Abstract
Myeloid cell leukemia-1 (MCL-1) acts as a key survival factor for chronic lymphocytic leukemia (CLL) cells. In addition, dissipation of cellular bioenergy may impose a lethal effect on these quiescent cells. Previously, in multiple myeloma cell lines we demonstrated that halogenated adenosine (8-Cl-Ado) was phosphorylated to triphosphate (8-Cl–adenosine triphosphate [ATP]), which preferentially incorporated into mRNA and inhibited RNA synthesis by premature transcription termination. Furthermore, 8-Cl-ATP accumulation was associated with a decline in cellular bioenergy. Based on these actions, we hypothesized that 8-Cl-Ado would be ideal to target CLL lymphocytes. In the present study we demonstrate that leukemic lymphocytes incubated with 8-Cl-Ado display time- and dose-dependent increase in the accumulation of 8-Cl-ATP, with a parallel depletion of the endogenous ATP pool. Inhibition of global RNA synthesis resulted in a significant decline in the expression of transcripts with a short half-life such as MCL1. Consistent to this, protein expression of MCL-1 but not B-cell lymphoma–2 (BCL-2) was decreased. Furthermore, 8-Cl-ATP induced programmed cell death, as suggested by caspases activation, cleavage of caspase 3, and PARP (poly–adenosine diphosphate [ADP]–ribose polymerase), and increased DNA fragmentation. In conclusion, 8-Cl-Ado induces apoptosis in CLL lymphocytes by targeting cellular bioenergy as well as RNA transcription and translation of key survival genes such as MCL1.
Introduction
Chronic lymphocytic leukemia (CLL) is presently an incurable disease representing the most common form of leukemia in North America and Europe.1 In recent years, purine analogs or alkylating agents used as a single drug or in combination have shown improved efficacy in B-CLL, as measured by complete remission (CR) rates and progression-free survival.1-5 However, overall survival has not improved. The disease is characterized as a neoplastic disorder with disrupted apoptosis, as opposed to aberrant proliferation, that leads to a gradual accumulation of leukemia cells.6,7 Lack of proliferative properties make these cells inherently quiescent; thus, cell cycle–specific agents and other drugs that act against actively dividing and proliferating cells may not be effective in this disease. Therefore, the identification and clinical development of agents that overcome the antiapoptotic state of B-CLL cells constitute a major area of preclinical and clinical investigation in this disease.
The survival advantage of CLL lymphocytes may be due to the presence of antiapoptotic proteins of the B-cell lymphoma–2 (BCL-2) family.8,9 Among the antiapoptotic proteins of this family, the most common members are BCL-2, BCL-XL, and myeloid cell leukemia-1 (MCL-1). These proteins inhibit apoptosis by several mechanisms, including the formation of dimers with proapoptotic family members. It has been demonstrated that MCL-1 is essential during early lymphoid development and then later for the maintenance of mature T and B lymphocytes.10 Immunohistochemical and quantitative immunoblotting studies have determined the levels of expression of BCL-2 and MCL-1 in normal and malignant lymphocytes. Specifically, lymphocytes express high levels of either MCL-1 (germinal center lymphocytes) or BCL-2 (mantle zone lymphocytes).11
Cell fate including proliferation, differentiation, viability, and tumorigenesis have been directly associated with expression of BCL-2 antiapoptotic proteins in general and MCL-1 in particular.12 High levels of MCL1 and BCL2 mRNA9,13 and proteins14 have been found in most cases of B-CLL. Furthermore, MCL-1 levels in CLL lymphocytes inversely correlate with in vitro and clinical response to chemotherapy. Finally, high levels of MCL-1 are associated with the failure of B-CLL patients to achieve CR to initial therapy with fludarabine.14 Taken together, these reports suggested that MCL-1 may act as a key survival factor for CLL.
The inherent resistance of B-CLL cells to traditional cytotoxic therapy arises from their apoptotic defects.14 For CLL patients with resistance to conventional agents, efforts are ongoing to evaluate different therapeutic approaches for induction of apoptosis.15,16 Ideally, such treatments would work through DNA replication–independent pathways, such as the inhibition of transcription of the survival genes for CLL lymphocytes and/or the bioenergetic pathway essential for physiologic function of these cells, or through direct effect on the mitochondria.17
Based on these findings, we hypothesized that the cytotoxicity in CLL lymphocytes could be induced by inhibiting the RNA transcription in these cells, leading to a decline in transcripts of antiapoptotic genes, resulting in a diminution of antiapoptotic proteins involved in the survival of these cells. An alternate or additional pathway would be to target the energetic metabolism of these nondividing lymphocytes thereby affecting the cellular functionality, which should induce cell death. These events should be independent of p53 status.
Previous in vitro investigations in human leukemia,18 multiple myeloma,19,20 and solid tumor cell lines21,22 reported that a halogenated adenosine analog such as 8-Cl-Ado has an obvious inhibitory effect in these cell types. Mechanistic studies have established the intracellular phosphorylation of the halogenated analog to its triphosphate, with a concomitant decline in cellular bioenergy.20 Furthermore, the actions of the analog were DNA independent and RNA directed.20,23 Finally, incorporation of the analog into RNA results in inhibition of global RNA synthesis with maximal effect on mRNA production.24 Taken together these characteristics make the halogenated adenosine triphosphate (8-Cl ATP) an ideal drug to target transcription and cellular bioenergy of replicationally quiescent CLL lymphocytes.
To test our postulates, we used leukemic lymphocytes isolated from peripheral blood obtained from patients with CLL and treated with 8-Cl-Ado. Our data demonstrate that these indolent leukemia cells phosphorylate 8-Cl-Ado to 8-Cl-ATP, which results in a decrease in ATP pool and inhibition of RNA synthesis. Furthermore, 8-Cl-Ado treatment affected MCL1 mRNA and protein expression levels and the survival gene of CLL and was associated with induction of cell death.
Patients, materials, and methods
Materials
For in vitro investigation, 8-Cl-Ado was obtained from Dr V. Rao at the Drug Development branch of the National Cancer Institute. For high-pressure liquid chromatography (HPLC) analyses, 8-Cl-ATP was custom synthesized by Bio Log (La Jolla, CA). [3H] Uridine was purchased from Moravek Biochemicals (Brea, CA). All other chemicals were reagent grade.
Patients and healthy donors
Present in vitro studies were carried out in leukemic lymphocytes obtained from previously treated or untreated CLL patients (n > 50) with a median white blood cell (WBC) count of 60 000 × 109/L (range, 35 000-219 000 × 109/L). The percentage of leukemic lymphocytes and prolymphocytes was high in these patients (median, 96%; range, 83%-100%). Cohorts of 5 to 12 patients' samples were used for different pharmacologic, biochemical, and molecular end points. To compare studies in normal hematopoietic cells, lymphocytes from 3 healthy donors were included in the study. To compare accumulation of 8-Cl-ATP in red blood cells (RBCs), a population of RBCs obtained from 3 patients was also studied.
Clinical pharmacology
Blood samples were obtained from CLL patients who agreed to and signed the institutional review board (IRB)–approved informed consent for blood drawing. Samples (10 mL) were collected in heparin-containing vacutainer tubes and transported to the laboratory. Control studies have demonstrated that leukemia cells are stable under these conditions with respect to size and membrane integrity. The cellular nucleotide content is stable for at least 15 hours under these conditions.25
Isolation of CLL and normal lymphocytes
Blood samples were diluted with phosphate-buffered saline and mononuclear cells were isolated using ficoll-hypaque density gradient centrifugation method. Cells were enumerated using a Coulter counter, and a Coulter electronics channelizer (Coulter, Hialeah, FL) was used to determine the mean cell volume. For in vitro investigations, cells were suspended in the RPMI medium containing 10% fetal calf serum at a density of 1 × 107 cells/mL.
Incubation of cells with 8-Cl-Ado
The primary CLL or normal lymphocytes were incubated either with the indicated concentration of 8-Cl-Ado for different time points or with different concentrations of 8-Cl-Ado for 4 hours. To determine the effect of in vitro culturing, CLL lymphocytes were incubated in medium without 8-Cl-Ado for different time points. Cultures were maintained and aliquots (1 × 107cells/mL) were removed at the indicated times. After being washed with phosphate-buffered saline, cells were processed for nucleotide extraction. Nucleotides were extracted using perchloric acid and the extracts were neutralized with KOH and stored at -20°C until analyzed.26
Measurement of intracellular nucleoside triphosphate by HPLC
The intracellular concentration of normal nucleotides and 8-Cl-ATP was quantitated using HPLC as described previously.20 The lower limit of sensitivity of this assay was 25 pmol in an extract of 1 × 107 cells corresponding to a cellular concentration of 10 μM.
Inhibition of RNA synthesis by 8-Cl-Ado
CLL or normal lymphocytes were either untreated or treated with 10 μM 8-Cl-Ado for 2 hours and 4 hours and synthesis of RNA was measured. Prior to removal of the aliquot, 1 μCi/mL (0.037 MBq/mL) of [3H] uridine was added to these cultures and the incubation was continued for an additional 30 minutes. The cells were then collected and the radioactivity in the acid-insoluble material was measured by scintillation counting and expressed as the percentage of control (untreated) value of cells.24
Analyses of gene expression
Total RNA was isolated from untreated and 8-Cl-Ado–treated primary CLL cells by either extraction with RNAzol B (Tel-test, Friendswood, TX) or by using RNeasy mini kit (QIAGEN S.A., Courtabeuf, France) with the optional RNase-free DNase step (to avoid contamination with genomic DNA), as instructed by the manufacturer. Poly(A)+-enriched RNA was isolated from 1 × 107 cells using GeneStrips hybridization tubes (RNAture, Irvine, CA). The expression level of different genes was measured on an ABI prism 7900 sequence detection system (Applied Biosystems, Foster City, CA) by using 1-step TaqMan real-time reverse transcriptase–polymerase chain reaction (real-time RT-PCR) and/or by a 2-step TaqMan real-time RT-PCR procedure in which SuperScript II (Invitrogen, Carlsbad, CA) was used to synthesize cDNA from the RNA with random oligonucleotide primers. The primers and probes for MCL1 and β2-microglobulin were purchased from Applied Biosystems Assays on Demand program and the BCL2 and 18S primers and probes were purchased from their “Predeveloped Assay Reagents.” The relative gene expression levels were quantitated by using either standard curves generated from known dilutions of cDNA from an untreated multiple myeloma cell line, MM.1S, and normalized with 18S ribosomal RNA as described by the manufacturer or by using the ΔΔCT method27 normalizing to β2-microglobulin. The results are presented as a percentage of the gene expression level in the untreated sample from the same patient.
Immunoblot analysis
Cells were lysed to extract protein, and the supernatant was stored at -70°C. Protein content was determined using DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). Aliquots (30 μg) of total cell protein were loaded onto 8% sodium dodecyl sulfate (SDS)–polyacrylamide gels and transferred to immobilon-p membranes (Millipore, Bradford, MA). Membranes were incubated with primary antibodies for 2 hours followed by species-specific horseradish peroxidase–conjugated secondary antibody (diluted 1:5000) for 1 hour. The blots were visualized by enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL) and normalized to the actin levels. Rabbit polyclonal antibody to MCL-1 (sc-819) and mouse monoclonal antibody to BCL-2 (sc-509) from Santa Cruz Biotech (Santa Cruz, CA) and mouse monoclonal antibody to PARP (poly–adenosine diphosphate–ribose polymerase) from BD Pharmingen International (San Diego, CA) were used. For anti–procaspase-3 (Transduction Laboratories, Pharmingen, San Diego, CA) and antiactive caspase-3 (Cell Signaling, Beverly, MA), detection was performed by an enhanced chemiluminescence method.
Cell-death assays
For cellular assay of caspase activity, cells were washed and resuspended in caspase buffer (50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4; 100 mM NaCl; 1 mM EDTA [ethylenediaminetetraacetic acid]; 0.1% Chaps [3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid]; and 5 mM dithiothreitol). Lysates were stored at -80°C and caspase enzymatic assays were carried out in 96-well plates. Lysates (10-20 μg of total protein) were mixed and reactions were initiated by addition of 100 μM of the respective specific substrates. Caspase-3–like protease activity was measured with the substrate Ac-DEVD-AMC. Activity was quantified by monitoring fluorescence at excitation and emission wavelengths of 380 and 460 nm on a Cytofluor 2000 (Millipore) fluorometer.
Cell viability and mitochondrial transmembrane potential were concomitantly analyzed by DiOC6 (3,3′ dihexyloxacarbocyanine iodide) and propidium iodide (PI) staining as previously described.17 Briefly, after 8-Cl-Ado treatment, B-CLL cells were incubated for 10 minutes at 37°C in culture medium containing 40 nM of DiOC6 and 5 μg/mL PI. After suitable compensation, fluorescence was recorded at different wavelengths: DiOC6 at 525 nm and PI at 600 nm. Viable cells were DiOC6-bright and PI-low; early apoptotic cells were DiOC6-low and PI-low; nonviable/dead cells were DiOC6-low and PI-bright. For the DNA fragmentation, a flow-based assay was used by staining DNA with PI in fixed and permeabilized CLL lymphocytes as previously described.17
Results
Time-dependent accumulation of 8-Cl-ATP and effect on cellular bioenergy
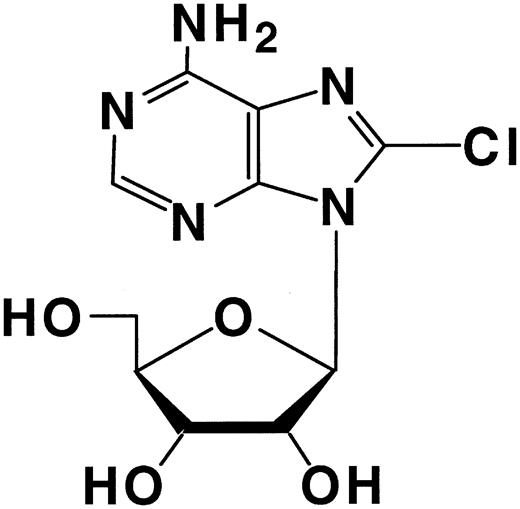
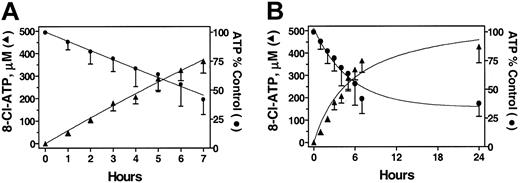
Previously, we have shown that 8-Cl-Ado (Figure 1) is cytotoxic to multiple myeloma cells19 and this cytotoxicity was dependent on conversion to its triphosphate.20 To determine the efficacy of 8-Cl-Ado for CLL, we first wanted to assess the ability of these cells to accumulate analog triphosphate. Primary CLL cells were incubated with 10 μM 8-Cl-Ado at different time points and the accumulation of 8-Cl-ATP was measured. 8-Cl-Ado was rapidly metabolized to its triphosphate in these cells. During the first 7 hours the accumulation of 8-Cl-ATP displayed a linear pattern of accumulation (r = 0.9270). The rate of accumulation of the analog triphosphate was 54 ± 2 μM/hour (Figure 2A). However, after this point the levels were maintained about the same at 24 hours (Figure 2B).
Accumulation of 8-Cl-ATP and depletion of ATP pool with the treatment of 8-Cl-Ado in primary CLL cells from 7 patients (1, 3-8). The CLL lymphocytes were incubated with 10 μM 8-Cl-Ado for indicated times and PCA-extracted nucleotides were analyzed by HPLC. The accumulation of 8-Cl-ATP (▴) and the depletion of ATP (•) are plotted together for 7 hours (A) and up to 24 hours (B). Symbols indicate mean ± standard deviation.
Accumulation of 8-Cl-ATP and depletion of ATP pool with the treatment of 8-Cl-Ado in primary CLL cells from 7 patients (1, 3-8). The CLL lymphocytes were incubated with 10 μM 8-Cl-Ado for indicated times and PCA-extracted nucleotides were analyzed by HPLC. The accumulation of 8-Cl-ATP (▴) and the depletion of ATP (•) are plotted together for 7 hours (A) and up to 24 hours (B). Symbols indicate mean ± standard deviation.
Concomitant with the accumulation of analog triphosphate, there was a decline in cellular bioenergy in the leukemic lymphocytes (Figure 2A). The starting value of ATP was a median 2660 μM (range, 2120-3650 μM), and at 7 hours the concentration declined to a median 1100 μM (range, 730-1540 μM). The decline was maintained at 24 hours (Figure 2B).
Dose-dependent accumulation of 8-Cl-ATP and its relationship to the ATP pool
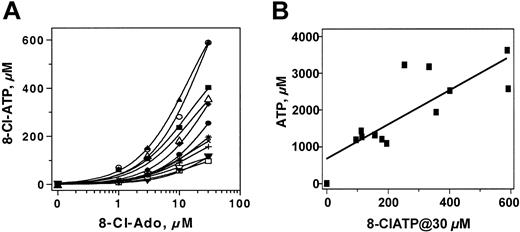
Primary CLL cells from 12 patients were incubated with 1, 3, 10, and 30 μM 8-Cl-Ado for 4 hours. There was a dose-dependent accumulation of 8-Cl-ATP. At 1 μM of 8-Cl-Ado, the level of 8-Cl-ATP was a median 15 μM (range, 5-70 μM), which increased to 45 μM with 3 μM drug (range, 15-150 μM). Variations in the 8-Cl-ATP levels were evident at each concentration of the drug; at 10 μM 8-Cl-ATP it was 110 μM (range, 56-354 μM), and at 30 μM it further increased to 188 μM (range, 30-592 μM; Figure 3A).
The heterogeneity among these patients suggested that the accumulation of 8-Cl-ATP might be dependent either on cellular kinases that phosphorylate 8-Cl-Ado to its monophosphate or on the final step in phosphorylation, which has been shown to involve ATP synthase. In such a scenario, the accumulation of 8-Cl-ATP should mimic the cellular level of ATP (ie, cells that are active in ATP synthesis would accumulate more 8-Cl-ATP). To determine this, a comparison of 8-Cl-ATP concentration and cellular ATP pool was made in the cells of 12 patients (Figure 3B). There was a direct relationship (r = 0.6597; P < .001) between endogenous ATP pool in untreated cells and accumulation of 8-Cl-ATP at 30 μM 8-Cl-Ado.
Dose-dependent accumulation of 8-Cl-ATP and its relation to the ATP pool. CLL lymphocytes from 12 patients (8, 23-33) were incubated with indicated concentration of 8-Cl-Ado for 4 hours and PCA-extracted nucleotides were analyzed by HPLC. Dose-dependent increase of 8-Cl-ATP was plotted (A) and accumulation of 8-Cl-ATP at 30 μM exogenous 8-Cl-Ado was compared with endogenous ATP pool from each patient (B); each symbol represents one patient.
Dose-dependent accumulation of 8-Cl-ATP and its relation to the ATP pool. CLL lymphocytes from 12 patients (8, 23-33) were incubated with indicated concentration of 8-Cl-Ado for 4 hours and PCA-extracted nucleotides were analyzed by HPLC. Dose-dependent increase of 8-Cl-ATP was plotted (A) and accumulation of 8-Cl-ATP at 30 μM exogenous 8-Cl-Ado was compared with endogenous ATP pool from each patient (B); each symbol represents one patient.
To determine if the decline in the ATP level was not due to in vitro culturing of cells, CLL cells from 3 patients were incubated without 8-Cl-Ado for 0, 2, 4, and 24 hours and the ATP levels were measured. Compared with control value, the ATP levels in all 3 patients at these different times varied between 95% ± 3% and 119% ± 6%. Thus there was no significant decrease in the ATP levels, suggesting that the decline in ATP pool is particularly due to the accumulation of 8-Cl-ATP in response to the addition of 8-Cl-Ado.
Inhibition of RNA synthesis by 8-Cl-Ado
To determine if 8-Cl-ATP accumulation results in inhibition of RNA synthesis, samples were assessed for [3H] uridine incorporation. Data from CLL cells of 5 patients suggest heterogeneity among patients regarding endogenous level of RNA synthesis (compare disintegrations per minute [dpm] values at 0 h). However, in all cases there was a time-dependent decrease in uridine incorporation with 10 μM 8-Cl-Ado and the percentage of the decrease was comparatively similar despite the differences in their synthesis rates. The percentage of inhibition ranged between 33% and 45% at 2 hours and between 58% and 68% at 4 hours (Table 1). When the same cells were analyzed for other macromolecule synthesis, there was no effect on DNA by 8-Cl-Ado (data not shown). This was expected, as CLL lymphocytes are quiescent and not replicating DNA.
Effect of 8-Cl-Ado on the RNA inhibition
. | RNA synthesis, % mean . | . | . | ||
|---|---|---|---|---|---|
| Patient no. . | 0 h (dpm/106 cells) . | 2 h . | 4 h . | ||
| 1 | 100 (1360) | 67 | 40 | ||
| 2 | 100 (1502) | 60 | 35 | ||
| 3 | 100 (2805) | 57 | 32 | ||
| 4 | 100 (648) | 55 | 42 | ||
| 5 | 100 (3450) | 62 | 37 | ||
. | RNA synthesis, % mean . | . | . | ||
|---|---|---|---|---|---|
| Patient no. . | 0 h (dpm/106 cells) . | 2 h . | 4 h . | ||
| 1 | 100 (1360) | 67 | 40 | ||
| 2 | 100 (1502) | 60 | 35 | ||
| 3 | 100 (2805) | 57 | 32 | ||
| 4 | 100 (648) | 55 | 42 | ||
| 5 | 100 (3450) | 62 | 37 | ||
Primary CLL cells of patients 1 to 5 were isolated and the cells (5 × 106/500 μL) were incubated without drug or with 10 μM of 8-Cl-Ado for 2- and 4-hour periods and pulsed with [3H] uridine for 30 minutes. At the end of incubation the uridine incorporation into RNA was measured. The values are expressed as percentage of control (untreated) cells.
To rule out that the decline in RNA synthesis was not due to culturing of cells, CLL lymphocytes from 3 patients were incubated without 8-Cl-Ado for 0, 2, and 4 hours and the RNA synthesis was measured. At 4 hours the inhibition of RNA synthesis was similar to that in the matched untreated sample for all 3 patients (99.7%, 85.3%, and 105% of respective control value), suggesting that the inhibition of RNA synthesis is particularly in response to 8-Cl-Ado.
Effect of 8-Cl-Ado on MCL1 and BCL2 mRNA transcript
The global RNA synthesis inhibition in CLL lymphocytes by 8-Cl-Ado should affect the synthesis of new transcripts. This should result in a decline in transcripts with a short half-life such as MCL1. To investigate this, primary CLL cells from 6 patients were incubated with 8-Cl-Ado for 4 and 24 hours and total RNA was isolated (Table 2, first 6 rows). Regarding MCL1 transcripts, there was heterogeneity among patients. The first 3 patients showed a strong response as the levels of MCL1 decreased by 40% by 24 hours. For the remaining 3 patients there was no discernable decrease in the MCL1 mRNA levels, suggesting heterogeneity among patients.
Effect of 8-Cl-Ado on RNA transcripts of apoptotic proteins
. | % of control . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | MCL-1 . | . | BCL-2 . | . | |||
| Patient no. . | 4 h . | 24 h . | 4 h . | 24 h . | |||
| 15 | 72 | 49 | ND | ND | |||
| 16 | 62 | 59 | 92 | 114 | |||
| 17 | 81 | 64 | 107 | 49 | |||
| 18 | 143 | 118 | 108 | 152 | |||
| 19 | 113 | 129 | 80 | 118 | |||
| 20 | 89 | 98 | 98 | 105 | |||
| 21 | 4* | 0 | ND | ND | |||
| 22 | 68* | 0 | ND | ND | |||
. | % of control . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | MCL-1 . | . | BCL-2 . | . | |||
| Patient no. . | 4 h . | 24 h . | 4 h . | 24 h . | |||
| 15 | 72 | 49 | ND | ND | |||
| 16 | 62 | 59 | 92 | 114 | |||
| 17 | 81 | 64 | 107 | 49 | |||
| 18 | 143 | 118 | 108 | 152 | |||
| 19 | 113 | 129 | 80 | 118 | |||
| 20 | 89 | 98 | 98 | 105 | |||
| 21 | 4* | 0 | ND | ND | |||
| 22 | 68* | 0 | ND | ND | |||
Total RNA was isolated from CLL lymphocytes (1 × 107/mL) after treatment with 10 μM 8-Cl-Ado at the indicated times. Real-time RT-PCR experiments were carried out to measure the RNA transcripts of antiapoptotic proteins MCL-1 and BCL-2. The values are expressed as percentage of control (untreated) cells.
ND indicates not done.
These values were measured at 18 hours in poly(A)-enriched RNA rather than total RNA
8-Cl-Ado treatment should generate nonfunctional short-length transcripts by prematurely terminating transcription.24 If not degraded, the partial transcripts would be present and detectable in total RNA. To quantify only the functional full-length MCL1 transcripts, real-time RT-PCR assay was done on poly(A)+-enriched RNA from 2 CLL patients' lymphocytes (Table 2 last 2 rows). A greater reduction in MCL1 mRNA levels was observed in the poly(A)+ RNA (over 70% in the first patient and over 90% in the second patient sample) after 18 hours of 8-Cl-Ado treatment. By 24 hours, the levels of MCL1 mRNA in both patients fell below the limit of detection. In contrast to the MCL1 levels in total RNA, on average BCL2 did not show any significant decrease at any time point (Table 2). This is consistent with other studies28 that show that BCL2 mRNA is more stable than MCL1 mRNA.
Effect of 8-Cl-Ado on MCL-1 and BCL-2 protein levels
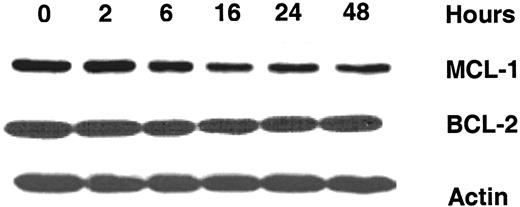
To determine if the decline in the transcript of MCL1 results in knocking down the protein, protein levels for MCL-1 and BCL-2 were measured. As shown in Figure 4 (from 1 representative patient), a time-dependent decrease in MCL-1 protein expression was noted in CLL lymphocytes starting at 6 hours and continued up to 48 hours after incubation with 10 μM 8-Cl-Ado. As expected from the BCL2 transcript data, there was no significant change in BCL-2 protein expression.
Similar studies were done in primary CLL cells from 9 patients and results from densitometry analysis are presented in Table 3. In all cases there was a significant down-regulation of MCL-1 protein. At 4 hours, the value compared with control (0 h, untreated) was a median 83% (range, 48%-98%) and at 24 hours the median was 39% (range, 3%-52%). In contrast to MCL-1 there was no discernable decrease in the expression level of BCL-2, whose median was 98% and 92% at 4 hours and at 24 hours, respectively.
Effect of 10 μM 8-Cl-Ado on antiapoptotic proteins
. | % of control . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | MCL-1 . | . | BCL-2 . | . | |||
| Patient no. . | 4 h . | 24 h . | 4 h . | 24 h . | |||
| 3 | 88 | 3 | 100 | 99 | |||
| 4 | 98 | 6 | 98 | 97 | |||
| 5 | 57 | 52 | 97 | 90 | |||
| 9 | 83 | ND | 96 | ND | |||
| 10 | 65 | 39 | 99 | 92 | |||
| 11 | 73 | 40 | 99 | 93 | |||
| 12 | 48 | 13 | 96 | 91 | |||
| 13 | 86 | 49 | ND | ND | |||
| 14 | 85 | 30 | ND | ND | |||
. | % of control . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | MCL-1 . | . | BCL-2 . | . | |||
| Patient no. . | 4 h . | 24 h . | 4 h . | 24 h . | |||
| 3 | 88 | 3 | 100 | 99 | |||
| 4 | 98 | 6 | 98 | 97 | |||
| 5 | 57 | 52 | 97 | 90 | |||
| 9 | 83 | ND | 96 | ND | |||
| 10 | 65 | 39 | 99 | 92 | |||
| 11 | 73 | 40 | 99 | 93 | |||
| 12 | 48 | 13 | 96 | 91 | |||
| 13 | 86 | 49 | ND | ND | |||
| 14 | 85 | 30 | ND | ND | |||
Proteins were separated and immunoblotted for MCL-1 and BCL-2 and quantitated using a densitometer. The values are normalized based on the levels of actin and the value in untreated cells. Value in untreated cells is referred to as control, which is expressed as 100%.
ND indicates not done.
Effect of 8-Cl-Ado on expression of antiapoptotic proteins MCL-1 and BCL-2 in CLL lymphocytes. Immunoblot analysis was carried out for the expression of MCL-1 and BCL-2 proteins after incubating CLL lymphocytes with 10 μM 8-Cl-Ado with varying times. Actin was used as a loading control.
Effect of 8-Cl-Ado on expression of antiapoptotic proteins MCL-1 and BCL-2 in CLL lymphocytes. Immunoblot analysis was carried out for the expression of MCL-1 and BCL-2 proteins after incubating CLL lymphocytes with 10 μM 8-Cl-Ado with varying times. Actin was used as a loading control.
To determine if higher concentration results in further decline in protein level, CLL lymphocytes were incubated with 10, 30, and 100 μM 8-Cl-Ado. These data indicate minor (86%) decrease at 4 hours but much greater down-regulation of MCL-1 protein at 24 hours. At both time points, the extent of decrease was similar at all 3 concentrations (data not shown). Taken together, these data illustrate that 8-Cl-Ado had a different effect on antiapoptotic proteins MCL-1 and BCL-2, which was consistent to the differential effect on the mRNA of both of these genes.
Induction of CLL cell death by 8-Cl-Ado
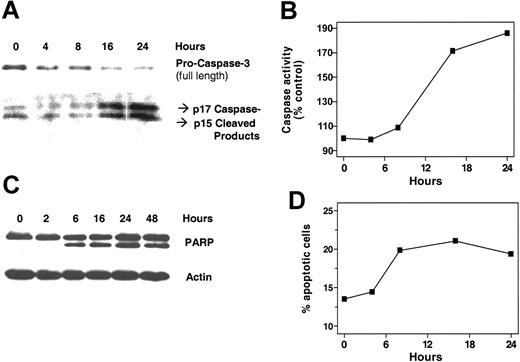
Several experimental approaches were used to measure drug-induced cell death. Treatment of CLL lymphocytes with 10 μM 8-Cl-Ado leads to an increase of the proteolytic cleavage of procaspase-3 into the enzymatically active fragments of caspase-3 (p15 and p17) detected by immunoblotting using a “cleavage-specific” antibody (Figure 5A, bottom panel). Concomitantly, the amount of full-length procaspase-3 gradually declined starting at 4 hours after incubation.
The results obtained with the immunoblots were confirmed by measuring the enzymatic activity of caspase-3 using a fluorometric biochemical assay based on the DEVD substrate (Figure 5B). The enzymatic activity of caspase-3 (and the related caspases that recognize DEVD as a substrate) increased after 8 hours of incubation and reached a plateau after 24 hours.
Activation of caspase-3 was further confirmed by the detection of cleaved PARP, a substrate of caspase-3–like enzymes. When primary CLL cells were incubated with 10 μM 8-Cl-Ado for indicated time points, cleavage of PARP was obvious starting at 6 hours (Figure 5C).
Flow cytometry assays were observed to determine the percentage of CLL lymphocytes undergoing apoptosis and cell death. A double-staining assay was used to concomitantly determine the membrane permeability of the CLL lymphocytes (using propidium iodide [PI]) and the mitochondrial transmembrane. This assay was previously shown17 to be able to distinguish apoptotic, healthy viable, and necrotic or dead cells. Incubation of primary CLL cells with 10 μM 8-Cl-Ado for 24 hours lead to a reduction in the percentage of viable cells. Concomitantly, an increase of the percentage of apoptotic cells was visible after 24 hours, followed by the increase of the percentage of necrotic/dead cells at 48 hours after incubation (data not shown).
Finally, the kinetics of 8-Cl-Ado–induced endonucleosomal DNA fragmentation were detected by measuring the percentage of cells with the sub-G0G1 DNA content using PI staining in permeabilized and fixed CLL lymphocytes (Figure 5D). The percentage of cells with fragmented DNA started to increase as early as 4 hours and reached a plateau by 8 hours. When CLL primary cells were incubated for 0, 2, and 4 hours in culture media without 8-Cl-Ado, there was no PARP cleavage (data not shown), suggesting that the induction of cell death in CLL lymphocytes is in response to 8-Cl-Ado. Culturing for longer time (24 hours), however, resulted in some PARP in these lymphocyte extracts.
Effect of 8-Cl-Ado on normal lymphocytes and erythrocytes
To evaluate the accumulation of 8-Cl-Ado and the level of ATP in the normal cells, lymphocytes from 2 healthy individuals were treated with 10 μM 8-Cl-Ado at 6 hours and the accumulation of 8-Cl-ATP was measured. The HPLC data showed lesser accumulation of analog triphosphate in normal lymphocytes (210 μM of 8-Cl-ATP at 6 hours incubation compared with CLL lymphocytes, which was 300 μM). Furthermore, the decline in ATP pool was substantially lower in normal lymphocytes (92% and 70% of control, respectively).
Similarly, when normal lymphocytes from 2 healthy individuals were incubated with increasing concentration of 8-Cl-Ado (3, 10, and 30 μM) for 3 hours, the HPLC data showed lesser accumulation of analog triphosphate compared with CLL lymphocytes. At 3, 10, and 30 μM drug levels, the 8-Cl-ATP concentrations were 15 and 29 μM, 48 and 97 μM, and 96 and 167 μM, respectively. At these concentrations of the drug there was not much decrease in the endogenous ATP pool; levels were 85% to 100% of control value.
To further determine if the accumulated 8-Cl-ATP inhibits RNA synthesis in normal lymphocytes, the uridine incorporation experiments were performed in 2 healthy individuals whose triphosphate accumulation was measured. There was no significant inhibition of RNA synthesis with the incubation of 10 μM 8-Cl-Ado at 4 hours (111% and 120%, respectively), suggesting that the drug is targeting preferably leukemic lymphocytes.
In order to see if 8-Cl-Ado gets accumulated in cells other than lymphocytes, RBCs from 3 CLL patients were incubated with 8-Cl-Ado for 4 hours and the 8-Cl-ATP accumulation was measured after perchloric acid (PCA) extraction. Accumulation of analog triphosphate in red blood cells ranged from 30 μM to 60 μM; however, this had a very minor effect on ATP pool (0% to 25% decrease compared with control untreated value).
Induction of CLL cell death by 8-Cl-Ado. Several experimental approaches were used to determine cell death of primary leukemic lymphocytes by 8-Cl-Ado. Immunoblot analysis of cleavage of procaspase 3 to active caspase 3 (A), caspase activation detection by fluorescent assay (B), time-dependent cleavage of PARP (C), and apoptotic death measured by sub GO-G1 DNA content (D).
Induction of CLL cell death by 8-Cl-Ado. Several experimental approaches were used to determine cell death of primary leukemic lymphocytes by 8-Cl-Ado. Immunoblot analysis of cleavage of procaspase 3 to active caspase 3 (A), caspase activation detection by fluorescent assay (B), time-dependent cleavage of PARP (C), and apoptotic death measured by sub GO-G1 DNA content (D).
Discussion
It is widely agreed that impaired apoptosis leading to a survival advantage of the leukemic lymphocytes is of central importance to the pathogenesis of CLL. The discrepancy between the prolonged survival of CLL lymphocytes in vivo and their predilection to undergo spontaneous apoptosis in vitro suggests that cell death is prevented in vivo by antiapoptotic factors operating within the tissue microenvironment.8,9,11,12 Even though several antiapoptotic genes have been identified, it appears that MCL-1 plays a pivotal role in CLL cell survival as described in detail in “Introduction.”14 Hence, abrogating this “survival” mechanism for leukemic lymphocytes represents a viable therapeutic strategy for the treatment of CLL.
Previous studies demonstrated that 8-Cl-Ado is cytotoxic in a number of multiple myeloma cell lines that are resistant to traditional chemotherapeutic agents.19,20,24,29 Additional studies established that the mechanism of cytotoxicity was due to several factors; however, it was dependent on metabolism of 8-Cl-Ado to its cytotoxic triphosphate. Pharmacologically, 8-Cl-Ado resembles a classical nucleoside analog, which must be converted to its phosphorylated form prior to incorporation into nucleic acids or for its actions on other cellular targets.20 In contrast to other clinically active nucleoside analogs, 8-Cl-Ado is phosphorylated to the monophosphate 8-Cl–adenosine monophosphate (8-Cl AMP) by adenosine kinase and not by deoxycytidine kinase.30 Studies using an adenosine kinase–deficient cell line further demonstrated the need of this enzyme and accumulation of the triphosphorylated metabolite 8-Cl-ATP for 8-Cl-Ado–induced cytotoxicity.20 The high specific activity of this enzyme in lymphocytes31 and substantial substrate specificity30 for 8-Cl-Ado may be responsible for high levels of 8-Cl-ATP in leukemic lymphocytes (Figures 2, 3).
In addition to their correlation with adenosine kinase activity, the level of 8-Cl-ATP accumulated in the CLL primary cells was strongly associated with the basal concentration of ATP pool (Figure 3B), suggesting that 8-Cl-ATP accumulation was associated with metabolic activity of cells. Parallel to the formation of intracellular 8-Cl-ATP there was a decline in the ATP pool in CLL lymphocytes (Figure 2A-B). Decrease in cellular bioenergy has been strongly associated with cell death via either necrosis or apoptosis.32-34 ATP depletion or energy failure was proposed as an important step in chemotherapy-induced tumor regression.33 Recently, ATP depletion of varying magnitudes has been linked specifically to the necrotic phenotype in different tissues.34 Because the physiologic functionality of these quiescent cells is dependent on cellular bioenergy, loss of the majority of the intracellular ATP pool should result in cell death.
Previous studies have demonstrated that cladribine triphosphate can cooperate with cytochrome c and apoptotic protease-activating factor–1 (APAF-1) to trigger a caspase pathway in a Hela cell–free system.35 Similarly, 8-Cl-ATP was able to induce APAF-1–dependent caspase activation in a cell-free system.36 After induction of apoptotic sequelae, 8-Cl-ATP could substitute for dATP in an apoptosome complex in whole cells; such action would result in enhancement of cell death.
Studies in multiple myeloma cell lines also established that the actions of 8-Cl-Ado were directed toward RNA. It appears that in these cell lines this analog has a multifactorial route for inhibition of RNA synthesis. First, the effect may be in part due to an overall decline in the ATP pool, a precursor for RNA synthesis.20 Second, the action may be due at least partially to the incorporation of a fraudulent nucleotide into RNA and premature termination of transcription. There was preferential incorporation into mRNA and the incorporated analog was at the 3′ terminus of the transcripts.24 Third, the preferred syn configuration of 8-Cl-AMP, rather than the conventional anticonfiguration of normal nucleotides in RNA, would prevent further polymerization.37 Finally, in vitro investigations using yeast poly(A)+ polymerase suggested a decline in polyadenylation of transcripts after 8-Cl-ATP incorporation in poly(A) tails.38 Collectively, these actions are likely to result in a significant loss of expression of functional full-length polyadenylated transcripts.
Although differential effect of 8-Cl-Ado on mRNA synthesis and processing was not studied in CLL primary cells, there was a global and consistent inhibition of total RNA synthesis after treatment of these lymphocytes with 8-Cl-Ado (Table 1). The consequence of the action of 8-Cl-Ado on mRNA should be most evident for transcripts with short half-lives.28 The half-lives of antiapoptotic transcripts MCL1 and BCL2 are less than 3 hours and more than 10 hours, respectively.39-42 Hence, 8-Cl-Ado–mediated RNA inhibition should result in an earlier decrease in MCL1 transcript without much effect on BCL2 mRNA. Our observations (Table 2) in primary CLL cells were in agreement with this postulate and indicated that even though some short-length MCL1transcripts are being generated, functional full-length MCL1 mRNA is largely lost in CLL lymphocytes after 8-Cl-Ado treatment.
As expected, there was a high level of BCL-2 and MCL-1 protein expression in leukemic lymphocytes from patients with CLL.14,43 However, the decline in the expression of MCL1 mRNA was associated with a significant depletion of MCL-1 protein after treatment of primary CLL cells by 8-Cl-Ado (Table 3; Figure 4). On the contrary, BCL-2 remained practically constant over this time. BCL-2 is a longer-lived protein, having a half-life of 10 to 14 hours,43 whereas MCL-1 has a very short half-life ranging from 20 minutes to 3 hours, depending on the cell type considered.39,40,42 Furthermore, BCL2 mRNA was not affected, whereas MCL1 functional transcripts were reduced after analog treatment.
Down-regulation in the expression of MCL-1 protein should affect survival of CLL and other cell types. For example, depletion of MCL-1 by antisense oligonucleotides facilitates entry into apoptosis.44,45 As expected for a short-term survival regulator, MCL-1 decreases when cells undergo apoptosis with various stimuli.46-48 The reduction of MCL-1 levels during apoptosis can be explained either by transcriptional or translational inhibition, since both the protein and mRNA have very short half-lives. The down-regulation of MCL-1 protein during apoptosis agrees with other studies49 and suggests that MCL-1 might be important for survival. Consistent with this statement, overexpression of MCL-1 prolongs the survival of cells exposed to a variety of apoptosis-inducing stimuli including cytokine withdrawal; c-myc overexpression; and treatment with staurosporine, etoposide, calcium ionophore, and UV irradiation.50-53 Similarly, MCL1 was induced at both the transcript and protein level after cytokine or growth factor stimulation of leukemia cells.49 Thus, 8-Cl-Ado–induced cytotoxicity may be the result of diminished expression of key survival gene to below critical levels for sustaining cell viability. Decline in cell viability through activation of the cyclic AMP (cAMP) signaling pathway has been observed both by forskolin and 8-Cl-phenylthio-cAMP.54 These pathways also decreased expression of MCL-1 protein, further strengthening the importance of MCL-1 in survival. Even in the clinical situation, increased expression of MCL-1 is observed in leukemia after relapse.55
Taken together, our data support the proposed mechanism of action of 8-Cl-Ado involving the following sequence of events: (1) accumulation of its triphosphate form, (2) ATP depletion, (3) transcriptional repression, (4) decline in the mRNA and protein levels of MCL-1, and (5) activation of cell death pathways. Based on this unique mechanism, 8-Cl-Ado should be considered for further investigation as a novel therapeutic strategy for treatment of B-CLL.
Prepublished online as Blood First Edition Paper, February 17, 2005; DOI 10.1182/blood-2004-05-1699.
Supported in part by the National Cancer Institute (grants CA57629, CA81534, and CA85915), Department of Health and Human Services, and a Translational Research Grant 6208-05 from the Leukemia and Lymphoma Society and from the CLL Global Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are thankful to Laura Rassenti (Tissue Core, CLL Research Consortium, University of California at San Diego, La Jolla, CA) for the CLL patient samples.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal