Abstract
Defects in the X-linked DNA-binding megakaryocyte transcription factor GATA1 cause thrombocytopenia and abnormal platelet function. However, detailed studies of GATA1 function in platelet activation are lacking. Here, we studied platelets from GATA1-deficient mice and from a male patient (S14) with a bleeding diathesis attributed to a single amino acid substitution (R216Q) in the N-terminal GATA1 zinc finger that alters binding to DNA. In both cases there was inhibition of aggregation to collagen and decreased tyrosine phosphorylation of glycoprotein VI (GPVI)–signaling proteins. This effect was more marked in GATA1-deficient murine platelets, where it was associated with a significant reduction in surface GPVI expression. Moreover, both human and murine GATA1-mutant platelets showed reduced adhesion and aggregate formation on a collagen matrix at an intermediate rate of shear, although this could not be accounted solely by the thrombocytopenia and altered GPVI expression, indicating that GATA1 regulates additional factors important for platelet activation under shear. In contrast, there was no inhibition of responses to G protein–coupled receptor agonists in GATA1-perturbed platelets. Our results are consistent with GATA1 regulating some but not all pathways of platelet activation, leading to an impairment of aggregate formation under flow, which cannot be attributed solely to the thrombocytopenia.
Introduction
Megakaryopoiesis describes the differentiation of hematopoietic stem cells into mature megakaryocytes, triggering the production and subsequent release of platelets into blood. The molecular mechanisms underlying this process are tightly regulated by lineage-affiliated transcription factors exemplified by the hematopoietic GATA proteins (GATA1-GATA3). Each member has a distinct developmental expression profile and function.1 GATA1 is expressed in red cells, megakaryocytes, eosinophils, and mast cells where it is required for terminal differentiation.1 GATA2 is expressed in early hematopoietic progenitors and mast cells, while GATA3 is expressed widely in embryogenesis and adult hematopoiesis in T cells.2,3 The GATA transcription factors recognize the consensus target sequence (T/A)GATA(A/G).3 GATA DNA-binding sites have been identified in promoter and enhancer sequences of a number of megakaryocyte-specific genes, including those encoding platelet glycoproteins (GPs), namely, GPIbα, GPIbβ, GPIIb (αIIb), GPIX, and GPVI.4-10
Defects in GATA1 cause megakaryocyte and red cell disorders. These include transient leukemia and acute megakaryoblastic leukemia in Down syndrome,11-15 X-linked thrombocytopenia with thalassemia,16 familial dyserythropoietic anemia,17 and X-linked macrothrombocytopenia.5,18 Many of the thrombocytopenias are caused by mutations in the bifunctional N-terminal zinc finger of GATA1, which not only mediates interactions with key GATA1-interacting proteins but also modulates binding of GATA1 to a subset of GATA DNA-binding sites in vitro. For example, glycine for aspartate substitution in amino acid 218 (D218G)18 in a patient with X-linked macrothrombocytopenia alters GATA1 interaction with its cofactor FOG-1 (Friend of GATA1). The platelets from this patient showed a partial reduction in aggregation to ristocetin and collagen.18 Electron microscopy of the platelets showed structural abnormalities, including increased size, spherical appearance, cytoplasmic clusters of smooth endoplasmic reticulum, and a reduction in α-granules.18 In contrast, another study identified a family with a glutamine for an arginine substitution at position 216 (R216Q)19 that alters DNA binding. In those patients, bleeding times were increased and clot retraction was defective.19
GATA1 function has also been studied in murine platelets. As deletion of GATA1 in mice causes embryonic lethality at embryonic day 10.5 from anemia,20 aspects of GATA1 function in platelets have been studied in mice (ΔneoΔHS) with a targeted mutation of a cis element required for megakaryocyte GATA1 expression. ΔneoΔHS mice express approximately 5% of normal GATA1 mRNA. They are thrombocytopenic, with a platelet count of 15% compared with that of age-matched controls.20,21 These mice demonstrate deregulated megakaryocyte maturation and proliferation and increased mean platelet volume, abnormal platelet and megakaryocyte ultrastructure with marked heterogeneity in number and distribution of granules and organelles, and a decrease in mRNA expression of a number of platelet-specific genes, including GPIbα and GPIbβ.20,21 Although ΔneoΔHS mice do not bleed spontaneously,20 they exhibit excessive bleeding following tail biopsy compared with littermate controls, suggestive of a platelet defect.21
A thorough investigation of the effect of GATA1 deficiency in mice on platelet activation has not been performed, in part because of the small numbers of platelets that can be obtained as a result of the thrombocytopenia. In the present study, we have carried out an extensive investigation of the contribution of GATA1 to platelet activation by a number of G protein–coupled and adhesion receptor agonists, with special emphasis on the collagen receptor glycoprotein VI (GPVI), as GATA1 binding sequences have been described in its promoter region.4,6 We have compared these results with those from a patient, S14, who is the third person described with the R216Q point mutation. The studies on murine platelets reveal an important role for GATA1 in regulating activation through GPVI that is partly explained by reduced surface expression of GPVI. In contrast, aggregation to the G protein–coupled agonist thrombin was maintained. The platelets of S14 displayed a similar pattern of reactivity, although the defects were less pronounced. Further studies indicated a reduction in aggregate formation on a collagen matrix in GATA1-perturbed platelets under shear, which could not be explained solely by the thrombocytopenia and reduced GPVI expression.
Materials and methods
Materials
CRP (GCO[GPO]10GCOG; single-letter code, where O is hydroxyproline) and collagen reagent Horm, as native type I fibrils from equine tendons, were obtained from sources described.22 Lotrafiban was a gift from GlaxoSmithKline (Middlesex, United Kingdom). Factor VIII concentrate and Haemate P were a gift from Paul Harrison (Oxford Haemophilia Centre, The Churchill Hospital, Oxford, United Kingdom), and prostacyclin (PGI2) was supplied by Alexis Biochemicals (Alexis, Nottingham, United Kingdom). Thrombin-activating peptide (TRAP) was synthesized by Alta Bioscience (Birmingham, United Kingdom). Anti-CD42b, 204-11 (anti-GPVI), anti-CD49b, anti-CD61, and anti-CD41 were from previously described sources.23 Fluorescein isothiocyanate (FITC)–labeled rat anti–mouse GPVI, 6.E10/rat immunoglobulin G2a (IgG2a), and the FITC-labeled rat IgG–negative control were obtained from Emfret Analytics (Wurzburg, Germany). All other reagents were from Sigma (St Louis, MO) or have been described.24-28
Mouse strains
Preparation of human and murine platelets
Whole human blood was collected in citrate anticoagulant from healthy consenting volunteers who had not taken any antiplatelet medication in the preceding 2 weeks. Whole human blood was supplemented with ACD (acid-citrate-dextrose; 90 mM sodium citrate, 7 mM citric acid, 140 mM D-glucose) and centrifuged at 200g for 20 minutes to obtain platelet-rich plasma (PRP). Prostacyclin (0.1 μg/mL) was added to the PRP prior to centrifugation at 1000g for 10 minutes. The platelet pellet was resuspended in Tyrode buffer (134 mM NaCl, 2.9 mM KCl, 0.34 mM Na2HPO4. 12H2O, 12 mM NaHCO3, 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.3, 1 mM MgCl2, 5 mM glucose), containing prostacyclin (0.1 μg/mL) and centrifuged at 1000g for 10 minutes. Murine platelets were obtained and prepared as previously described.29 Platelets were resuspended in Tyrode buffer at 2 to 5 × 108/mL. Donors provided written informed consent for participation and ethical approval was obtained through the Universities of Birmingham and Oxford.
Static adhesion assays
Static adhesion assays were performed by using a modified method of Inoue et al30 and Yuan et al.31 Briefly, glass coverslips were coated with von Willebrand factor (VWF; 10 μg/mL), Horm collagen (100 μg/mL), or fibrinogen (200 μg/mL). Nonimmobilized protein was removed by washing with phosphate-buffered saline, and coverslips were blocked with fatty acid–free bovine serum albumin (BSA; 5 mg/mL) for 60 minutes at room temperature. Washed murine (3-5 × 107/mL) or human platelets (1-3 × 107/mL) were incubated on the coverslips for 45 minutes at 37°C. Nonadherent platelets were removed with washing, and adherent platelets were fixed (3.7% formaldehyde, 10 minutes), permeabilized (0.2% Triton-X-100), and stained with rhodamine-phalloidin overnight. Platelets were imaged on an axiovert S100 fluorescent microscope with monochromatic light source and CCD camera (Zeiss, Jena, Germany), and images were captured using OpenLab (v3.0) software for Macintosh as previously described.22,30
Platelet aggregation assays
Human PRP derived from citrate anticoagulated blood was stimulated with adenosine diphosphate (ADP; 10 μM) or arachidonic acid (500 μg/mL), and aggregation was observed with stirring at 1200 rpm at 37°C (BioData PAP-4; Alpha Laboratories, Eastleigh, United Kingdom). The platelet count of control PRP was normalized to that of S14 by using autologous platelet-poor plasma (PPP). For washed platelet studies, murine (1 × 108/mL) and human platelets (2 × 108/mL) were stimulated with thrombin (0.1 or 1.0 U/mL), CRP (1 or 10.0 μg/mL), or collagen (2 or 10 μg/mL) and aggregated. All aggregations were monitored for 5 to 10 minutes and determined by maximum change in light transmission. Platelets were then lysed using 2 × lysis buffer (300 mM NaCl2, 20 mM Tris (tris(hydroxymethyl)aminomethane), 2 mM EGTA (ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid), 2 mM EDTA (ethylenediaminetetraacetic acid), pH 7.5, 2% NP-40 ([Octylphenoxy]polyethoxyethanol) detergent [vol/vol], 5 mM Na3VO4, 2 mM AEBSF (4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride), 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 μg/mL pepstatin).
Immunoprecipitation and Western blotting
Immunoprecipitation studies were performed as described previously.26 Specifically, anti-Syk (spleen tyrosine kinase), anti-PLCγ2 (phospholipase C-γ2), and anti-LAT (linker for activation of T cells) antibodies were added to the supernatant in concert with Protein A-Sepharose beads. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electrically transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked in 10% (wt/vol) BSA dissolved in Tris buffered saline-Tween (TBS-T), for 1 hour at room temperature or overnight at 4°C. Antibodies were incubated with the membranes for 1 to 2 hours at room temperature (RT). Membranes were washed thrice with TBS-T and incubated with the appropriate secondary antibody conjugated to horseradish peroxidase. In all studies, membranes were developed using the Enhanced Chemiluminescence system (Amersham Pharmacia Biotech, Cardiff, United Kingdom).
Platelet adhesion to collagen or VWF under flow conditions
Human and murine platelets in whole blood were allowed to adhere to immobilized proteins under shear conditions as previously described.22,23 Capillary tubes (CamLab, Cambridge, United Kingdom) were coated with Horm collagen (100 μg/mL) or VWF (100 μg/mL) overnight at 4°C. The capillaries were rinsed with Tyrode buffer and attached to the flow adhesion set-up. Whole murine or human blood was collected into citrate anticoagulant (3.2%). Blood was then perfused through the capillary tubes at a shear rate of 800 s-1 or 1800 s-1 for 2 or 5 minutes. Nonadherent cells were removed during perfusion with Tyrode buffer. Platelet thrombi were imaged as described,23 using a CCD camera (Sony, Tokyo, Japan) and 63× objective.
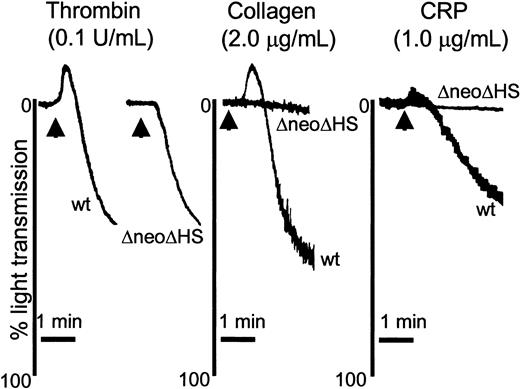
Absence of GATA1 leads to a selective reduction in GPVI-mediated platelet aggregation at low concentrations of agonist. Washed murine platelets (1 × 108/mL) obtained from wild-type (WT; n = 3) and ΔneoΔHS (ΔneoΔHS; n = 8) mice were stimulated with thrombin (0.1 U/mL; left), collagen (2.0 μg/mL; middle), or CRP (1.0 μg/mL; right) and allowed to aggregate for 2.5 minutes, with stirring at 1200 rpm. Platelet aggregation was demonstrated by the change in light transmission. An arrow marks addition of agonist for thrombin 2 arrows are used, to assist in delineating between the WT and ΔneoΔHS tracings. Representative aggregation tracings are shown.
Absence of GATA1 leads to a selective reduction in GPVI-mediated platelet aggregation at low concentrations of agonist. Washed murine platelets (1 × 108/mL) obtained from wild-type (WT; n = 3) and ΔneoΔHS (ΔneoΔHS; n = 8) mice were stimulated with thrombin (0.1 U/mL; left), collagen (2.0 μg/mL; middle), or CRP (1.0 μg/mL; right) and allowed to aggregate for 2.5 minutes, with stirring at 1200 rpm. Platelet aggregation was demonstrated by the change in light transmission. An arrow marks addition of agonist for thrombin 2 arrows are used, to assist in delineating between the WT and ΔneoΔHS tracings. Representative aggregation tracings are shown.
Measurement of platelet-surface glycoprotein levels
Surface expression of human platelet GPs Ib, VI, IIb, and IIIa were measured as previously described, using a platelet calibrator kit (Biocytex, Marseille, France).23 Surface expression of murine GPVI was measured in individual platelet preparations. Briefly, 2 × 106 platelets were labeled with 5μL 6.E10 or rat IgG for 15 minutes at RT. The reaction was stopped by addition of Tyrode buffer, and samples were analyzed using a flow cytometer (Becton Dickinson, San Jose, CA).
Statistical analysis
Significant differences were detected using one-way analysis of variation (ANOVA), using the Prism software package (GraphPAD Software for Science, San Diego, CA).
Results
ΔneoΔHS murine platelets exhibit a reduction in GPVI-mediated platelet aggregation
To address the role of GATA1 in platelet regulation, we used lineage-selective GATA1-mutant mice (ΔneoΔHS). ΔneoΔHS mice have a marked reduction in platelet count to approximately 15% of controls, necessitating the use of groups of up to 10 mice per experiment. Initially, we assessed the ability of murine platelets to aggregate in response to thrombin, collagen, and CRP, a GPVI-selective agonist. Supramaximal concentrations of all 3 agonists elicited maximal aggregation of platelets from both wild-type and ΔneoΔHS mice (data not shown). In contrast, aggregation responses to lower concentrations of CRP and collagen were severely compromised in the absence of GATA1 (Figure 1), whereas the aggregation response of ΔneoΔHS murine platelets to a lower concentration of thrombin (0.1 U/mL) (42% aggregation, slope 16%) was similar to controls (42% aggregation, slope 20%) (Figure 1). It was noted that the aggregation traces to all 3 agonists reveal a loss of shape change in ΔneoΔHS mice, which can be explained by the alteration in platelet morphology from a disc to a sphere because of the absence of expression of the GATA1-regulated gene β1-tubulin.32 These results demonstrate a selective loss of aggregation to low but not high concentrations of GPVI agonists, whereas the response to a G protein–coupled receptor agonist, thrombin, is maintained.
GATA1 deficiency leads to differential protein tyrosine phosphorylation
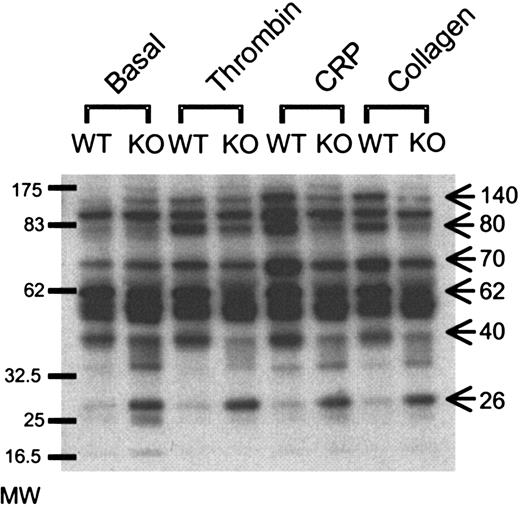
To examine the molecular basis of the defect in GPVI-mediated platelet activation, we measured protein tyrosine phosphorylation in whole cell lysates of aggregated samples. Under these conditions, an increase in tyrosine phosphorylation is mediated downstream of activation of the protease-activated receptor 1 thrombin receptor and GPVI in combination with integrin αIIbβ3 outside-in signaling. A number of differences could be seen under basal conditions and in response to stimulation. The most dramatic difference under basal conditions was the appearance of a constitutively tyrosine phosphorylated band of 26 to 28 kDa in the GATA1-mutant lysates, which did not change in response to thrombin, CRP, or collagen. In addition, reductions in phosphorylation of a number of bands were observed in GATA1-mutant lysates, including those at approximately 40, 62, 70, 80, and 140 kDa (Figure 2). Of interest, the extent of these differences was further increased upon stimulation with CRP and to a lesser extent with collagen. Taken together, these differences in tyrosine phosphorylation may provide a biochemical explanation for the defect in GPVI-dependent platelet aggregation.
GATA1 contributes to the surface expression of GPVI on murine platelets
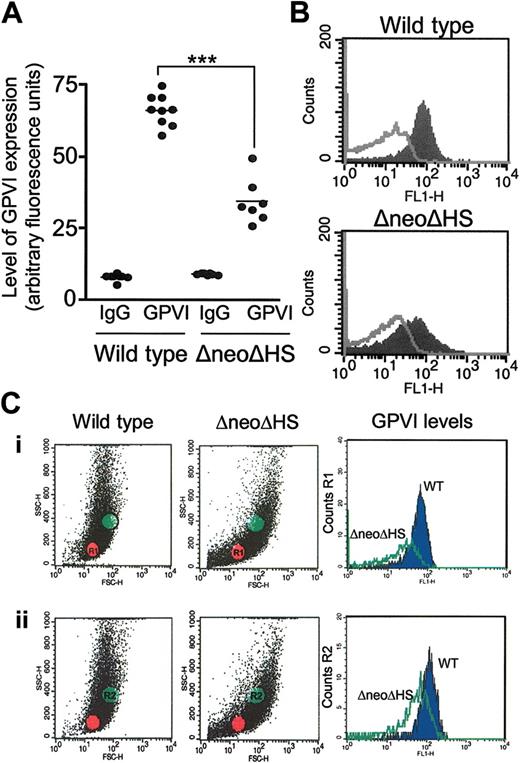
GATA1 DNA-binding sites are present in the promoter and enhancer sequences of GPVI,4,6 and a reduction in expression of the glycoprotein may, therefore, account for the inhibition of response to collagen and CRP. Measurement of surface expression of GPVI by using a FITC-conjugated murine-specific antibody to GPVI did indeed reveal a reduction in expression of approximately 55% relative to wild-type platelets (Figure 3A-B). This fluorescence profile was also observed by gating on small (Figure 3Ci) or large platelets (Figure 3Cii), suggesting that the reduction in glycoprotein expression is independent of platelet size.
Adhesion and spreading of ΔneoΔHS platelets on immobilized collagen and fibrinogen
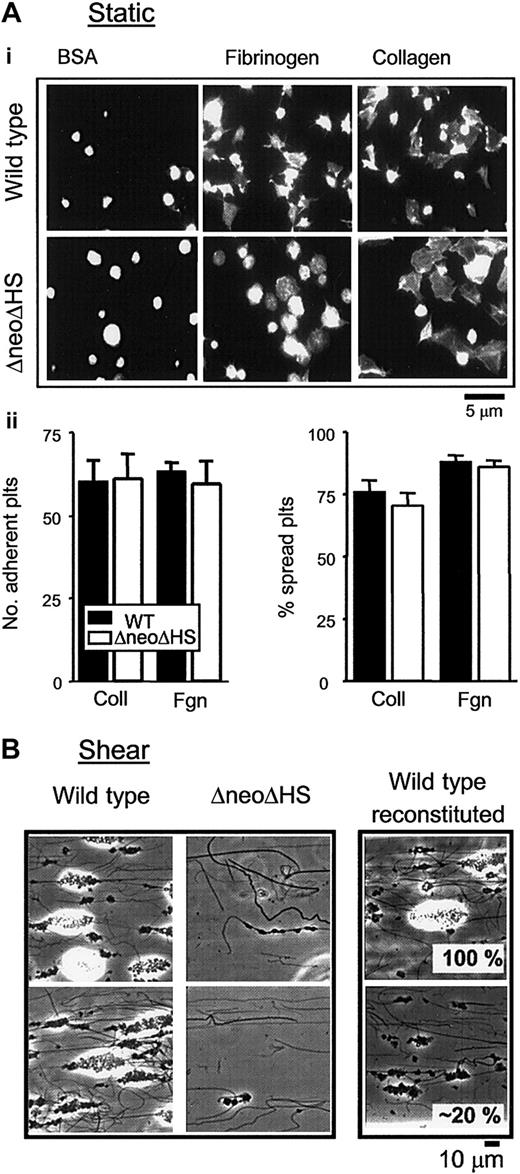
The physiologic consequence of the defect in GPVI expression on platelet adhesion and spreading on collagen was investigated alongside the response to fibrinogen, under static conditions. As demonstrated in Figure 4A, formation of filopodia and lamellipodia structures on collagen and fibrinogen could be seen in ΔneoΔHS and control platelets. There were no significant differences between wild-type and ΔneoΔHS platelets in the extent of adhesion or the ability of platelets to spread on fibrinogen and collagen (Figure 4A, histograms), although it was noticeable that the ΔneoΔHS platelets on fibrinogen were rounder in appearance and had fewer filopodia than controls, most likely reflecting the absence of β1-tubulin.32 It was also noted that a significant proportion of the ΔneoΔHS platelets were larger than control cells, as previously reported.20,21 These studies demonstrate that ΔneoΔHS platelets undergo spreading upon adhesion to immobilized collagen and fibrinogen.
Absence of GATA1 leads to differential protein tyrosine phosphorylation. Platelets from wild-type (WT) and ΔneoΔHS (KO) mice were aggregated with thrombin (1.0 U/mL), collagen (10 μg/mL), or CRP (10 μg/mL) prior to lysis with 2 × Lysis buffer. Proteins were separated on SDS-PAGE, using 4% to 12% precast gradient gels, transferred onto PVDF membranes, and blotted with anti-phosphotyrosine Ab, 4G10. Bands of interest are highlighted by arrows, including a constitutively tyrosine phosphorylated band at approximately 26 to 28 kDa, and phosphorylated proteins at 40, 62, 70, 80, and 140 kDa. Molecular weight (MW) markers are provided on the left-hand side.
Absence of GATA1 leads to differential protein tyrosine phosphorylation. Platelets from wild-type (WT) and ΔneoΔHS (KO) mice were aggregated with thrombin (1.0 U/mL), collagen (10 μg/mL), or CRP (10 μg/mL) prior to lysis with 2 × Lysis buffer. Proteins were separated on SDS-PAGE, using 4% to 12% precast gradient gels, transferred onto PVDF membranes, and blotted with anti-phosphotyrosine Ab, 4G10. Bands of interest are highlighted by arrows, including a constitutively tyrosine phosphorylated band at approximately 26 to 28 kDa, and phosphorylated proteins at 40, 62, 70, 80, and 140 kDa. Molecular weight (MW) markers are provided on the left-hand side.
GATA1 contributes to the surface expression of GPVI on murine platelets. Washed murine platelet suspensions (2 × 106 platelets) were labeled with murine-specific FITC-conjugated anti-GPVI antibody (6 E.10) or appropriate negative control (FITC-conjugated rat IgG) for 15 minutes at RT. Suspensions were diluted with Tyrode buffer prior to detection of fluorescence using a flow cytometer. GPVI expression is represented in arbitrary fluorescence units. (A) Graph shows fluorescence values for individual mice (WT, n = 9; ΔneoΔHS, n = 7), and the matched negative control. Horizontal bar represents mean value. Statistical significance is shown, P < .001, ***. (B) A representative fluorescence-activated cell sorting (FACS) tracing for a wild-type and ΔneoΔHS mouse platelet suspension is shown. The filled curve shows the binding of 6 E.10, and the open curve represents the binding of rat IgG. (C) A representative fluorescence profile demonstrates the scatter of platelets in 1 wild-type and 1 ΔneoΔHS mouse suspension. (Ci) The fluorescence of region 1 (R1, red dots), representing smaller platelets, has been measured and plotted on FL1-H. (Cii) The fluorescence of region 2 (R2, green dots), representing larger platelets, has been measured and plotted on FL1-H. The blue filled curve shows the binding of 6 E.10 to wild-type platelets, and the green unfilled curve represents the binding 6 E.10 to ΔneoΔHS platelets.
GATA1 contributes to the surface expression of GPVI on murine platelets. Washed murine platelet suspensions (2 × 106 platelets) were labeled with murine-specific FITC-conjugated anti-GPVI antibody (6 E.10) or appropriate negative control (FITC-conjugated rat IgG) for 15 minutes at RT. Suspensions were diluted with Tyrode buffer prior to detection of fluorescence using a flow cytometer. GPVI expression is represented in arbitrary fluorescence units. (A) Graph shows fluorescence values for individual mice (WT, n = 9; ΔneoΔHS, n = 7), and the matched negative control. Horizontal bar represents mean value. Statistical significance is shown, P < .001, ***. (B) A representative fluorescence-activated cell sorting (FACS) tracing for a wild-type and ΔneoΔHS mouse platelet suspension is shown. The filled curve shows the binding of 6 E.10, and the open curve represents the binding of rat IgG. (C) A representative fluorescence profile demonstrates the scatter of platelets in 1 wild-type and 1 ΔneoΔHS mouse suspension. (Ci) The fluorescence of region 1 (R1, red dots), representing smaller platelets, has been measured and plotted on FL1-H. (Cii) The fluorescence of region 2 (R2, green dots), representing larger platelets, has been measured and plotted on FL1-H. The blue filled curve shows the binding of 6 E.10 to wild-type platelets, and the green unfilled curve represents the binding 6 E.10 to ΔneoΔHS platelets.
To further assess the significance of the reduction in expression of GPVI upon adhesion, as well as other possible defects in the GATA1-deficient platelets, we assessed the ability of control and ΔneoΔHS platelets to adhere and form aggregates on immobilized collagen at an intermediate rate of shear. In these experiments, whole anticoagulated blood was used in preference to isolated washed platelets, as efficient platelet tethering requires the presence of erythrocytes. Further, whole blood more closely reflects the platelet adhesion process as it occurs in vivo. Wild-type platelets exhibited robust adhesion and aggregate formation on immobilized collagen at a shear rate of 800 s-1 (Figure 4B). In contrast, a marked reduction in adhesion and aggregate formation was seen in ΔneoΔHS platelets (Figure 4B). This reduction was not due solely to the reduction in expression of GPVI, because a 50% reduction in the level of the glycoprotein does not have a significant effect on adhesion and aggregate formation under shear conditions.23 To evaluate the contribution of thrombocytopenia to the defect seen in the ΔneoΔHS mice, wild-type platelet samples were reconstituted with platelet-poor plasma and red blood cells to have only 20% of their original platelet count (Figure 4B, right) and, thus, represent a similar concentration as that for ΔneoΔHS platelets. Despite the reduced platelet count, reconstituted control blood still developed a greater degree of aggregate formation than ΔneoΔHS platelets. Taken together these data demonstrate that the observed differences in aggregate formation could not be solely accounted for by the thrombocytopenia or GPVI expression.
Adhesion and spreading of ΔneoΔHS platelets on collagen under static and flow conditions. (Ai) Washed murine platelets (1 × 107/mL) from wild-type (top row) and ΔneoΔHS (bottom row) mice were allowed to adhere and spread on immobilized fibrinogen (Fibrinogen) and collagen (Collagen). Adherent platelets were fixed, permeabilized, and stained with rhodamine-phalloidin. Platelets were also plated on BSA-covered coverslips (BSA). Adherent platelets were imaged using fluorescence microscopy, and cropped representative images are shown. Images taken are from 1 experiment, representative of 3. (ii) Graphs show the number of platelets adherent to the thrombogenic matrix (left) and the number of platelets that had undergone some change in shape, recorded as a percentage of spread platelets (right), mean ± SEM. ▪ indicates WT platelets; □, ΔneoΔHS platelets. (B) Whole blood was obtained from individual wild-type (Wild type) or ΔneoΔHS (ΔneoΔHS) mice and perfused over collagen-coated (100 μg/mL) capillary tubes at a shear rate of 800 s-1 for 2 minutes followed by Tyrode buffer for 5 minutes. Five fields were imaged (× 63 objective), and representative images of adherent platelets and aggregates from 2 individual wild-type and ΔneoΔHS mice are shown. These data are from 1 flow experiment representative of 2. On the right, red blood cells, PRP and PPP were isolated from wild-type whole blood. Blood was reconstituted to represent either 100% (Wild type reconstituted, 100%) or 20% (Wild type reconstituted, 20%) of the original wild-type platelet count in whole blood. These reconstituted samples were perfused and treated as whole blood flows, described in earlier part of (B). Note that the white regions of the images correspond to regions of substantial and high aggregates. The increased height has induced flare in the phase-contrast image.
Adhesion and spreading of ΔneoΔHS platelets on collagen under static and flow conditions. (Ai) Washed murine platelets (1 × 107/mL) from wild-type (top row) and ΔneoΔHS (bottom row) mice were allowed to adhere and spread on immobilized fibrinogen (Fibrinogen) and collagen (Collagen). Adherent platelets were fixed, permeabilized, and stained with rhodamine-phalloidin. Platelets were also plated on BSA-covered coverslips (BSA). Adherent platelets were imaged using fluorescence microscopy, and cropped representative images are shown. Images taken are from 1 experiment, representative of 3. (ii) Graphs show the number of platelets adherent to the thrombogenic matrix (left) and the number of platelets that had undergone some change in shape, recorded as a percentage of spread platelets (right), mean ± SEM. ▪ indicates WT platelets; □, ΔneoΔHS platelets. (B) Whole blood was obtained from individual wild-type (Wild type) or ΔneoΔHS (ΔneoΔHS) mice and perfused over collagen-coated (100 μg/mL) capillary tubes at a shear rate of 800 s-1 for 2 minutes followed by Tyrode buffer for 5 minutes. Five fields were imaged (× 63 objective), and representative images of adherent platelets and aggregates from 2 individual wild-type and ΔneoΔHS mice are shown. These data are from 1 flow experiment representative of 2. On the right, red blood cells, PRP and PPP were isolated from wild-type whole blood. Blood was reconstituted to represent either 100% (Wild type reconstituted, 100%) or 20% (Wild type reconstituted, 20%) of the original wild-type platelet count in whole blood. These reconstituted samples were perfused and treated as whole blood flows, described in earlier part of (B). Note that the white regions of the images correspond to regions of substantial and high aggregates. The increased height has induced flare in the phase-contrast image.
Description of patient with R216Q substitution in GATA1
The phenotype of the murine platelets was compared with that of a male patient with defective GATA1/DNA binding. Described in detail elsewhere (P.V. and A.T., manuscript in preparation), S14 has a mild bleeding diathesis, on occasion requiring therapeutic platelet transfusions, which is attributed to a single amino acid substitution (R216Q) in the N-terminal zinc finger of GATA1 that destabilizes in vitro binding of GATA1 to complex GATA sites.16 This mutation has been previously described in patients presenting with X-linked thrombocytopenia and β-thalassemia16,33 and X-linked thrombocytopenia with dyserythropoietic anemia.19 S14 has a mean platelet count of 46 × 109/L (normal range, 150-450 109/L) and a mean platelet volume of 7.7 μm3 (normal range, 6-10 μm3), which is made up of 2 populations of platelets, one with normal size distribution and a second, larger population.
Examination of surface expression of major platelet glycoproteins
We examined the surface expression of platelet glycoproteins of control and S14 platelets. Using a recently published assay from our laboratory,23 we showed that levels of platelet glycoproteins Ib and the α2 subunit of α2β1, fell within the normal range (mean ± 2 SD) (α2β1 expression: S14, 1973 receptors/platelet, normal range, 867-2595; GPIb expression: S14, 19 212 receptors/platelet, normal range, 15 098-23 922). In comparison, the level of expression of GPVI was approximately 70% of controls (GPVI expression: S14, 2624 receptors/platelet, normal range, 2823-4637), falling outside of the 95% confidence limits relative to the mean measured in a population of 101 controls.23
Aggregation and protein tyrosine phosphorylation of S14 platelets shows an impairment of GPVI-mediated platelet signaling
To investigate the functional importance of the R216Q mutation, we measured platelet aggregation. There was no defect in aggregation of S14 platelets under washed conditions following stimulation with high concentrations of TRAP or collagen (data not shown). There was, however, a slight reduction in the extent and rate of aggregation to a submaximal concentration of collagen in S14 platelets, whereas both of these responses were increased slightly for a submaximal concentration of TRAP (Figure 5A). There was also a dramatic reduction in shape change in S14 platelets to all agonists, possibly consistent with the altered morphology of the GATA1-mutant platelets. Nonetheless, a similar level of aggregation in PRP was observed in control and S14 platelets in response to ADP and arachidonic acid (data not shown).
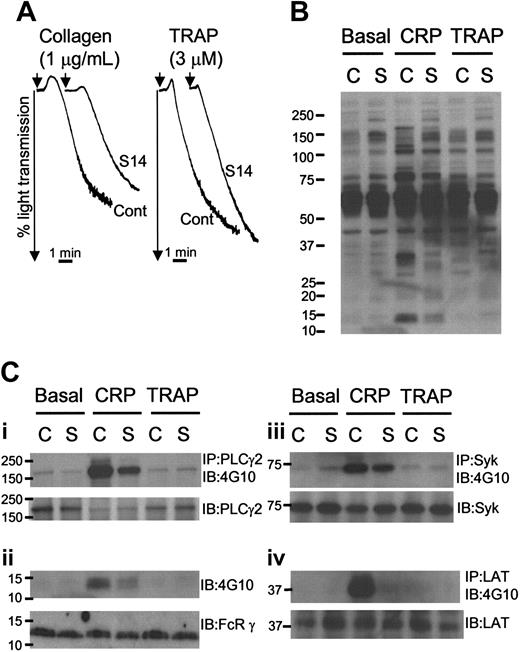
GPVI-specific platelet aggregation and tyrosine phosphorylation is reduced in S14 platelets. (A) Washed platelets were prepared from a control donor (Cont or C) and patient S14 (S14 or S) and aggregated with continuous stirring at 1200 rpm, with collagen (1 μg/mL; left) or TRAP (3 μM; right). Platelet aggregation was demonstrated by a change in light transmission. Representative aggregation tracings are shown. (B) Washed platelets were treated with lotrafiban (10 μM) and allowed to rest (Basal) or were stimulated with CRP (5 μg/mL for 90 seconds, CRP) or TRAP (30 μM for 90 seconds, TRAP) prior to lysis with 2 × Lysis buffer. Proteins were separated on SDS-PAGE, using 4% to 12% precast gradient gels, transferred onto PVDF membranes, and blotted with anti-phosphotyrosine antibody (Ab), 4G10. (C) Proteins PLCγ2 (Ci), Syk (Ciii), and LAT (Civ) were immunoprecipitated from whole platelet lysates samples and analyzed by immunoblotting with a monoclonal anti-phosphotyrosine antibody (4G10). Immunoblots were then stripped and reprobed with an antibody to PLCγ2, Syk, or LAT (lower). (Cii) Lysates were immunoblotted with 4G10 and then stripped and reprobed with an antibody to FcRγ chain. Numbers on left side of blot are molecular weight markers.
GPVI-specific platelet aggregation and tyrosine phosphorylation is reduced in S14 platelets. (A) Washed platelets were prepared from a control donor (Cont or C) and patient S14 (S14 or S) and aggregated with continuous stirring at 1200 rpm, with collagen (1 μg/mL; left) or TRAP (3 μM; right). Platelet aggregation was demonstrated by a change in light transmission. Representative aggregation tracings are shown. (B) Washed platelets were treated with lotrafiban (10 μM) and allowed to rest (Basal) or were stimulated with CRP (5 μg/mL for 90 seconds, CRP) or TRAP (30 μM for 90 seconds, TRAP) prior to lysis with 2 × Lysis buffer. Proteins were separated on SDS-PAGE, using 4% to 12% precast gradient gels, transferred onto PVDF membranes, and blotted with anti-phosphotyrosine antibody (Ab), 4G10. (C) Proteins PLCγ2 (Ci), Syk (Ciii), and LAT (Civ) were immunoprecipitated from whole platelet lysates samples and analyzed by immunoblotting with a monoclonal anti-phosphotyrosine antibody (4G10). Immunoblots were then stripped and reprobed with an antibody to PLCγ2, Syk, or LAT (lower). (Cii) Lysates were immunoblotted with 4G10 and then stripped and reprobed with an antibody to FcRγ chain. Numbers on left side of blot are molecular weight markers.
It is significant that the donor platelets used for comparison in these studies have been shown to exhibit reproducible and representative aggregation responses to collagen over several months, when compared alongside responses with 20 other controls (Ban Dawood and S.P.W., unpublished data, 2004), thereby giving increased confidence of the selective defect in response. Furthermore, Freson et al18 also reported a mild reduction in response to collagen in platelets from a patient with a different mutation in GATA1 (D218G).
Control and S14 samples were analyzed for the pattern of protein tyrosine phosphorylation under basal and stimulated conditions, in the presence of the αIIbβ3-blocking reagent lotrafiban. The patterns of tyrosine phosphorylation in control and S14 lysates were qualitatively similar under all conditions, although there was a slightly greater degree of staining in the patient sample (Figure 5B). The constitutively tyrosine phosphorylated band of 26 kDa observed in ΔneoΔHS platelets was not seen in S14 lysates. Nonetheless, the stimulation of tyrosine phosphorylation by CRP was markedly reduced in S14 platelets, suggesting a biochemical basis for the defect in GPVI-mediated aggregation. In comparison, neither TRAP (Figure 5B) nor thrombin (data not shown) stimulated a significant degree of tyrosine phosphorylation in control or S14 samples, due to the absence of aggregation.
Immunoprecipitation studies were performed to examine the reduction in response to CRP in further detail. Stimulation of tyrosine phosphorylation of PLCγ2, Syk, and LAT was markedly reduced in lysates from S14 (Figure 5Ci,iii,iv). The reduction in Syk phosphorylation suggests that this is due to a proximal effect on the GPVI signaling cascade. Consistent with this, a reduction in tyrosine phosphorylation of the band that comigrates with the FcR γ chain was observed in whole-cell lysates and in Syk immunoprecipitates (not shown) from S14. Western blotting studies confirmed that this band migrates with the FcR γ chain (Figure 5Cii). These reductions demonstrate a proximal effect on the stimulation of tyrosine phosphorylation by GPVI and are consistent with the reduced responsiveness of GATA1-perturbed platelets to GPVI signaling.
Adhesion and spreading of S14 platelets on collagen and VWF under static and flow conditions
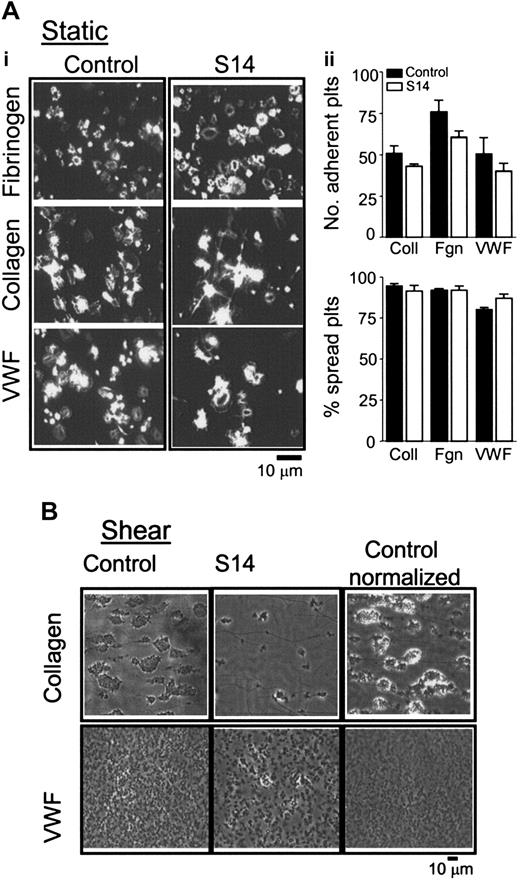
We examined the ability of S14 platelets to adhere and spread on immobilized collagen, fibrinogen, and VWF. As illustrated in Figure 6A, S14 platelets extend filopodia and generate extensive lamellipodia, consistent with observations in control platelets, suggesting that the GATA1 mutation had no effect on platelet adhesion or spreading. This was further supported by a quantitative analysis of the number of platelets that adhere and undergo cytoskeletal changes when plated on thrombogenic matrices (Figure 6A, histograms).
To examine the effect of the GATA1 mutation on adhesion and aggregate formation on a thrombogenic surface in a more physiologic setting, we assessed aggregate formation following perfusion of whole blood over immobilized collagen or VWF at 800 s-1 or 1800 s-1, respectively. Platelet aggregates were seen in whole blood along the length of collagen fibers, in comparison to much smaller aggregates of 2 to 3 cells on VWF. In sharp contrast, blood from S14 showed no substantial formation of aggregates on either matrix (Figure 6B), although a limited degree of platelet adhesion and spreading was observed. To differentiate between the effects of defective platelets versus thrombocytopenia on aggregate formation, we reduced the platelet count of control blood to that of the patient by using autologous platelet-poor plasma and red blood cells. Adjustment of the platelet count of control blood by approximately 75% to match that of S14 led to a reduction in the magnitude of aggregate formation on collagen and, to a lesser extent, on VWF. Of importance, induction of thrombocytopenia did not prevent control platelets from adhering and aggregating to collagen or VWF, in sharp contrast to the results for S14 (Figure 6C, Control - normalized). These data demonstrate that defective GATA1/DNA binding influences the ability of platelets to adhere and develop aggregates on collagen or VWF under shear conditions. Moreover, these data suggest that the defect in platelet aggregate development under shear conditions is not limited to a collagen surface.
Discussion
The studies reported here define a modulatory role for GATA1-regulated genes in the activation of human and murine platelets. Our studies demonstrate a reduction in GPVI platelet surface expression, which is linked to a reduction of aggregation and tyrosine phosphorylation of signaling proteins in the GPVI cascade. In comparison, we observed normal aggregation responses to a number of G protein–coupled receptor proteins in both species. The complex nature of the GATA1 defect was highlighted by the impairment in aggregate formation under shear conditions in both human and mouse platelets, which cannot be explained solely by the thrombocytopenia or reduction in GPVI.
Adhesion and spreading of S14 platelets on immobilized collagen under static and shear conditions. (Ai) Washed human platelets (1 × 107/mL) from control (Control) or patient (S14) were allowed to adhere and spread on immobilized fibrinogen (Fibrinogen), VWF (VWF), and collagen (Collagen) for 45 minutes at 37°C. Adherent platelets were fixed, permeabilized, and stained with rhodamine-phalloidin, then imaged using fluorescence microscopy, and cropped representative images are shown. Images are taken from 1 experiment, representative of 2. (ii) Graphs show the number of platelets adherent to the thrombogenic matrix and the number of platelets that had undergone some change in shape, recorded as a percentage of spread platelets, mean ± SEM. ▪ indicates control; □, S14. (B) Whole blood was obtained from control (Control); patient (S14) or whole blood from control donor was diluted with autologous platelet-poor plasma and red blood cells (Control - normalized) and perfused over collagen- (100 μg/mL; top row) or VWF-coated (100 μg/mL; bottom row) capillary tubes at a shear rate of 800 s-1 or 1800 s-1, respectively, for 2 to 5 minutes. Nonadherent platelets were removed by perfusion with Tyrode buffer. Three to 5 fields were imaged per capillary slide, and representative images are shown (× 63 objective).
Adhesion and spreading of S14 platelets on immobilized collagen under static and shear conditions. (Ai) Washed human platelets (1 × 107/mL) from control (Control) or patient (S14) were allowed to adhere and spread on immobilized fibrinogen (Fibrinogen), VWF (VWF), and collagen (Collagen) for 45 minutes at 37°C. Adherent platelets were fixed, permeabilized, and stained with rhodamine-phalloidin, then imaged using fluorescence microscopy, and cropped representative images are shown. Images are taken from 1 experiment, representative of 2. (ii) Graphs show the number of platelets adherent to the thrombogenic matrix and the number of platelets that had undergone some change in shape, recorded as a percentage of spread platelets, mean ± SEM. ▪ indicates control; □, S14. (B) Whole blood was obtained from control (Control); patient (S14) or whole blood from control donor was diluted with autologous platelet-poor plasma and red blood cells (Control - normalized) and perfused over collagen- (100 μg/mL; top row) or VWF-coated (100 μg/mL; bottom row) capillary tubes at a shear rate of 800 s-1 or 1800 s-1, respectively, for 2 to 5 minutes. Nonadherent platelets were removed by perfusion with Tyrode buffer. Three to 5 fields were imaged per capillary slide, and representative images are shown (× 63 objective).
While several papers have been published that describe the nature and functional consequences of GATA1 defects in human disease,16-19 the studies on platelets have been limited primarily to measurement of expression of a small number of glycoproteins (excluding GPVI) and platelet ultrastructure. The few aggregation and secretion studies that have been performed have used single concentrations of a limited number of agonists. Those studies have demonstrated a minimal or no change in functional responses to G protein–coupled receptor agonists and to collagen.18,19,21 In the present study, we have investigated a more extensive range of agonists and functional responses. This work has confirmed that responses to G protein–coupled receptor agonists are conserved, whereas there is a reduction in response to submaximal, but not to maximal, concentrations of collagen.
An important consideration in the present study is the extent to which it is valid to compare results in the human and mouse models of GATA1-perturbation, bearing in mind both the difference in species and in the nature of the mutation. In this context, the overall profile of the defect was surprisingly similar, in that there was a selective loss of response to low concentration of collagen, a reduction in expression of GPVI, a marked reduction in platelet adhesion and aggregation under flow conditions, and responses to G protein–coupled receptors were maintained. Furthermore, the defect observed under flow conditions could not be explained by the reduction in GPVI expression or thrombocytopenia. The severity of the defect in the GATA1-deficient murine platelets, however, was noticeably more pronounced. Specifically, ΔneoΔHS mice demonstrated a complete abrogation of aggregation to low concentrations of collagen/CRP and a 55% reduction in the surface expression of GPVI, whereas S14 showed a more mild reduction in aggregation to collagen and a 30% reduction of GPVI expression. These differences may be related to the genetic nature of the perturbation of GATA1 or the difference in species. In vitro, the R216Q mutation alters the affinity of GATA1 for a subset of GATA DNA-binding sites, tandem and inverted repeat GATA sites.34
Thus, this mutation is likely to perturb expression of only a subset of GATA1 target genes. In contrast ΔneoΔHS mice express a structurally normal GATA1 mRNA but only at 5% of wild-type levels. An important area for investigation, therefore, would be to evaluate the functional integrity of platelets obtained from mice bearing the same genetic defect in GATA1 as S14.
One striking difference between the human and mouse models of GATA1 perturbation was the constitutive phosphorylation of an unidentified protein of 26 kDa in mouse platelets. The appearance of this protein is reminiscent of studies performed on Motheaten viable (mev) mice that express a mutation in SHP-1 (SH2-domain containing tyrosine phosphatase). Mev mice have reduced functional responses to CRP; marked thrombocytopenia and an increase in tyrosine phosphorylation of a 26-kDa protein.35-37 Although we found no difference in the levels of SHP-1 protein in whole-cell lysates from GATA1-perturbed platelets versus control platelets (S.C.H. and S.P.W., unpublished observations, August 2004), it remains an interesting possibility that GATA1 may regulate platelet reactivity through transcriptional regulation of protein phosphatases or their upstream regulators.
It was somewhat surprising that a 50% reduction in the surface expression of GPVI has such a profound effect on aggregation to submaximal concentrations of GPVI agonists in the ΔneoΔHS murine platelets, since we have previously demonstrated that the dose-response curve for aggregation to collagen is shifted 2- and 5-fold to the right in platelets that express 50% and 20% normal GPVI.24 This finding indicates that an additional factor contributes to the abrogation of platelet aggregation to collagen, such as a defect in signaling. This possibility is supported by the reduction in tyrosine phosphorylation of ΔneoΔHS platelets that had undergone aggregation following stimulation by thrombin, thereby indicating a possible defect on “outside-in” signaling through αIIbβ3.
While the defect in response to collagen does not appear to play a role in supporting adhesion and spreading under static conditions, it may have contributed to the reduction in these responses observed under flow conditions. However, the defect in aggregate growth that is observed does not appear to be due solely to a defect in GPVI expression, because platelets with a 50% reduction in GPVI expression develop normal thrombi under shear conditions.23 This is further supported by the observation that S14 platelets are able to adhere and spread on immobilized VWF under static conditions, while being unable to form aggregates on this matrix under flow.
It is noteworthy that mice deficient in GPVI do not have a major increase in tail bleeding times,38 in comparison to the GATA1-deficient mice,21,39 although the latter mice are also thrombocytopenic. It is unlikely, however, that the thrombocytopenia is the sole explanation for the defect, as platelet aggregates are seen in control samples under the same conditions in which thrombocytopenia had been induced by dilution with platelet-poor plasma (Figures 4 and 6). Furthermore, an in vivo bleeding defect was still evident in another patient bearing the R216Q mutation, despite having a nearly normal (126 × 109 platelets/L; range, 150-400 × 109/L) platelet count,19 while ΔneoΔHS mice have a more pronounced bleeding defect than Tpo-null mice, despite the equivalent level of thrombocytopenia in the 2 mouse models.21 One factor that could influence aggregate formation under shear is the spherocytic nature of the GATA1-perturbed platelets. However, it is noteworthy that Italiano et al32 have shown that β1-tubulin-/- platelets, which like GATA1 platelets are spherocytic, are able to develop normal adhesive contacts and thrombi under shear. It, therefore, seems likely a number of factors have contributed to the defect in aggregate formation under shear conditions, including the reduced level of expression of GPVI, thrombocytopenia, and the change in the hemodynamic environment. The present data would suggest, however, that the defect in aggregate formation under shear conditions is not due to impaired inside-out activation of integrin αIIbβ3 because GATA1-perturbed platelets aggregate normally in response to G protein–coupled receptor agonists.
Our studies define a modulatory role for the transcription factor GATA1 in mediating platelet function and extend the literature that links the regulation of transcription factors to platelet activation. We demonstrate an important role for GATA1 in regulating GPVI-mediated platelet activation and phosphorylation events, which could be explained by a reduction in the surface expression of the glycoprotein. These observations resemble those made by Sun et al40 who demonstrated the association between the transcription factor core-binding factor A2 (CBFA2) and proteins linked to integrin αIIbβ3 activation in platelets. Those investigators described a patient bearing a heterozygous point mutation in CBFA2 who demonstrated impaired integrin αIIbβ3 activation, linked to impaired protein phosphorylation and decreased protein kinase C-θ (PKC-θ) mRNA levels.40 Similarly, Shiraga et al41 demonstrated a role for the transcription factor, nuclear factor-erythroid 2 (NF-E2), in agonist-induced integrin αIIbβ3 activation in megakaryocytes. These studies illustrate that understanding the intricacies of the transcriptional regulation of hematopoiesis may shed more light on the pathophysiology of bleeding and thrombosis.
Prepublished online as Blood First Edition Paper, February 8, 2005; DOI 10.1182/blood-2004-10-4098.
Supported in part by grants from the Wellcome Trust and British Heart Foundation. P.V. is a Wellcome Trust Senior Clinical Fellow. S.P.W. holds a British Heart Foundation Chair. S.C.H. holds a National Health and Medical Research Council (Aust) CJ Martin Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank patient S14 and family for their willingness to be involved in this study. We thank Ben Atkinson for bleeding mice, Stephanie Dumon for assistance with flow cytometry, Christiane Kuhl for providing ΔneoΔHS and control mice, and Prof Ludlam (Haemophilia and Thrombosis Centre, Royal Infirmary, Edinburgh) and Dr Simon Brown (Centre for Inflammation Research, University of Edinburgh) for use of laboratory space and equipment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal