Abstract
An experimental system to explore central tolerance in humans is unavailable. However, the human endogenous retrovirus K-18 (HERV-K18) region on chromosome 1 provides an excellent model: HERV-K18 encodes a superantigen (SAg) stimulating Vβ7CD4 T cells that is implicated in type 1 diabetes and Epstein-Barr virus persistence. In this study, we have addressed thymic HERV-K18 SAg expression, the capacity of SAg to induce negative selection, and the consequences of this for peripheral tolerance compared with SAg reactivity. We demonstrate that thymic HERV-K18 SAg expression is constitutive and is restricted in time and space such that it can induce negative selection. We developed an in vitro assay capable of detecting negative human thymocyte selection by bacterial SAgs presented on extrathymic antigen-presenting cells (APCs). Using this assay, the HERV-K18 SAg is necessary and sufficient for negative selection of immature or semimature Vβ7CD4 thymocytes. Decreases of SAg reactive Vβ7CD4 T cells generated in the thymus predict low or absent SAg reactivity. Therefore, these results indicate that negative thymic selection to HERV-K18 SAgs constitutes a first checkpoint controlling peripheral tolerance compared with SAg reactivity. This study now offers a framework to dissect negative selection and its interplay with viral persistence and autoimmunity in humans.
Introduction
Self-tolerance is a multistage process progressively established during T-cell development in the thymus and maintained in the periphery throughout the lifespan of mature T cells. Much of the initial concepts of T-cell tolerance emerged from studying the biology of superantigens (SAgs) in mice.1,2 SAgs stimulate 103 to 105 more T cells than do conventional antigens, a feature that is based on their capacity to selectively bind Vβ chains on T cells in a variety of different modes indiscriminately and without regard for their antigen specificity.3 Infectious and endogenous mouse mammary tumor viruses (MMTVs) encode several SAgs with different T-cell receptor (TCR) Vβ specificities.4,5 Multiple forms of T-cell tolerance develop to these SAgs; negative selection in the thymus is one of the most extensively studied. It results in partial or complete deletion of mature T cells expressing SAg-reactive Vβ chains from the peripheral repertoire.6 Thymic deletion reflects the susceptibility of immature and semimature thymocytes to undergo negative selection at the corticomedullary junction and in the thymic medulla replete of professional antigen-presenting cells (APCs).7,8 It requires that SAg expression exceed a threshold level during thymocyte development9,10 and that SAgs be presented by the appropriate thymic cell type, which includes medullary epithelial cells and hematopoietic APCs.11 Expression of some MMTV SAgs is restricted to the thymocyte lingeage,12 and efficient deletion in these instance relies on paracrine transfer to major histocompatibility complex (MHC) class 2+ thymic APCs.13,14 SAg-induced central tolerance, therefore, depends on the developmental stage of the reactive thymocytes and on the temporospatial regulation of SAg expression. The primary failure to eliminate self-reactive thymocytes can result in organ-specific autoimmunity,15 which illustrates the importance of the thymus for organ-specific tolerance.
The HERV-K18 envelope gene on chromosome 1 encodes for SAg stimulating Vβ7 and Vβ13 T cells16 implicated in the establishment of Epstein-Barr virus (EBV) persistence17 and in type 1 diabetes (T1D). For instance, HERV-K18 polymorphisms are associated with T1D.18 Vβ7 T cells in particular, and Vβ13 T cells to a lesser extent, are enriched in the inflammatory lesions19,20 and in the circulatory systems21 of patients at the clinical onset of T1D. HERV-K18 transcription and SAg function in cells efficiently presenting SAgs are inducible by proinflammatory stimuli such as viral infection22 and interferon-α (IFN-α).23 This provides an attractive model linking infection with the initiation or progression of autoimmunity.
Here we explored the mechanisms governing central T-cell tolerance to the HERV-K18 SAg. We found that thymic HERV-K18 SAg expression is constitutive and restricted in time and space such that it can induce negative selection. HERV-K18 SAg is necessary and sufficient for the negative selection of immature or semimature Vβ7CD4 thymocytes. Our data are consistent with the notion that the peripheral levels of Vβ7CD4 T cells are predictive of SAg reactivity and establish the premise to exploit the role of HERV-K18–induced negative selection for tolerance and autoimmunity in humans.
Materials and methods
Molecular cloning and ribonuclease protection analysis
The frameshift mutation Δ53 was generated by AvrII digestion and fill in of the HERV-K18.1 SAg. Ribonuclease protection analysis (RPA) was performed on approximately 25 μg total RNA of whole tissue samples or fluorescence-activated cell sorter (FACS) suspensions using previously described probes.23 The mouse TATA binding protein (mTBP) probe protected a 160–base pair (bp) fragment, and the SAg probe protected a 299-bp fragment from Δ53-based constructs and a 324-bp fragment from wild-type (wt) SAg-based constructs.
Purification of thymocyte subsets and FACS analysis
Double-positive (DP) thymocytes were pre-enriched by targeting the CD1+ subset with biotinylated anti-CD1A antibodies and streptavidin-coated paramagnetic microbeads, followed by magnetic sorting (Miltenyi Biotec, Bergisch Gladbach, Germany). The positive fraction was further labeled with CD8-APC and sorted on a flow cytometer to eliminate the contaminating CD8- immature CD4SP cells. The final purity of the DP cells was greater than 98%.
FACS analysis of thymocyte subsets was performed on freshly isolated cells. Cells were stained with monoclonal HERV-K18 antibodies and isotype control antibodies. After blocking with normal mouse serum, cells were labeled with secondary fluorescence isothiocyanate (FITC)–labeled antibodies, CD4-APC, and CD8-phycoerythrin (CD8-PE). This allowed gating on DP, CD4 single-positive (CD4SP), CD8SP, and double-negative (DN) cells after the exclusion of dead cells with propidium iodide (PI). Anti-hemagglutinin (anti-HA) antibodies (HA.11, clone 16B12; Covance Research Products, Princeton, NJ) were used as negative control in Figure 1B, and immunoglobulin G2a (IgG2a) isotype control antibodies (PharMingen, San Diego, CA) were used as negative control in Figure 1D.
Monoclonal HERV-K18 antibodies were produced at the Center for Biomedical Invention (Dr. X-H. Li, University of Texas at Dallas). They were used at 1:100 and were revealed with FITC-coupled secondary antibodies (PharMingen).
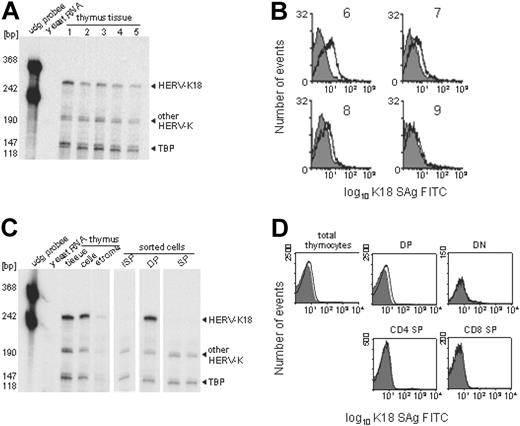
Constitutive HERV-K18 SAg expression restricted to thymocyte lineage. Thymic HERV-K18 expression was addressed by RPA and FACS analysis on total thymocytes and thymocyte subsets. (A) Shown is an RPA of whole thymus tissue from 5 donors using HERV-K18 SAg and human TBP control probes. The HERV-K18 SAg probe generates a full-length protected fragment from HERV-K18 transcripts and smaller internally digested fragments from other HERV-Ks. (B) Thymocyte suspensions from 4 donors (donors 6-9) were incubated with an isotype control (gray shaded curve) and with monoclonal antibodies against HERV-K18 (open curve), which were subsequently revealed by FITC-labeled secondary antibodies. (C) RPA was performed on whole thymus tissue, on single-cell suspensions, and on FACS-sorted samples. The cell fraction obtained after the elution of thymocytes is labeled stroma. Thymocyte suspensions were FACS sorted into the immediate precursors of DP cells (ISP = CD4+CD8-CD3-), into immature DP thymocytes (CD4+CD8+), and into mature SP thymocytes (CD4-CD8+, CD4+CD8-). (D) Freshly isolated thymocytes were labeled with isotype control antibodies (gray shaded curve) and monoclonal anti–HERV-18 antibodies (open curve). Gating on thymocyte subsets was based on CD4/CD8 expression, and exclusion of dead cells, which allowed to us address HERV-K18 expression of thymocyte subsets.
Constitutive HERV-K18 SAg expression restricted to thymocyte lineage. Thymic HERV-K18 expression was addressed by RPA and FACS analysis on total thymocytes and thymocyte subsets. (A) Shown is an RPA of whole thymus tissue from 5 donors using HERV-K18 SAg and human TBP control probes. The HERV-K18 SAg probe generates a full-length protected fragment from HERV-K18 transcripts and smaller internally digested fragments from other HERV-Ks. (B) Thymocyte suspensions from 4 donors (donors 6-9) were incubated with an isotype control (gray shaded curve) and with monoclonal antibodies against HERV-K18 (open curve), which were subsequently revealed by FITC-labeled secondary antibodies. (C) RPA was performed on whole thymus tissue, on single-cell suspensions, and on FACS-sorted samples. The cell fraction obtained after the elution of thymocytes is labeled stroma. Thymocyte suspensions were FACS sorted into the immediate precursors of DP cells (ISP = CD4+CD8-CD3-), into immature DP thymocytes (CD4+CD8+), and into mature SP thymocytes (CD4-CD8+, CD4+CD8-). (D) Freshly isolated thymocytes were labeled with isotype control antibodies (gray shaded curve) and monoclonal anti–HERV-18 antibodies (open curve). Gating on thymocyte subsets was based on CD4/CD8 expression, and exclusion of dead cells, which allowed to us address HERV-K18 expression of thymocyte subsets.
HERV-K18 transfectants
Bulk transfectants of A20 (TIB-208) were generated by electroporation of an SRα promoter–driven bicistronic enhanced green fluorescence protein (EGFP) expression cassette23 and were maintained at 1 to 2 μg/mL blasticidin concentrations (BSD; Invitrogen). To generate efficient SAg-presenting transfectants, clones of A20 bulk transfectants were FACS sorted for low EGFP of the bicistronic expression cassette (mean fluorescence intensity [MFI] greater than 3 and less than 5).22,23 These clones were kept for less than 4 weeks in continuous culture and were tested for T cell hybrid reactivity22 before their use in thymocyte assays.
Thymocyte assays
Single-cell suspensions of freshly isolated human thymocytes were obtained from children undergoing cardiac surgery, as described.24 To achieve consistent biologic behavior, samples from donors younger than 1 month, from donors with less than 70% DP thymocytes, and from donors with CD8 SP values equaling or exceeding those of CD4 SP were excluded. Thymocytes were analyzed by FACS, after culture for 24 or 48 hours at 37°C, with mitomycin C–inactivated and phorbol myristate acetate (PMA) (50 ng/mL for 16 hours at 37°C)–treated APCs at the indicated ratios. Before analysis, cell suspensions were treated with EDTA (ethylenediaminetetraacetic acid) to avoid clustering of thymocytes with APCs; dead cells were excluded by PI staining.
Mature T-cell expansions assays
Assays were performed with FACS-sorted A20 clones with low SAg expression for the most efficient SAg presentation. After 3 days in 96 round-bottomed well plates at the indicated stimulator/responder ratios, T cells were expanded in 10 U/mL interleukin-2 (IL-2) for 7 or more days before FACS analysis. Cells were split every 3 days, and IL-2 was freshly added at 10 U/mL. Staphylococcal enterotoxin B (SEB) (Toxin Technology, Sarasota, FL) was used at 1 μg/mL.
Blood donors
Twenty-four healthy donors were from the blood bank in Geneva, and 18 were from the blood bank in Lausanne (55 ± 13 years); 18 (28 ± 4 years) were from the Department of Genetics and Microbiology at the University of Geneva Medical School in Switzerland. The local ethics committee approved the study, and informed consent was obtained in accordance with the Declaration of Helsinki.
Results
Constitutive thymic HERV-K18 SAg expression
An absolute requirement for the deletion of SAg-reactive thymocytes in vivo is thymic HERV-K18 SAg expression. We indeed found significant levels of HERV-K18 SAg transcripts in total thymus tissue and in thymocyte suspensions from all donors analyzed (n = 5; Figure 1A). Compared with the loading control TBP, some variation in individual HERV-K18 expression levels was observed. Thymic HERV-K18 protein expression and its variability were corroborated by FACS analysis on thymocyte suspensions using monoclonal anti–HERV-K18 antibodies (n = 4; Figure 1B). The level of HERV-K18 cell surface expression matched that of another well-characterized endogenous retroviral envelope protein constitutively expressed in the placenta.25 HERV-K18 exists as 3 alleles, encoding secreted or cell-surface membrane proteins. Surface-protein complexes are unstable and are shed from cells.26 Thus, in all cases, SAg can become available for presentation by MHC class 2 in trans.
Constitutive HERV-K18 expression in the thymocyte lineage implied that it had to be shut down later during development because SAg is not constitutively expressed in mature T cells.23 This is effectively the case, as can be seen in Figure 1C. Interestingly, SAg transcription is restricted to immature DP thymocytes, but it is expressed neither in the immediate precursors of DP cells (ISP = CD4+CD8-CD3-)27 nor in mature SP T cells. The protected band derived from HERV-K transcripts other than HERV-K18 SAg demonstrates that this subtle differential regulation is not a general feature of HERV-K genes. The HERV-K18 signals detected in the stromal fraction could be derived from epithelial cells or from rare APCs associated with it. Alternatively, they represent transcription from contaminating thymocytes. Differential HERV-K18 expression on the surfaces of thymocyte subsets was confirmed by FACS analysis (Figure 1D). It was indeed restricted to DP thymocytes and was not detected in other subsets. In essence, constitutive thymic HERV-K18 expression was mainly found associated with the thymocyte lineage, analogous with certain MMTV SAgs.12
Although this regulatory feature drives SAg expression at the appropriate place and developmental stage to negatively select thymocytes, it does not imply that thymocytes present SAg in cis. Specialized cell types, such as medullary thymic epithelial cells and hematopoietic APCs, may present SAg in trans, and thymocytes may merely provide the SAg source. Consistent with this, the HERV-K18 SAg is a secreted or unstable membrane-associated glycoprotein (F. Meylan et al, manuscript submitted, January 2004) (Figure 1B-D). In conclusion, thymic HERV-K18 SAg expression was constitutive in all donors analyzed; it is differentially regulated during thymocyte development and is restricted to DP thymocytes.
In vitro assay for negative selection induced by soluble bacterial SAgs
We first developed an in vitro assay with freshly isolated human thymocytes and extrathymic APCs, analogous to one of the first systems for negative selection developed in mice.28 It was capable of specifically detecting the deletion of immature Vβ17+ thymocytes reactive with the soluble bacterial SAg SEB (Figure 2). Interestingly, SEB-induced Vβ17 deletions were equally effective in the presence (Figure 2A) or absence (Figure 2B) of exogenously added APCs. Although this suggests that human thymocytes are capable of autopresenting the bacterial SAg SEB in vitro, it remains to be elucidated whether this is generally true for SAgs, and it implies that human thymocytes autopresent the HERV-K18 SAg in vivo. If this were to be the case, one would anticipate that it is inefficient.
HERV-K18 SAg is necessary and sufficient for negative selection
To probe the HERV-K18 SAg for negative selection of deletion-sensitive immature or semimature thymocytes, we cultured freshly isolated thymocytes with SAg and control transfectants of the extrathymic hematopoietic A20 APCs. They represent the best-characterized HERV-K18 SAg presenters for which loss-of-function mutants and variants are available.22,23 We also chose A20 cells rather than primary human APCs to reduce complexity. For instance, A20 cells are devoid of human endogenous retroviruses; contrary to polymorphic human MHC class 2 proteins, which can modulate the Vβ repertoire of SAg reactive T cells,29,30 this is not the case for mouse MHC class 2.
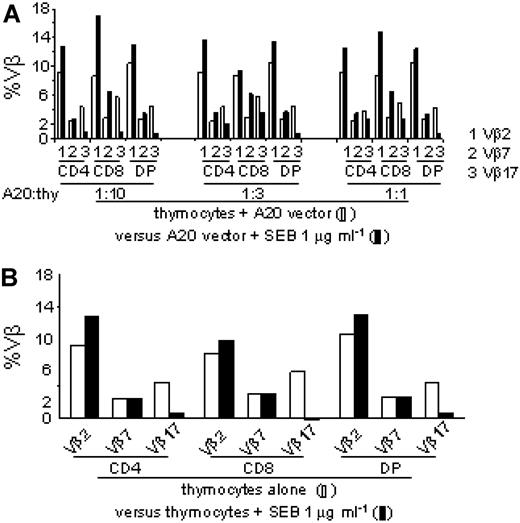
In vitro assay for SEB-induced Vβ17 deletions. We developed an in vitro assay capable of detecting negative selection of freshly isolated human thymocytes to bacterial SAgs. (A) Thymocytes were cultured with different ratios of vector-transfected A20APCs in the presence (▪) or absence (□) of the soluble bacterial SAg SEB. After culture, percentages of Vβ2 (1), Vβ7 (2), and the SEB reactive Vβ17 (3) were determined on DP, SPCD4, and SPCD8 thymocytes. (B) SEB was added directly to thymocytes (▪) or not (□) without exogenous APCs.
In vitro assay for SEB-induced Vβ17 deletions. We developed an in vitro assay capable of detecting negative selection of freshly isolated human thymocytes to bacterial SAgs. (A) Thymocytes were cultured with different ratios of vector-transfected A20APCs in the presence (▪) or absence (□) of the soluble bacterial SAg SEB. After culture, percentages of Vβ2 (1), Vβ7 (2), and the SEB reactive Vβ17 (3) were determined on DP, SPCD4, and SPCD8 thymocytes. (B) SEB was added directly to thymocytes (▪) or not (□) without exogenous APCs.
As anticipated, the percentages of DP thymocytes and their CD4/CD8 expression decreased in a SAg dose–dependent fashion compared with control transfectants, consistent with SAg-induced negative selection (Figure 3A). This was paralleled by significant and specific reductions in absolute thymocyte numbers, as shown in Supplemental Figure 1 (see the Supplemental Figure link at the top of the online article on the Blood website), corroborating HERV-K18 SAg–induced negative selection. Additional evidence supported the notion that the decrease in DP thymocytes was the consequence of cell death. First, given that only immature DP and semimature CD4SP thymocytes, but not mature SP thymocytes, are known to be deletion sensitive, we confirmed that purified immature DP (Supplemental Figure 2A) but not mature SP thymocytes (not shown) also lost coreceptor expression and decreased in a SAg dose–dependent fashion. Second, DP cells with down-regulated CD4/CD8 expression reappeared in the dead cell gate in a fashion complementary to their disappearance in the live gate and carried markers of cells undergoing apoptosis (not shown).
Deletion of thymocytes sensitive to negative selection was SAg dependent because the frameshift mutation that truncates SAg after 53 N-terminal amino acids (K18.1Δ53) completely abolished its capacity to delete (Figure 3B). This was not attributed to a loss of protein stability because Δ53 mutant transcripts and protein accumulated to levels comparable to those of the wt SAg (Supplemental Figure 2B). In addition, the deletion of thymocytes sensitive to negative selection was SAg dependent because deletion was consistent among different batches of SAg transfectants (Figure 3B, K18.1a+b) and among independently generated transfectants (Figure 3B, K18.12), indicating that the HERV-K18 SAg allele 1 is necessary and sufficient for the deletion of immature or semimature thymocytes.
Robust thymocyte deletion induced by the HERV-K18 SAg was found in all donors analyzed though to a varying extent, as anticipated by the polymorphic structure of human populations (not shown). As with mature T cells responding to the HERV-K18 SAg,22,23 more thymocytes (approximately 10%-25%) than predicted by Vβ use (5%-10%) were deleted. Thus, robust thymocyte deletion is in part an inherent characteristic of this SAg and could also be explained by the lower activation threshold of thymocytes and by the soluble proapoptotic mediators released by mature thymocytes, such as tumor necrosis factor-α (TNF-α) and glucocorticoids.31,32
The HERV-K18.1 SAg is necessary and sufficient for thymocyte deletion affecting primarily Vβ7CD4 cells. With the deletion assay, the capacity of the wt and mutant HERV-K18 SAg to negatively select deletion-sensitive human Vβ7 thymocytes was addressed. (A) Coculture of HERV-K18 SAg transfectants with freshly isolated thymocytes decreased percentages and absolute numbers of immature thymocytes (Supplemental Figure 1). Thymocytes were cultured with increasing numbers of vector- and SAg-transfected APCs (A20) and were subsequently analyzed for CD4/CD8 expression after the exclusion of dead cells. The percentages of DN, DP, SPCD4, and SPCD8 thymocytes are indicated in each quadrant. Respective reductions in absolute thymocyte numbers for 5 independent deletion assays are shown in Supplemental Figure 1. (B) Negative thymocyte selection was SAg dependent because it was present in independently generated SAg transfectants but was lost on SAg mutation. Thymocytes were cultured in the presence of vector-transfected A20 cells (horizontal bars), of A20 cells expressing the HERV-K18.1 SAg frameshift mutant (Δ53; ♦), of 2 distinct batches of HERV-K18.1 wt SAg-transfected A20 cells (K18.1 SAg1a+b; ▴), and of an independently generated second wt SAg transfectant (K18.1 SAg2; ▪). mt indicates mutant. (C) HERV-K18 SAg–induced negative selection affects primarily Vβ7CD4 thymocytes, when the extent of deletion (Figure 3C) and the specificity of deletion (Figure 3D) are jointly taken into account. Vβ use of DP and SP thymocytes from 5 donors was determined after 48-hour culture with vector and HERV-K18.1 SAg transfectants. Reductions obtained for SAg, compared with the vector control, are indicated in percentages for immature DP and mature SP thymocytes, and statistically significant results are annotated (t test, *P < .05; **P < .01). ♦ represent the mean ± 1 standard deviation of values obtained from 5 donors. (D) Absolute values of panel C are shown, and significant results are annotated (t test, *P < .05; **P < .01). Open and filled symbols represent individual values; horizontal bars represent the mean.
The HERV-K18.1 SAg is necessary and sufficient for thymocyte deletion affecting primarily Vβ7CD4 cells. With the deletion assay, the capacity of the wt and mutant HERV-K18 SAg to negatively select deletion-sensitive human Vβ7 thymocytes was addressed. (A) Coculture of HERV-K18 SAg transfectants with freshly isolated thymocytes decreased percentages and absolute numbers of immature thymocytes (Supplemental Figure 1). Thymocytes were cultured with increasing numbers of vector- and SAg-transfected APCs (A20) and were subsequently analyzed for CD4/CD8 expression after the exclusion of dead cells. The percentages of DN, DP, SPCD4, and SPCD8 thymocytes are indicated in each quadrant. Respective reductions in absolute thymocyte numbers for 5 independent deletion assays are shown in Supplemental Figure 1. (B) Negative thymocyte selection was SAg dependent because it was present in independently generated SAg transfectants but was lost on SAg mutation. Thymocytes were cultured in the presence of vector-transfected A20 cells (horizontal bars), of A20 cells expressing the HERV-K18.1 SAg frameshift mutant (Δ53; ♦), of 2 distinct batches of HERV-K18.1 wt SAg-transfected A20 cells (K18.1 SAg1a+b; ▴), and of an independently generated second wt SAg transfectant (K18.1 SAg2; ▪). mt indicates mutant. (C) HERV-K18 SAg–induced negative selection affects primarily Vβ7CD4 thymocytes, when the extent of deletion (Figure 3C) and the specificity of deletion (Figure 3D) are jointly taken into account. Vβ use of DP and SP thymocytes from 5 donors was determined after 48-hour culture with vector and HERV-K18.1 SAg transfectants. Reductions obtained for SAg, compared with the vector control, are indicated in percentages for immature DP and mature SP thymocytes, and statistically significant results are annotated (t test, *P < .05; **P < .01). ♦ represent the mean ± 1 standard deviation of values obtained from 5 donors. (D) Absolute values of panel C are shown, and significant results are annotated (t test, *P < .05; **P < .01). Open and filled symbols represent individual values; horizontal bars represent the mean.
When the Vβ use of DP and SP thymocytes was analyzed after culture with SAg transfectants, Vβ7 was in general more strongly reduced than control Vβ (Figure 3C). Negative selection of Vβ7 most manifestly affected CD4 thymocytes when the specificity (P < .05; Figure 3D) and the extent of the deletion (P < .01; Figure 3C) were jointly taken into consideration. This is consistent with HERV-K18–induced deletion primarily affecting semimature CD4SP cells, analogous to MMTV SAgs.7 Based on these experiments, we conclude that the HERV-K18 SAg predominantly deletes semimature CD4SP or immature DP thymocytes carrying Vβ7 chains.
Vβ7CD4 T-cell levels predict HERV-K18 SAg reactivity
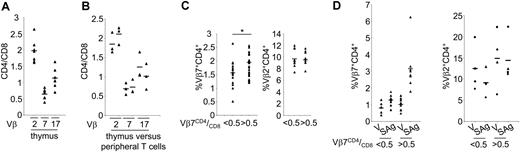
To be able to causally relate peripheral T cell levels with SAg reactivity, we first sought to establish a link between intrathymic and peripheral Vβ7CD4 cell levels and subsequently with SAg reactivity. The central premise was that if negative selection to HERV-K18 affected primarily Vβ7CD4 thymocytes, then low peripheral Vβ7CD4 levels would predict weak or absent SAg reactivity. To explore this, we compared the thymic and peripheral repertoires of 3 Vβ families heterogeneously distributed in the CD4/CD8 subsets. This analysis confirmed and extended the notion that these distributions are generated intrathymically and are maintained in the periphery. In fact, though Vβ2 expression was skewed toward CD4 use in the thymus and peripheral T cells, Vβ7 was variably reduced among CD4 cells, and Vβ17 was evenly distributed among subsets in both anatomic locations (Figure 4A-B). Reductions in Vβ7CD4 thymocyte and peripheral T-cell counts could have resulted from partial negative selection in the thymus.
If reduced thymic Vβ7CD4/CD8 ratios were to be the consequence of negative thymic selection by SAg, then peripheral Vβ7CD4/CD8 ratios must predict SAg reactivity. We first verified the differential Vβ7 distribution among CD4 and CD8 T cells in 47 healthy donors divided into 2 groups of similar age. As expected, donors with Vβ7CD4/CD8 ratios less than 0.5 had significantly lower percentages of Vβ7+ T cells in the CD4 subset, but this was not the case for other Vβ analyzed (P = .01; Figure 4C). Subsequently, 16 donors, who differed in the degree to which Vβ7 T cells were skewed in their coreceptor use, were probed for SAg reactivity. The group with Vβ7CD4/CD8 ratios greater than 0.5 more efficiently expanded Vβ7+ T cells (ratio SAg/vector greater than 2), whereas the group with ratios less than 0.5 weakly responded or did not respond (ratio SAg/vector less than 2) (Figure 4D).
Differential peripheral SAg responsiveness was associated with donors of each group—expander and weak expander—when analyzed periodically (Supplemental Figure 3) and was confirmed for 14 additional healthy blood donors (not shown). These results are consistent with the idea that low thymic and peripheral Vβ7CD4 T levels predict absent or diminished peripheral SAg reactivity and, conversely, that high thymic and peripheral Vβ7CD4 T levels predict strong peripheral SAg reactivity.
T-cell anergy does not likely account for the differential peripheral SAg reactivity
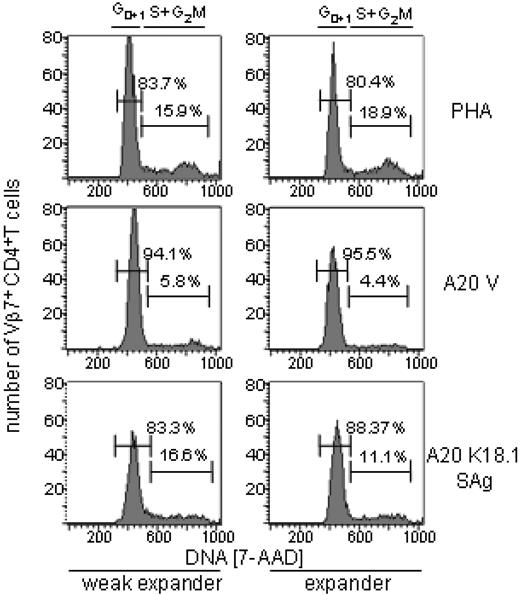
We wanted to exclude that other tolerance mechanisms could account to a significant extent for the differential peripheral T-cell reactivity. Anergic T cells in mice and in humans do not proliferate or secrete IL-2, and they do not progress through the cell cycle from G1 to S in response to appropriate stimuli.33,34 Therefore, we asked whether SAg-stimulated Vβ7CD4 T cells in weak responders were blocked in the G1 phase of the cell cycle. This was clearly not the case. As can be seen in Figure 5, in weak expanders a steady percentage of Vβ7CD4 T cells progressed from G1 to S in response to the SAg and the polyclonal activator phytohemagglutinin (PHA). The fact that a smaller fraction of Vβ7CD4 T cells entered the cycle in the expander group than in the weak expander group does not negate the presence of a higher frequency of SAg-reactive Vβ7CD4 T cells in the expander group. On the contrary, this is consistent with observations made for MMTV SAgs.35 For instance, with increasing numbers of SAg-reactive T cells, the fraction of those that entered the cell cycle on SAg stimulation diminished.
Vβ7CD4 T-cell levels predict HERV-K18 SAg reactivity. To causally relate peripheral T-cell levels with SAg reactivity, we first established a link between intrathymic and peripheral Vβ7CD4 cell levels and subsequently with SAg reactivity. (A) The thymic CD4/CD8 ratios of Vβ2, Vβ7, and Vβ17 were determined on CD1A- thymocytes of 7 donors. (B) For 3 donors, the thymic and peripheral T-cell Vβ-CD4/CD8 ratios were compared. (C) Forty-seven healthy donors were divided in 2 groups of similar age and with Vβ7CD4/CD8 ratios less than and 0.5, respectively. The Vβ7 percentages among CD4+ T cells were determined by FACS. For 19 other controls, Vβ families were also analyzed. (D) Expansions of Vβ7CD4 T cells from 16 donors with high or low proportions of SAg-reactive Vβ7CD4 T cells were analyzed. T cells from 8 donors with Vβ7CD4/CD8 ratios greater than 0.5 and from 8 with ratios less than 0.5 were analyzed after culture with vector and SAg-transfected A20 cells. For 8 donors, the control family Vβ2 was also determined. Differential reactivity was corroborated for another 14 donors (not shown). ▴ represent individual values; horizontal bars represent the mean.
Vβ7CD4 T-cell levels predict HERV-K18 SAg reactivity. To causally relate peripheral T-cell levels with SAg reactivity, we first established a link between intrathymic and peripheral Vβ7CD4 cell levels and subsequently with SAg reactivity. (A) The thymic CD4/CD8 ratios of Vβ2, Vβ7, and Vβ17 were determined on CD1A- thymocytes of 7 donors. (B) For 3 donors, the thymic and peripheral T-cell Vβ-CD4/CD8 ratios were compared. (C) Forty-seven healthy donors were divided in 2 groups of similar age and with Vβ7CD4/CD8 ratios less than and 0.5, respectively. The Vβ7 percentages among CD4+ T cells were determined by FACS. For 19 other controls, Vβ families were also analyzed. (D) Expansions of Vβ7CD4 T cells from 16 donors with high or low proportions of SAg-reactive Vβ7CD4 T cells were analyzed. T cells from 8 donors with Vβ7CD4/CD8 ratios greater than 0.5 and from 8 with ratios less than 0.5 were analyzed after culture with vector and SAg-transfected A20 cells. For 8 donors, the control family Vβ2 was also determined. Differential reactivity was corroborated for another 14 donors (not shown). ▴ represent individual values; horizontal bars represent the mean.
Although we cannot completely rule out a contribution of anergy to peripheral SAg reactivity, this appears to be unlikely. We conclude that the differences between strongly and weakly expanding Vβ7CD4 T cells is T-cell autonomous and qualitative and is by all criteria addressed here more accurately explained by thymic deletion than by T-cell anergy. Hence, we propose that thymic deletion affects primarily Vβ7CD4 thymocytes and determines weak SAg reactivity by reducing the peripheral Vβ7CD4 levels.
T cell-anergy did not account for the differential peripheral SAg reactivity. Vβ7CD4 T cells from responders and weak responders were activated with the polyclonal T-cell mitogen PHA (top row), with vector (A20 V, middle row), or with HERV-K18 SAg–transfected A20 cells (bottom row). Cell cycle analysis of Vβ7+CD4+ T cells was performed with 7-actinomycin D (7-AAD). The lower fraction of cells entering the cell cycle in the expander could reflect a regulatory mechanism encountered among samples with high proportions of SAg-reactive T cells, which was previously recognized for MMTV SAgs. Horizontal bars and percentages refer to the fraction of cells in Go1, and S+2M, respectively.
T cell-anergy did not account for the differential peripheral SAg reactivity. Vβ7CD4 T cells from responders and weak responders were activated with the polyclonal T-cell mitogen PHA (top row), with vector (A20 V, middle row), or with HERV-K18 SAg–transfected A20 cells (bottom row). Cell cycle analysis of Vβ7+CD4+ T cells was performed with 7-actinomycin D (7-AAD). The lower fraction of cells entering the cell cycle in the expander could reflect a regulatory mechanism encountered among samples with high proportions of SAg-reactive T cells, which was previously recognized for MMTV SAgs. Horizontal bars and percentages refer to the fraction of cells in Go1, and S+2M, respectively.
Discussion
Our results show that transcriptional regulation of HERV-K18 expression in the thymus is such that HERV-K18 SAgs can induce negative selection in vivo and that HERV-K18 SAg is necessary and sufficient for negative selection of immature or semimature Vβ7 thymocytes, affecting primarily Vβ7CD4 cells. The data further show that reduced thymic and peripheral levels of Vβ7CD4 T cells predict low or absent SAg reactivity. HERV-K18 can, therefore, be exploited to study central tolerance and its potential role for viral persistence and autoimmunity in humans.
Our data fit best a model stipulating that HERV-K18–induced negative selection in vivo primarily affects Vβ7CD4 thymocytes by acting through the deletion-sensitive semimature CD4SP thymocytes, analogous to MMTV SAgs. Although we have not addressed this directly in the present study, this scenario is closest to our observation that peripheral Vβ7CD4 T cells are variably reduced and that low proportions predict weak SAg reactivity.
The model system presented here is a first step toward dissecting the complexity of negative selection in humans. It must be expanded stepwise to account for HERV-K18,23 MHC class 2,29 and TCR polymorphisms,36 all of which are anticipated to modulate thymocyte deletion. This can only be achieved once the genetic and biochemical interactions of variants at these 3 loci have been defined, which is far beyond the scope of the present study.
The extent of thymocyte deletion induced by the transfected HERV-K18 SAg exceeds the theoretical number of thymocytes carrying SAg-reactive Vβ chains. This suggests that more than thymocytes with nominally SAg-reactive Vβ chains are negatively selected and in part explains why the Vβ specificity of the HERV-K18 SAg in this assay is imperfect. Four reasons, not mutually exclusive, may account for this observation. First, T-cell stimulatory activity exceeding its precise Vβ specificity is an inherent characteristic of this SAg.22,23 Second, T-cell stimulation by SAgs is quantitative. It implies that at high SAg doses a considerable number of T cells that do not carry SAg-reactive Vβ chains are stimulated.37 Third, substances such as TNF-α or glucocorticoids31 released by SAg-activated mature thymocytes could at least in part explain excess apoptosis of DP thymocytes. Fourth, the imperfect specificity may reflect the experimental conditions used and may not be relevant for negative selection in vivo.
Two observations suggest that factors beyond HERV-K18, MHC class 2, and TCR polymorphisms also modulated the extent of negative selection in our assay, challenging the tantalizing prediction that simple correlations exist between the HERV-K18 genotype and the peripheral levels of SAg-reactive Vβ7+ T cells. They are that donors differed in their capacity to undergo SAg-mediated negative selection despite the rigorous selection of thymocyte samples for consistent biologic behavior and that thymic HERV-K18 SAg expression levels are anticipated to influence the efficiency and the kinetics of deletion, analogous to MMTV SAgs.9,10 Our data indeed lend support to this idea (Figure 1). We are in the process of dissecting the respective role of each parameter by large-scale genetic analyses of the interactions among HERV-K18, MHC class 2, and TCR polymorphisms and by repopulation assays in immunodeficient mice reconstituted with human hematopoietic precursors that can give rise to thymocytes plus APCs capable of negative selection.38 The repopulation assays will allow us to demonstrate the causality of the HERV-K18 genotype on the T-cell repertoire by knocking it down with RNA interference.
In sum, we propose that HERV-K18 SAg–induced negative thymocyte selection is operational in vivo. Analysis of the mature human T-cell repertoire is indeed consistent with this view. Healthy persons have variably reduced peripheral Vβ7CD4 T-cell levels39,40 that are predictive of SAg reactivity and that may be attributed to partial negative selection by HERV-K18 SAgs. We propose that negative thymic selection to HERV-K18 SAgs is a first checkpoint controlling peripheral tolerance compared with SAg reactivity.
Prepublished online as Blood First Edition Paper, January 11, 2005; DOI 10.1182/blood-2004-07-2596.
Supported by the Helmut Horten Foundation (B.C.), a START fellowship and grants from the Swiss National Science Foundation (B.C.), and grants from NFWO-Flanders and the Special Research Fund of Ghent University (G.L., J.P.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Christian Van Delden and all students in the department for their blood donations. We thank Drs A. Necker (Coulter, Marseille) for αVβ antibodies, J. White, P. Marrack, N. Sutkowski, and B. Huber for T-cell hybrids, and J. Curran for critically reading the manuscript, and we thank the reviewers for useful comments and suggestions.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal