A, B, D, E and × 200 for panels C and F. The microscope was an Axioskop (Zeiss, Germany) with Plam-Neofluar objective lens ×20, numerical aperture 10. Immersion oil was not used. The camera was a JVC model 3-CCT, and the acquisition software was Analysis Software (Softimaging Systems, Germany).
COX-2 plays a key role in the pathogenesis of several human cancers, including colo-rectal, non-small cell lung, breast, skin, head, and neck, as is clearly demonstrated by numerous epidemiologic, experimental, and clinical reports.2,5-9,24-26 On the basis of these observations, we hypothesized that the COX-2 pathway might also be involved in the pathogenesis of hematologic cancers. Several biologic considerations suggest a role for COX-2 and prostaglandins in the deregulated cytokine network associated to MM: (1) interleukin 1 (IL-1) and IL-6 induce COX-2 expression in several tissues, particularly brain27,28 ; (2) prostaglandin E2 induces IL-6 synthesis in hematopoietic and neural tissues29,30 ; (3) prostaglandin E2 modulates vascular endothelial growth factor (VEGF) synthesis, suggesting a possible link between COX-2 expression and myeloma-associated angiogenesis31 ; (4) prostaglandins actively induce bone reabsorption through osteoclast activation, possibly mediated by the RANK (receptor activation of nuclear factor kB) ligand.32
Western blotting on CD138-selected cell population. Positive control was the HT-29 cell line, negative was normal BM. The percentage of CD138+ cells was 99% in the CD138+ fraction and 0.3% in the CD138- fraction. The β-actin loading control is included. Pos indicates positive control; Neg, negative control (normal BM).
Western blotting on CD138-selected cell population. Positive control was the HT-29 cell line, negative was normal BM. The percentage of CD138+ cells was 99% in the CD138+ fraction and 0.3% in the CD138- fraction. The β-actin loading control is included. Pos indicates positive control; Neg, negative control (normal BM).
Involvement of COX-2 in MM would also provide an explanation for some as-yet-unexplained epidemiologic observations. Fernandez et al and Fritschi et al established a negative association between MM and fish intake.33,34 Fish consumption has a well-known negative effect on prostaglandin metabolism induced by ω-3 fatty acids.35 Brown et al demonstrated a positive association between obesity and MM,36 an association that echoes findings in other cancers characterized by a deregulated prostaglandin network, such as breast and colo-rectal.37,38 The link between obesity and prostaglandin metabolism has several potential explanations, including increased availability of substrate and IGF (insulin growth factor)-mediated up-regulation of prostaglandin metabolism.39 Finally, the beneficial properties of some drugs employed in the treatment of MM, most notably thalidomide and immunomodulatory drugs (IMiDs) might also depend on effective inhibition of the COX-2 pathway, as outlined in a recent paper by Fujita et al.40
COX-2 involvement in MM is obviously a strong indication of this pathway's potential role in other hematologic cancers, but the literature is limited in this field.13-16 Giles et al13 noticed that COX-2 expression is associated to advanced phases of chronic myeloid leukemia (CML) natural history; Wun et al15 found COX-2 expression in non-Hodgkin lymphoma-derived cell lines. More recently, some in vivo data have been published, based on IC alone, suggesting the potential involvement of COX-2 in non-Hodgkin lymphoma.16 However, this study is limited by sample heterogeneity and lack of multiple-approach validation. Our study clearly establishes an association between COX-2 expression and disease progression, and shows COX-2 expression to have a negative impact on outcome, paralleling the reports relating several different solid tumors.5,8 We would not be surprised if COX-2 is also found to be involved in other blood-related cancers, particularly myelodysplastic syndromes and Hodgkin lymphoma, which share with MM the presence of a highly disordered cytokine network.41,42
A novel pathogenetic model for MM has recently been proposed,18,43 which suggests that cyclin D deregulation is a major event in MM pathogenesis, as either cyclin D1, D2, or D3 appear to be consistently overexpressed in MM. Although this might come about directly through a chromosomal translocation such as the t(11;14), this does appear to be the most frequent situation.43 Indeed, COX-2 might be involved in cyclin D deregulation. A number of studies have positively correlated COX-2 and cyclin D overexpression in solid tumors and have shown that COX-2 inhibitors can down-regulate cyclin D expression.44,45 Future research will address the problem of determining whether a similar interaction might also be relevant in MM pathogenesis.
Increased COX-2 expression acts as an independent adverse prognostic factor in MM, as also observed in other cancers such as lung and breast.8,46,47 COX-2 expression is not related to a number of widely employed prognostic indicators, such as stage, β-2 microglobulin, and BM plasma cells. Recently, additional prognostic indicators have been described in MM, most notably cytogenetics and gene profiling.48-50 Thirty-six patients from our panel were evaluated for the presence of chromosome 13 deletion but no association was found. Additional studies are required to verify whether COX-2 expression might somehow be related to other cytogenetic abnormalities (particularly chromosomal translocations) or prognostic models obtained using high-throughput gene profiling approaches.49
COX-2 expression assessed by WB was found to have high prognostic significance. However, WB is probably not an ideal strategy for routine clinical examination. It is quite complex, requires fresh or frozen tissue, and does not provide adequate quantification of protein levels. IC also detected COX-2 expression in malignant plasma cells and showed good concordance with WB. Since IC is more user-friendly and can be applied to formalin-fixed tissue, it would of interest to determine whether it possesses the same prognostic value as WB. Real-time PCR was also highly concordant with WB and the high quantitative power of this assay could make it very useful to quantitatively correlate COX-2 expression levels and disease aggressiveness. However, the high cost and potential methodologic biases associated with RNA manipulation do not suggest its use in routine evaluation. Finally, although our series was small, we found no difference between results on purified populations and those on whole BM cells. It would be interesting to examine a larger series of patients to determine whether COX-2 expression analysis on CD138-purified cells has a higher prognostic value, by eliminating potential biases related to the excessive dilution of COX-2-positive plasma cells on a background of COX-2-negative cells.
Univariate and multivariate analysis showed COX-2 expression to be the most important predictor of outcome in our series. This observation is intriguing but should be considered with caution: our series has some features that are unusual and somehow difficult to explain, particularly the lack of prognostic significance in univariate analysis of several well-established parameters, such as stage and creatinine level. Our series was quite heterogeneous in terms of age and clinical features, and patients had been treated through different approaches over a long period of time. Thus our findings require further validation on large, independent, and uniformly treated patient series.
The prognostic value of COX-2 expression and its increased expression in advanced disease phases suggest that disordered prostaglandin metabolism might confer increased aggressiveness to malignant plasma cells. However, it is not yet clear how this may take place. In particular, we do not know whether prostaglandins act directly on plasma cells by an autocrine mechanism or whether the paracrine effect on BM microenvironment prevails. We are currently working to clarify the roles of COX-2 and COX-2-derived metabolites, particularly prostaglandin E2 (PGE2), within the deregulated cytokine network typical of MM.
The simple observation that COX-2 is expressed in MM does not indicate that it might be a useful therapeutic target. However, this is a worthwhile investigation hypothesis, since considerable success has been achieved with specific and nonspecific COX-2 inhibitors in solid tumors.10-12 As previously mentioned, there are several reports indicating that COX-2 inhibition might result in effective down-regulation of pathways that are relevant to MM pathogenesis. A recent study by Fujita et al40 shows that thalidomide strongly inhibits COX-2 expression at the mRNA level, and suggests that some of the many clinical effects of this drug may be due to inhibition of this pathway. Due to its severe toxic effects, thalidomide is probably not an ideal drug for long-term maintenance or chemoprevention in MM. On the other hand, several drugs targeting COX-2 are currently employed for chemopreventive and therapeutic intervention in other settings.10-12 Some of these drugs have limited toxicity and acceptable costs, and are actively being evaluated as anticancer agents in several human neoplasms, with very promising results. MM is an attractive model for both chemopreventive and chemotherapeutic trials: (1) MGUS is a well-defined, high-risk preneoplastic condition and there is no useful intervention to reduce the risk of developing a fatal disease; (2) MM often responds to chemotherapy but invariably relapses and thus effective nontoxic maintenance treatments are required. Novel drugs including thalidomide, IMiDs, and bortezomib have produced encouraging results, and other agents targeting both the malignant plasma cells and the microenvironment are under extensive evaluation.51-56 It is thought that a cocktail of these agents might afford effective control of MM in the near future.57 COX-2 has been targeted (whether consciously or not) by several generations of physicians in many different diseases. It would be fascinating to find a role for specific or nonspecific COX-2 inhibitors in the therapeutic cocktail holding promise of effective control over this still devastating and fatal disease.
Prepublished online as Blood First Edition Paper, February 24, 2005; DOI 10.1182/blood-2004-11-4201.
Supported by the International Myeloma Foundation (IMF), Los Angeles, CA (L. Monitillo, D.D., G.P.); Fondazione Angela Bossolasco (L. Monitillo, D.D., G.P.) and Compagnia di San Paolo, Turin, Italy; Consiglio Nazionale delle Ricerche (CNR) and Ministero dell' Università e della Ricerca (MIUR), Rome, Italy. M.D.A. is a recipient of a fellowship from Comitato Piemontese Luigi Ghirotti. I.R. is a recipient of a fellowship from Fondazione Italiana Ricerca sul Cancro (FIRC), Milan, Italy. M.A. and R.R. are recipients of a fellowship from Fondazione San Paolo, Turin, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Brian G. Durie for critical revision of the manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal