Abstract
Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1/CD66a), expressed on leukocytes, epithelia, and endothelia mediates homophilic cell adhesion. It plays an important role in cell morphogenesis and, recently, soluble CEACAM1 isoforms have been implicated in angiogenesis. In the present study, we investigated the function of long transmembrane isoform of CEACAM1 (CEACAM1-L) in cultured rat brain endothelial cells. We observed that expression of CEACAM1-L promotes network formation on basement membrane Matrigel and increased cell motility after monolayer injury. During cell-matrix adhesion, CEACAM1-L translocated into the Triton X-100–insoluble cytoskeletal fraction and affected cell spreading and cell morphology on Matrigel and laminin-1 but not on fibronectin. On laminin-1, CEACAM1-L–expressing cells developed protrusions with lamellipodia, showed less stress fiber formation, reduced focal adhesion kinase (FAK) tyrosine phosphorylation, and decreased focal adhesion formation leading to high motility. CEACAM1-L–mediated morphologic alterations were sensitive to RhoA activation via lysophosphatidic acid (LPA) treatment and dependent on Rac1 activation. Furthermore, we demonstrate a matrix protein–dependent association of CEACAM1-L with talin, an important regulator of integrin function. Taken together, our results suggest that transmembrane CEACAM1-L expressed on endothelial cells is implicated in the activation phase of angiogenesis by affecting the cytoskeleton architecture and integrin-mediated signaling.
Introduction
Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) was originally identified as a homophilic cell-cell adhesion receptor and has been implicated in several cellular functions.1,2 Studies with epithelial cells revealed the importance of CEACAM1 in cell morphogenesis and proliferation, while analysis of transgenic mice showed a function for CEACAM1 in insulin clearance.3-5 Stimulation of CEACAM antigens in leukocytes modulates signaling and integrin-mediated adhesion.6-8 Apart from its homophilic adhesion capacities, it serves as a receptor for different bacterial strains and for the mouse hepatitis virus.9,10 This immunoglobulin (Ig)–like molecule is expressed as transmembrane and soluble isoforms.11-13 The cytoplasmic domain of the long isoform of rat CEACAM1 (CEACAM1-L) contains consensus sequences for an immunoreceptor tyrosine-based inhibitory motif (ITIM)14 and an immunoreceptor tyrosine-based switch motif (ITSM),15 implicated in signal regulation.
In endothelial cells, transmembrane CEACAM1 is found in small blood vessels and capillaries in most mature tissues in the rat and in the developing rat central nervous system, as well as in angiogenic blood vessels.16-18 Angiogenesis is a multistep process that can be roughly divided into 2 phases. In the activation phase, endothelial cells degrade the perivascular basement membrane, migrate into the extracellular space, proliferate, and form capillary sprouts and tubular structures. In the maturation phase, endothelial cells cease migration and proliferation, reconstitute a basement membrane, and recruit smooth muscle cells, thereby permitting the maintenance of vessel wall integrity.19 Growth factors, such as vascular endothelial growth factor (VEGF), have been extensively characterized as responsible stimulatory agents, and different cell surface molecules, such as integrins, vascular endothelial (VE)–cadherin, or platelet-endothelial cell adhesion molecule-1 (PECAM-1), have been implicated at various steps in vascular assembly.20,21 Recently, a functional role for soluble CEACAM1 in angiogenesis was demonstrated. Stimulation of endothelial cells with soluble CEACAM1 led to a significant increase in VEGF-induced tube formation and increased neovascularization of chick chorioallantoic membranes.18
The aim of the present study was to determine the biologic functions of transmembrane CEACAM1-L expressed on endothelial cells. We were able to demonstrate that transmembrane CEACAM1-L regulates cell morphology during adhesion to extracellular matrix proteins, promotes network formation, and enhances migration of endothelial cells. These processes were dependent on the activation state of the Rho family of small guanosine triphosphatases (GTPases) and involved altered integrin signaling. Finally, we could demonstrate a cytoskeletal anchorage of CEACAM1-L during cell spreading and present data for a matrix protein–regulated in vivo association with talin, a major cytoskeletal protein implicated in integrin activation.
Materials and methods
Materials, antibodies, vectors, and cell culture
Anti-CEACAM1 monoclonal antibody (mAb) Be9.2 and polyclonal anti-CEACAM1 rabbit serum were described previously.22,23 Primary mouse monoclonals were as follows: antiphosphotyrosine (PY99; Santa Cruz Biotechnology, Santa Cruz, CA); antipaxillin (no. 349), anti–beta1-integrin (no. 18), and anti–focal adhesion kinase (FAK) (no. 77) (BD/Transduction-Laboratories, San Jose, CA); anti–alpha6-integrin (4E9G8; DPC-Biermann, Nauheim, Germany); anti–E-selectin (5D11; R&D Systems, Wiesbaden, Germany); and antitalin antibody (8d4), antivinculin (hVIN-1), anti–beta-actin (AC-15), and anti–c-myc (9E10) (Sigma, Deisenhofen, Germany). Polyclonal antibodies were as follows: goat anti–mouse cyanin 2 (Cy-2) conjugate (Dako, Hamburg, Germany), rabbit anti–PECAM-1, rabbit anti-FAK, and goat anti–VE-cadherin (Santa Cruz Biotechnology). Colorimetric bromodeoxyuridine (BrdU) cell proliferation enzyme-linked immunosorbent assay (ELISA) kit and laminin-1 were purchased from Roche (Mannheim, Germany). Fibronectin, tumor necrosis factor α (TNF-α), phalloidin-TRITC, and lysophosphatidic acid (LPA) were from Sigma. Matrigel was purchased from Becton Dickinson (Heidelberg, Germany) and ROCK-inhibitor Y27632, from Merck (Darmstadt, Germany).
Eukaryotic expression vectors encoding wild-type (wt), constitutive active (Q61L), or dominant-negative variants (T17N) of Rac1 were from Dr Keith Burridge (Chapel Hill, NC). Rac1 sequences were inserted into the EcoRI and XhoI site of pCMV-Myc (Clontech, Palo Alto, CA), resulting in Rac/myc-fusion proteins. The expression vector pRAX/C-CAM1 encoding the long CEACAM1 isoform and the generation of the CEACAM1 tyrosine double-mutant Y488F/Y513F were described previously.24,25
Rat endothelial cells were generated according to the method of Folkman et al.26 Briefly, the meninges were prepared from rat cerebral cortex; large leptomeningeal blood vessels were isolated, cut, and incubated in OptiMEM/Ham/F12 (1:1) medium and 10% fetal calf serum (FCS), at 37°C/5% CO2. Fibroblasts and other cells were removed 3 hours after plating. After 4 days, microcolonies of endothelial cells with a cobblestone morphology became visible. Cell debris and contaminating cells were removed and remaining colonies were further incubated and cloned as described.26 Cloned RBE (rat brain endothelial) cells were cultured in RPMI medium/10% FCS containing 50 IU/mL penicillin and 50 μg/mL streptomycin at 37°C. Transfection was done using lipofectamine (Invitrogen, Karlsruhe, Germany); selection, by G418-resistance; and cloning, by limiting dilution. Culturing of rat MTC cells was performed as described.13 Rat IEC-6 cells were cultured in Dulbecco modified Eagle medium (DMEM)/10% FCS.
Immunohistochemistry
Freshly removed rat brains (without removal of the meninges) were immersed in phosphate-buffered saline (PBS), snap frozen in liquid nitrogen, cut into 5-μm–thick sections, air dried, fixed in acetone, and blocked with bovine serum. CEACAM1 was detected with mAb Be9.2 (10 μg/mL in PBS) and secondary alkaline phosphatase–conjugated antibodies (1:50; Dako). Detection was done by incubation with naphthol AS-BI phosphate (Sigma) and New Fuchsin (Merck). Endogenous alkaline phosphatase activity was blocked by levamisole (Sigma). Counterstaining was done with Mayer hemalaum (Merck).
RNA isolation and RT-PCR
RNA isolation and reverse-transcriptase–polymerase chain reaction (RT-PCR) were carried out as described previously.13 Primer combination R+L detects all rat CEACAM1 isoforms, whereas 9+L is specific for CEACAM1-L and 22+L is specific for the short isoform of CEACAM1 (CEACAM1-S). PCR products after 25 PCR cycles (transfectants) or additional 25 cycles after a second PCR amplification reaction (wt cells) were analyzed by agarose gel electrophoresis.
Immunoprecipitation and Western blotting
For tyrosine phosphorylation, cells were scraped into lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane, pH 7.2], 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS]) containing phosphatase inhibitors (1 mM NaVO3, 10 mM Na4P2O6, 100 mM NaF), 1 mM phenylmethylsulfonyl fluoride (PMSF), and a protease inhibitor cocktail (AEBSF, E-64, betastatin, leupeptin, aprotinin; Sigma). In all other cases, cells were lysed in HBSMC (HEPES-buffered saline-magnesium-calcium; 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.2], 150 mM NaCl, 5 mM MgCl2, 1 mM CaCl2) containing 0.5% Triton X-100, PMSF, and protease inhibitor cocktail. After solubilization at 4°C, lysates were cleared by centrifugation. Equivalent amounts of protein-containing lysates were immunoprecipitated using protein A– or G–Sepharose, or directly subjected to SDS–polyacrylamide gel electrophoresis (PAGE). Insoluble material after Triton X-100 (TX-100) lysis, containing cytoskeletal-associated proteins, was washed with HBSMC and boiled in Laemmli buffer. DNA was degraded with Benzonase (Sigma). For detection of soluble CEACAM1 isoforms, cell culture supernatants from confluent cells, incubated for 4 days, were cleared by stepwise centrifugation (100-100 000g). Overnight, 5 mL was subjected to immunoprecipitation with polyclonal anti-CEACAM1 serum. Western blotting and subsequent protein detection were performed as described.27 Chemiluminescence and quantification were done using the Fujifilm-LAS-1000 digital system (Fujifilm Europe, Düsseldorf, Germany). For reprobing, filters were stripped with Re-Blot-Plus solution (Chemicon, Hofheim, Germany).
Fluorescence-activated cell sorter (FACS) analysis
RBE cells were grown to subconfluency. For detection of CD62E, cells were stimulated or not with 10 ng/mL TNF-α for 20 hours. Trypsin/EDTA (ethylenediaminetetraacetic acid)–detached cells were washed in 1% bovine serum albumin (BSA)/PBS and incubated with primary antibodies (Be9.2 and 5D11, 10 μg/mL). After incubation with secondary fluorescein isothiocyanate (FITC)–conjugated antibodies, flow cytometry was performed with a FACScan instrument (Becton Dickinson).
Capillary network formation and migration after monolayer injury
Matrigel was thawed overnight on ice and 100 μL was added per well in a 96-well plate. The plate was transferred to a 37°C CO2 incubator for one hour. Trypsinized RBE cells (3 × 104) were plated onto the gel and incubated for the indicated times. Cell motility was measured in plain tissue culture dishes by monolayer injury with a pipet tip. After washing, closure of the cell-free area was monitored after incubation for different time intervals. Network formation and migration of RBE cells were examined using a phase contrast microscope equipped with a Nikon F-601 camera (Nikon, Düsseldorf, Germany). Quantification was done using Adobe Photoshop software (Adobe, San Jose, CA).
Adhesion measurement, morphology, and proliferation
The 96-well plates were coated with fibronectin (20 μg/mL PBS), laminin-1 (20 μg/mL PBS), or dilutions of Matrigel (1:20-1:100) overnight, followed by blocking in 1% BSA/PBS. Cells were serum starved for 60 minutes and plated in serum-free RPMI medium (3 × 104 cells/well). After 75 minutes at 37°C, nonattached cells were removed with PBS washing; attached cells were fixed (4% paraformaldehyde/PBS) and stained with 0.1% crystal violet. Quantification was done in a microplate reader at 570 nm after Triton X-100 dye solubilization. Images were taken using a Zeiss Axiovert 200 microscope (Carl Zeiss, Göttingen, Germany) equipped with a digital camera AxioCam (Zeiss Vision, Halbergmoos, Germany). For proliferation measurement, 2 × 104 serum-starved RBE cells were plated per well in 1% FCS and BrdU-containing medium. After 24 hours of incubation, cells were fixed and BrdU incorporation was measured in a microplate reader at 405 nm according to the manufacturer's instructions (Roche).
Random motility
Serum-starved RBE cells (2 × 105) were plated on fibronectin- or laminin-1–coated, BSA-blocked, 25-cm2 cell culture flasks in serum-free medium and allowed to adhere for one hour. Cell migration was monitored using the digital system of a Zeiss-Axiovert 200 microscope equipped with the AxioCam digital system at 20-minute intervals. The positions of nuclei were tracked to quantify cell motility. Velocities were calculated in micrometers per 8 hours using AxioVision software (Zeiss Vision).
Immunofluorescence
Serum-starved RBE cells (3 × 104) were plated on extracellular matrix protein–coated slides (20 μg/mL) in serum-free RPMI medium. In some cases, 10% FCS, LPA (50-500 ng/mL), ROCK-inhibitor Y27632 (10 μM), or antibody (50 μg/mL) was added. For experiments with Rac1 constructs, cells were transfected by nucleofection (Amaxa, Cologne, Germany) according to the manufacturer's instructions and allowed to recover and express myc-tagged proteins for 7 hours in normal culture medium prior to serum starvation for 1 hour. At 75 minutes after plating, cells were fixed (4% paraformaldehyde/PBS or 4% paraformaldehyde/PBS/0.025% saponin). After blocking, cells were incubated with TRITC-conjugated phalloidin (1 ng/mL) or primary antibodies (anti-CEACAM1 Be9.2, 10 μg/mL; antipaxillin mAb, 10 μg/mL) in PBS, washed, and incubated with Cy-2–labeled antimouse antibody (1:100). Immunofluorescence was visualized using the digital system of a Zeiss Axiovert 200 microscope equipped with the AxioCam digital system. Protrusion length was calculated from phalloidin-stained cells using AxioVision software.
Statistics
Statistical analysis was performed using Student t test.
Results
Generation and characterization of a rat brain endothelial cell line
Immunohistochemistry of cross-sections of adult rat brain showed CEACAM1 expression on endothelia of the meninges (Figure 1A) and in capillaries from inner parts of the rat brain. To establish a rat endothelial cell model, we prepared leptomeningeal blood vessels and generated primary endothelial cells by the method of Folkman et al.26 Cultured under standard conditions, these rat brain endothelial (RBE) cells consisted of a homogeneous population similar in morphology to other primary endothelial cells. They grew in a cobblestone-like monolayer characterized by cell-cell contact-dependent growth arrest, and expressed the endothelial marker proteins PECAM-1 (CD31) and VE-cadherin (Figure 1B) as well as TNF-α–inducible CD62E (Figure 1C). Although detectable in vivo on endothelial cells of the meninges, RBE cells in culture no longer expressed CEACAM1, as revealed by isoform-specific RT-PCR for CEACAM1-L and CEACAM1-S (Figure 1D). Because no CEACAM1 transcript could be visualized even after a second amplification reaction of 25 PCR cycles, we restored CEACAM1 expression by stable transfection of RBE wild-type (wt) cells with cDNA for CEACAM1-L and subsequent cloning. RT-PCR and flow cytometric analysis confirmed isoform-specific expression of CEACAM1-L on the cell surface of RBE transfectants (Figure 1D-E). Immunoprecipitation with polyclonal anti-CEACAM1 antibodies did not reveal any shedded or secreted CEACAM1 in conditioned media from wt or CEACAM1-L–transfected RBE cells (Figure 1F). Rat MTC cells secreting soluble CEACAM1-4C1 were used as a positive control.13
Characterization of wild-type and CEACAM1-L–transfected rat brain endothelial (RBE) cells. (A) Immunohistochemistry of vascular endothelia in rat meninges using rat CEACAM1-specific monoclonal antibody Be9.2 or isotype control IgG (insert in the upper left); representative staining of a cross-section is shown. Original magnification, × 10. (B) Expression of VE-cadherin and PECAM-1 in RBE cells. Immunoblotting of cell lysates (50 μg) of (lane 1) IEC-6 (small intestine epithelial cells, negative control) or (lane 2) RBE cells. (C) Flow cytometric detection of E-selectin on the surface of RBE cells. Isotype control IgG staining of untreated cells (gray shading), anti–E-selectin staining of untreated cells (–), or 20-hour TNF-α–treated cells (—). (D) Detection of CEACAM1 mRNA by RT-PCR in wild-type (wt) and CEACAM1-L–transfected RBE cells (CEACAM1-L); the primer combinations recognized all isoforms of rat CEACAM1 (L/S), the long isoform specifically (L), or the short isoform specifically (S). (E) Cell surface expression of CEACAM1-L after transfection. Wt (top) or CEACAM1-L–transfected and cloned cells were incubated with isotype control (gray shading) or mAb Be9.2 (—) and FITC-coupled secondary antibody for flow cytometric analysis. (F) Detection of soluble and membrane-bound CEACAM1-L. Immunoprecipitated soluble isoforms of CEACAM1 from cell culture supernatants (top) or membrane-bound CEACAM1 (bottom) of rat RBE and MTC cells detected by immunoblotting with mAb Be9.2. A representative immunoblot of 3 independent experiments is shown.
Characterization of wild-type and CEACAM1-L–transfected rat brain endothelial (RBE) cells. (A) Immunohistochemistry of vascular endothelia in rat meninges using rat CEACAM1-specific monoclonal antibody Be9.2 or isotype control IgG (insert in the upper left); representative staining of a cross-section is shown. Original magnification, × 10. (B) Expression of VE-cadherin and PECAM-1 in RBE cells. Immunoblotting of cell lysates (50 μg) of (lane 1) IEC-6 (small intestine epithelial cells, negative control) or (lane 2) RBE cells. (C) Flow cytometric detection of E-selectin on the surface of RBE cells. Isotype control IgG staining of untreated cells (gray shading), anti–E-selectin staining of untreated cells (–), or 20-hour TNF-α–treated cells (—). (D) Detection of CEACAM1 mRNA by RT-PCR in wild-type (wt) and CEACAM1-L–transfected RBE cells (CEACAM1-L); the primer combinations recognized all isoforms of rat CEACAM1 (L/S), the long isoform specifically (L), or the short isoform specifically (S). (E) Cell surface expression of CEACAM1-L after transfection. Wt (top) or CEACAM1-L–transfected and cloned cells were incubated with isotype control (gray shading) or mAb Be9.2 (—) and FITC-coupled secondary antibody for flow cytometric analysis. (F) Detection of soluble and membrane-bound CEACAM1-L. Immunoprecipitated soluble isoforms of CEACAM1 from cell culture supernatants (top) or membrane-bound CEACAM1 (bottom) of rat RBE and MTC cells detected by immunoblotting with mAb Be9.2. A representative immunoblot of 3 independent experiments is shown.
CEACAM1-L expression on RBE cells promotes network formation on Matrigel
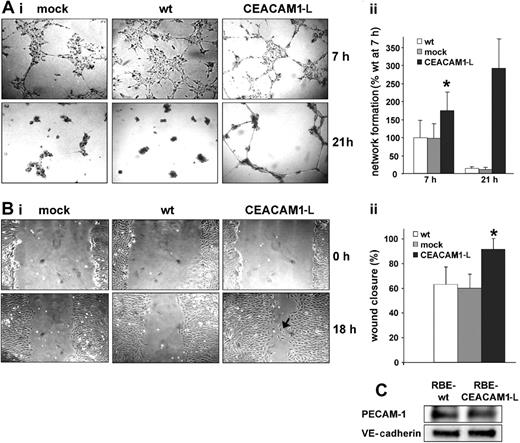
RBE cells spontaneously assembled into an interconnected network clearly visible after 7 hours when plated on basement membrane Matrigel (Figure 2A). This process began 3 hours after plating when the cells had spread, moved, and started to form small interconnected clusters. During the first 2 hours when the cells adhered and began to reorganize, no differences were observed between wt, mock-, and CEACAM1-L–transfected cells (not shown). At 7 hours after plating, wt-RBE, mock-transfected, and CEACAM1-L–expressing cells showed a reticular multicellular pattern. Compared with wt and mock-transfected cells, CEACAM1-L transfectants appeared more stretched and elongated, and reached an advanced netlike structure with larger discrete Matrigel areas devoid of cells and pronounced interconnections between cell clusters (Figure 2Aii). After 21 hours of incubation, the wt and mock-transfected cells formed clustered aggregates, while the CEACAM1-L transfectants remained in a continuous network that lasted for approximately 36 hours.
Enhanced migration of CEACAM1-L–expressing RBE cells
To analyze, whether CEACAM1-L expression induced changes in cell migration, monolayers of RBE wt, mock, and CEACAM1-L transfectants were injured and monolayer restitution was analyzed at different time intervals (Figure 2B). By 18 hours, the migration of CEACAM1-L–expressing cells was significantly faster compared with wt cells and mock-transfected cells, resulting in increased closure of the wounded area (91%, compared with 67% and 63%, respectively; Figure 2Bii). Interestingly, wt and mock-transfected cells remained tightly associated with neighboring cells during monolayer restitution, whereas the CEACAM1-L–expressing cells often detached from their neighbors, resulting in single cells migrating into the cell-free area. However, expression levels of the proadhesive proteins VE-cadherin and PECAM-1 were unaltered in CEACAM1-L–expressing cells (Figure 2C), as revealed by immunoblotting.
CEACAM1-L enhances network formation on Matrigel and cell migration after monolayer injury. (Ai) Single-cell suspensions of 3 × 104 RBE/wt (wt), mock-transfected (mock), or CEACAM1-L–expressing (CEACAM1-L) cells were transferred on Matrigel-coated plates. Images were taken at the indicated time points after plating. Original magnification, × 10. (ii) Quantification of network formation. Length of interconnections between cell clusters were measured at 7 hours and 21 hours. Length of interconnections relative to wt cells after 7 hours are represented as means ± SD of 3 experiments done in duplicate. *P < .01. (Bi) RBE wild-type (wt), mock-transfected (mock), and CEACAM1-L–expressing (CEACAM1-L) cells were cultured to confluence. Monolayers were scratched with a pipette tip to create a wound, washed with medium, and recultured. Closure of the cell-free area was followed by phase contrast microscopy and photographed at the indicated time points after scratching. CEACAM1-L–expressing RBE cells show single cells migrating into the wounded area (arrow). Original magnification × 25. (ii) The width of the cell-free area was measured at time 0 (scratching) and 18 hours after scratching. Relative closure of the cell-free area is shown in means ± SD (n = 8). *P < .01. (C) Immunoblotting of total cell lysates to determine the expression levels of PECAM-1 and VE-cadherin in RBE wt and CEACAM1-L–expressing cells. The data shown are representative of 2 independent experiments.
CEACAM1-L enhances network formation on Matrigel and cell migration after monolayer injury. (Ai) Single-cell suspensions of 3 × 104 RBE/wt (wt), mock-transfected (mock), or CEACAM1-L–expressing (CEACAM1-L) cells were transferred on Matrigel-coated plates. Images were taken at the indicated time points after plating. Original magnification, × 10. (ii) Quantification of network formation. Length of interconnections between cell clusters were measured at 7 hours and 21 hours. Length of interconnections relative to wt cells after 7 hours are represented as means ± SD of 3 experiments done in duplicate. *P < .01. (Bi) RBE wild-type (wt), mock-transfected (mock), and CEACAM1-L–expressing (CEACAM1-L) cells were cultured to confluence. Monolayers were scratched with a pipette tip to create a wound, washed with medium, and recultured. Closure of the cell-free area was followed by phase contrast microscopy and photographed at the indicated time points after scratching. CEACAM1-L–expressing RBE cells show single cells migrating into the wounded area (arrow). Original magnification × 25. (ii) The width of the cell-free area was measured at time 0 (scratching) and 18 hours after scratching. Relative closure of the cell-free area is shown in means ± SD (n = 8). *P < .01. (C) Immunoblotting of total cell lysates to determine the expression levels of PECAM-1 and VE-cadherin in RBE wt and CEACAM1-L–expressing cells. The data shown are representative of 2 independent experiments.
Cell morphology and cytoskeletal reorganization on Matrigel and laminin-1 are affected by CEACAM1-L expression
We next analyzed cell spreading of RBE cells on coatings of Matrigel and purified matrix proteins. Under serum-free conditions, CEACAM1-L transfectants showed pronounced differences in cell morphology compared with wt and mock-transfected cells when plated on laminin-1 or Matrigel (Figure 3Ai-ii), but not on fibronectin. RBE wt cells and mock transfectants adhering on fibronectin, laminin-1, or diluted Matrigel spread by extending lamellipodia along the entire cell periphery, acquiring a polygonal cell shape within 75 minutes after plating. The same cell shape was displayed by CEACAM1-L transfectants adhering to fibronectin. In contrast to the wild-type and mock-transfected cell phenotype, CEACAM1-L–expressing cells adhering to laminin-1 acquired an elongated, often stellate morphology with extended protrusions arising from the cell periphery. Coatings of Matrigel yielded comparable results, consistent with laminin-1 being a major component of Matrigel.
Phalloidin staining (Figure 3B) revealed that adhesion to fibronectin promoted organization of prominent actin stress fibers in wt, mock-transfected, and CEACAM1-L–expressing cells. Mock-transfected and wt cells plated on laminin-1 or Matrigel (not shown) coatings also displayed prominent actin stress fibers. In contrast, CEACAM1 transfectants adhering to laminin-1 or Matrigel (not shown) coatings displayed reduced actin stress fiber formation. A few remaining stress fibers ran parallel to the protrusions, but essentially no stress fibers were found in the cell bodies. At the leading edges of the extended protrusions, the CEACAM1-L–expressing cells formed prominent lamellipodia (shown by strong cortical actin staining), which were not found in wt or mock-transfected cells on any matrix protein. Addition of the CEACAM1-specific homophilic binding-blocking mAb Be9.2 did not affect cell shape or F-actin organization during spreading. In contrast, addition of rabbit polyclonal IgG against rat CEACAM1 (α-CC1 pAb) resulted in elongated protrusions on laminin-1 (+ 45%, Figure 3Bii) and, interestingly, the induction of small cortical actin-rich protrusions on fibronectin (arrows). Cell morphology and stress fiber formation of mock-transfected/wild-type RBE cells were unchanged by α-CC1 pAb treatment, and nonfunctional control rabbit immunoglobulins did not affect cell morphology, demonstrating specificity of the α-CC1 serum.
The cytoplasmic domain of CEACAM1-L associates with signaling molecules and the actin cytoskeleton. Because some interacting proteins require phosphorylated tyrosine residues within the CEACAM1-L cytoplasmic sequence, we analyzed F-actin organization of the CEACAM1-L tyrosine double-mutant Y448F/Y513F during cell spreading. On fibronectin, cell morphology and stress fiber formation were comparable with wt-, mock-, and CEACAM1-L–transfected cells. On laminin-1, Y448F/Y513F transfectants showed protrusion formation and stress fiber assembly almost comparable with wild-type CEACAM1-L, but fewer lamellipodia were found at the leading edges. Again, α-CC1 pAb treatment during adhesion induced small protrusions on fibronectin and led to extended protrusions on laminin-1 in Y448F/Y513F cells (Figure 3Bii).
Morphology and cytoskeletal reorganization of RBE cells adhering on extracellular matrix proteins. Serum-starved wild-type (wt), mock-transfected (mock), or CEACAM1-L–expressing (CEACAM1-L) cells (3 × 104) were plated on fibronectin (FN), laminin-1 (LN), or Matrigel coatings (Matrigel, diluted 1:100) under serum-free conditions for 75 minutes. (Ai) Nonadherent cells were removed by washing, and adherent cells were fixed and visualized with crystal violet. Original magnification, × 100. (ii) For discrimination of differential cell morphologies, cells with or without at least one protrusion longer than the intersection of the cell body were counted using the AxioVision software. Relative numbers of cells with extended protrusions are presented as means ± SD of 2 independent experiments done in triplicate. *P < .001. (Bi) Visualization of the actin cytoskeleton by phalloidin-TRITC in wt (wt), mock-transfected (mock), tyrosine double-mutant (CEACAM1-L/Y448F/Y513F), or CEACAM1-L–expressing (CEACAM1-L) RBE cells during spreading on fibronectin (FN) and laminin-1 (LN). CEACAM1-L + α-CC1 pAb indicates treatment with polyclonal rabbit anti-CEACAM1 antiserum (50 μg/mL). Note the occurrence of small protrusions with lamellae after α-CC1 pAb treatment of CEACAM1-expressing cells on fibronectin (arrows). Original magnification, × 400. (ii) Protrusion length of adhering cells (distance in μm from cell body to the tip) was measured with AxioVision software and means ± SD of 2 experiments done in duplicate are shown (n = 20). Antibodies (mAb Be9.2 IgG and rabbit polyclonal anti-CEACAM1 IgG [α-CC1 pAb]) as well as control rabbit IgG were added at 50 μg/mL. *Increase in protrusion length of CEACAM1-L–expressing cells by α-CC1 treatment represents significant difference, P < .001.
Morphology and cytoskeletal reorganization of RBE cells adhering on extracellular matrix proteins. Serum-starved wild-type (wt), mock-transfected (mock), or CEACAM1-L–expressing (CEACAM1-L) cells (3 × 104) were plated on fibronectin (FN), laminin-1 (LN), or Matrigel coatings (Matrigel, diluted 1:100) under serum-free conditions for 75 minutes. (Ai) Nonadherent cells were removed by washing, and adherent cells were fixed and visualized with crystal violet. Original magnification, × 100. (ii) For discrimination of differential cell morphologies, cells with or without at least one protrusion longer than the intersection of the cell body were counted using the AxioVision software. Relative numbers of cells with extended protrusions are presented as means ± SD of 2 independent experiments done in triplicate. *P < .001. (Bi) Visualization of the actin cytoskeleton by phalloidin-TRITC in wt (wt), mock-transfected (mock), tyrosine double-mutant (CEACAM1-L/Y448F/Y513F), or CEACAM1-L–expressing (CEACAM1-L) RBE cells during spreading on fibronectin (FN) and laminin-1 (LN). CEACAM1-L + α-CC1 pAb indicates treatment with polyclonal rabbit anti-CEACAM1 antiserum (50 μg/mL). Note the occurrence of small protrusions with lamellae after α-CC1 pAb treatment of CEACAM1-expressing cells on fibronectin (arrows). Original magnification, × 400. (ii) Protrusion length of adhering cells (distance in μm from cell body to the tip) was measured with AxioVision software and means ± SD of 2 experiments done in duplicate are shown (n = 20). Antibodies (mAb Be9.2 IgG and rabbit polyclonal anti-CEACAM1 IgG [α-CC1 pAb]) as well as control rabbit IgG were added at 50 μg/mL. *Increase in protrusion length of CEACAM1-L–expressing cells by α-CC1 treatment represents significant difference, P < .001.
CEACAM1-L expression does not affect cell matrix adhesion and proliferation, but does affect cell migration on laminin-1
Because the extracellular matrix exerts an extraordinary control of cell behavior, we analyzed whether CEACAM1-L expression affects matrix-dependent cellular functions. Under serum-free conditions, the RBE wt and CEACAM1-L–expressing cells showed similar degrees of adhesion to diluted Matrigel as well as to fibronectin and laminin-1 coatings (Figure 4A). We also compared cell proliferation of RBE wt and CEACAM1-L–expressing cells on extracellular matrix proteins. BrdU incorporation over a period of 24 hours did not reveal any influence of CEACAM1-L expression on cell proliferation on plastic (data not shown), fibronectin, or laminin-1 (Figure 4B). Moreover, cell death and apoptosis on plastic, fibronectin, or laminin-1 were unchanged (data not shown).
Furthermore, cell migration on extracellular matrix proteins was determined by time-lapse microscopy (Figure 4C). On plastic and fibronectin, RBE wt cells showed marginal motility. Moderate motility (45 ± 12 μm/8 hours) was observed only on laminin-1. Negligible random migration on fibronectin and on plastic was also tracked for CEACAM1-L–expressing cells, whereas on laminin-1, CEACAM1-L–expressing cells showed an accelerated tempo (134 ± 37 μm/8 hours), exceeding the motility of wt RBE cells 2.9-fold.
Differential activity of Rho and Rac1 during adhesion of RBE cells to laminin-1
The Rho family of small GTPases regulates structural rearrangements of the actin cytoskeleton. During readhesion on laminin-1, addition of 10% FCS or 50 to 500 ng/mL LPA, a physiologic activator of Rho,28 to wt RBE cells did not significantly change the cell morphology but led to increased stress fiber formation (Figure 5A). In contrast, the morphology of CEACAM1-L–expressing cells was remarkably changed by serum addition and LPA treatment in a concentration-dependent manner. Protrusions shortened and lamellipodia were no longer detectable, instead the cells took on a polygonal shape and displayed prominent stress fiber formation, comparable with the phenotype of untreated wt cells (Figure 5Ai-ii).
An important Rho effector is the Rho-associated serine/threonine kinase p160 (ROCK). Rho activation leads to ROCK activity, which indirectly stimulates the formation of F-actin and stress fibers. Dependent on the incubation time, addition of the ROCK-inhibitor Y27632 during adhesion reduced stress fiber formation in CEACAM1-L–expressing cells and increased the length of protrusions (Figure 5Ai-ii). In contrast, wt cells treated with Y27632 kept their cell shape and did not form protrusions, although stress fiber formation was strongly reduced during adhesion. Reversibility of the ROCK inhibition was shown by wash-out experiments.
Attachment, proliferation, and motility of RBE cells on extracellular matrix proteins. (A) Cell attachment; Serum-starved wild type (wt; □) or CEACAM1-L–expressing (CEACAM1-L; ▦) cells (3 × 104) were plated on fibronectin (FN), laminin-1 (LN), or Matrigel coatings under serum-free conditions for 75 minutes. Nonadherent cells were removed by washing, and adherent cells were fixed and visualized with crystal violet. Quantification of attachment after 75 minutes was measured at 570 nm after dye release. The data represent means ± SD of 3 independent experiments done in triplicate. (B) Cell proliferation. Serum-starved wt (□) or CEACAM1-L–expressing (▦) cells (2 × 104) were plated on matrix protein–coated 96-well plates in medium containing 1% FCS and BrdU for 24 hours. Incorporation of BrdU was measured at 405 nm as described in “Materials and methods.” The results are shown as means ± SD of 2 independent experiments done in triplicate. (Ci) Representative paths of cell migration on plastic, fibronectin (FN), or laminin-1 (LN) of RBE wt (wt) or CEACAM1-L–expressing cells (CEACAM1-L) tracked at 20-minute intervals over a span of 8 hours. (ii) Quantification of cell migration represented as means ± SD in duplicate assays. *P < .001.
Attachment, proliferation, and motility of RBE cells on extracellular matrix proteins. (A) Cell attachment; Serum-starved wild type (wt; □) or CEACAM1-L–expressing (CEACAM1-L; ▦) cells (3 × 104) were plated on fibronectin (FN), laminin-1 (LN), or Matrigel coatings under serum-free conditions for 75 minutes. Nonadherent cells were removed by washing, and adherent cells were fixed and visualized with crystal violet. Quantification of attachment after 75 minutes was measured at 570 nm after dye release. The data represent means ± SD of 3 independent experiments done in triplicate. (B) Cell proliferation. Serum-starved wt (□) or CEACAM1-L–expressing (▦) cells (2 × 104) were plated on matrix protein–coated 96-well plates in medium containing 1% FCS and BrdU for 24 hours. Incorporation of BrdU was measured at 405 nm as described in “Materials and methods.” The results are shown as means ± SD of 2 independent experiments done in triplicate. (Ci) Representative paths of cell migration on plastic, fibronectin (FN), or laminin-1 (LN) of RBE wt (wt) or CEACAM1-L–expressing cells (CEACAM1-L) tracked at 20-minute intervals over a span of 8 hours. (ii) Quantification of cell migration represented as means ± SD in duplicate assays. *P < .001.
To examine the role of Rac1 in the process of CEACAM1-L–induced formation of protrusions and lamellipodia on laminin-1, we transiently transfected wt and CEACAM1-L–expressing RBE cells with myc-tagged dominant-negative (N17Rac1), dominant-active (L61Rac1), or wt Rac1. As shown by phalloidin-TRITC staining, overexpression of wt/Rac1 in RBE-wt and CEACAM1-L–transfected cells did not change the cell morphology or protrusion length during adhesion to laminin-1 (Figure 5Bi-ii), whereas overexpression of L61Rac1 resulted in the formation of a large, circular lamellipodium, which extended from the entire cell circumference in both wt and CEACAM1-L–expressing cells. Overexpression of dominant-negative Rac1 in wt cells did not affect cell morphology. In contrast, CEACAM1-L–expressing cells transfected with N17Rac1 no longer formed extended protrusions or lamellipodia on laminin-1. The CEACAM1-L transfectants now adhered and spread in like wt cells transfected with N17Rac1. According to these data, the observed morphologic changes of CEACAM1-L–expressing RBE cells adhering on laminin-1 are at least partly due to activation of Rac1 and inhibition of Rho.
Reduced focal contact assembly and FAK tyrosine phosphorylation during adhesion to laminin-1
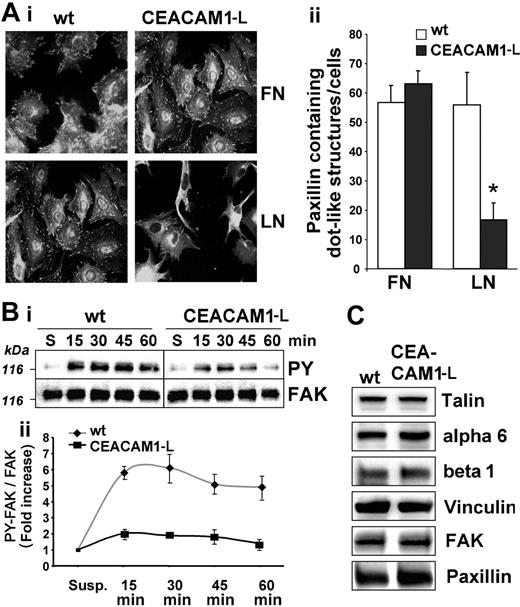
We analyzed focal adhesion formation by indirect immunofluorescence of paxillin during adhesion. Plated on fibronectin, wt- and CEACAM1-L–expressing RBE cells displayed well-developed focal complexes as well as more centrally located large focal adhesions (Figure 6Ai-ii). Plated on laminin-1, wt cells displayed focal complex formation that was comparable with that on fibronectin. In contrast, CEACAM1-L transfectants showed reduced focal complex formation on laminin-1. Centrally located focal adhesions were no longer detectable and the few remaining focal complexes were small and located in the periphery and at the leading edges of protrusions (Figure 6Ai-ii).
Focal adhesion kinase (FAK) is a key component in integrin signaling and focal adhesion assembly and is activated and tyrosine phosphorylated in response to integrin–extracellular matrix (ECM) interactions. Therefore, we determined FAK tyrosine phosphorylation during adhesion to laminin-1 (Figure 6B). CEACAM1-L–expressing cells showed a transient increase in FAK tyrosine phosphorylation. In comparison, wt cells showed a stronger and more sustained tyrosine phosphorylation of FAK (Figure 6Bii).
To monitor for differential expression of adhesion proteins in wt and CEACAM1-L–transfected cells, we analyzed the expression levels of α6/β1 integrin (the major laminin-1 receptor) and the focal adhesion complex–associated proteins talin, vinculin, FAK, and paxillin (Figure 6C). Immunoblotting did not reveal any differences in expression levels of any of these proteins between wt or CEACAM1-L–expressing cells. Also flow cytometry showed identical expression levels of α6 and β1 integrins in wt cells and CEACAM1-L transfectants (data not shown).
The CEACAM1-L distribution and association with the cytoskeleton and talin are differentially regulated by fibronectin and laminin-1
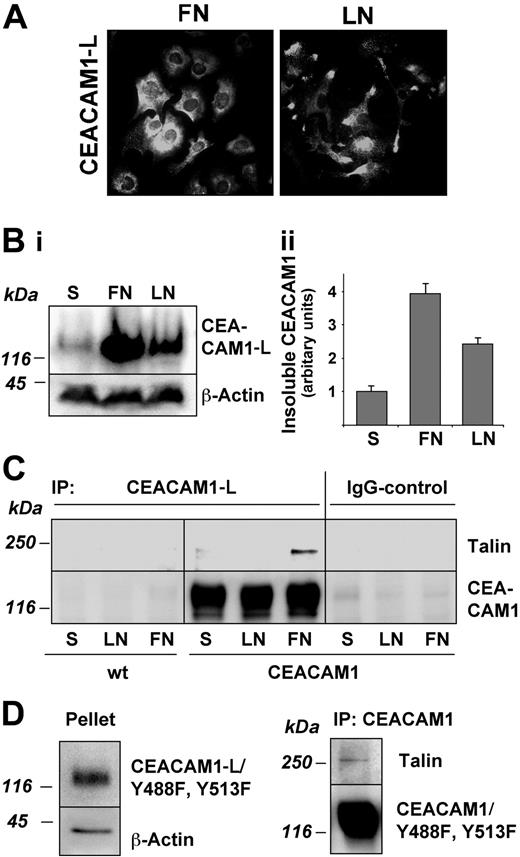
Indirect immunofluorescence of unpermeabilized cells with anti-CEACAM1 mAb Be9.2 revealed a random CEACAM1-L distribution over the entire cell surface after adhesion to fibronectin. In contrast, during adhesion to laminin-1, the majority of CEACAM1-L accumulated in the protrusions in a punctuated fashion behind the region of strong cortical actin staining (Figure 7A). No CEACAM1-L was found in the focal adhesion structures.
Cytoskeletal proteins constitute a major part of the Triton X-100–insoluble cell fraction. To analyze whether CEACAM1-L was associated with the cytoskeleton during adhesion, its partition into the TX-100–insoluble fraction was determined by immunoblotting. In cells kept in suspension, most of the CEACAM1-L was present in the 0.5% Triton X-100–soluble fraction, whereas very little was found in the Triton X-100–insoluble cytoskeletal fraction (Figure 7B). However, during adhesion, the level of insoluble CEACAM1-L increased, and a significant amount of CEACAM1-L specifically translocated into the cytoskeletal fraction. At 75 minutes after plating on fibronectin, the insoluble CEACAM1-L fraction increased 3.9-fold compared with cells in suspension and 2.4-fold on laminin-1 (Figure 7Bii).
We then examined whether CEACAM1-L might be associated with proteins known to mediate binding to the actin cytoskeleton. As shown in Figure 7C, immunoprecipitation of CEACAM1-L specifically brought down a significant amount of talin in cells that were spreading on fibronectin, whereas no coprecipitating talin was detected in cells spreading on laminin, or in cells kept in suspension. Other integrin-associated cytoskeletal proteins such as vinculin or alpha-actinin were not coprecipitated with CEACAM1-L (data not shown).
Rho- and Rac-dependent morphology of RBE cells during adhesion to laminin-1. (Ai) Serum-starved wild-type (wt) and CEACAM1-L–expressing cells were plated with or without manipulation on laminin-1 for 75 minutes, fixed, and stained for actin filaments with phalloidin-TRITC. During adhesion, cells were treated without (control, -FCS) or with 10% FCS (+FCS) or LPA (50 and 500 ng/mL) or Rho-kinase inhibitor Y27632 (10 μM) to activate or inhibit Rho-dependent signaling pathways. For demonstration of the reversibility of ROCK inhibition, cells were preincubated with Y27632 (10 μM) for one hour and plated in new serum-free medium without the inhibitor (wash-out). Original magnification, × 400. (ii) Protrusion length in RBE/wt (□) and CEACAM1-L–expressing (▪) cells after treatment with FCS, LPA, and Y27632 was measured with AxioVision software. Means ± SD of 2 independent experiments are shown (n ≥ 20). *Altered protrusion length of CEACAM1-L–expressing cells by the indicated treatment represents significant difference, P < .001. (Bi) Morphology of RBE cells overexpressing Rac1 wt, L61Rac1 (dominant active), or N17Rac1 (dominant negative) on laminin-1. Wild type (wt) and CEACAM1-L–expressing cells were transiently transfected with myc-tagged Rac1 constructs by nucleofection. Then 8 hours later, serum-starved cells were plated on laminin-1 for 75 minutes, fixed, and stained for actin filaments with phalloidin-TRITC (left panels) and with anti–myc-tag antibodies and anti–mouse Cy2-conjugated secondary antibodies (right panels) for visualization of Rac1-construct expression. Original magnification, × 400. (ii) Protrusion length of untransfected (control) or Rac1-construct–transfected RBE cells was measured with AxioVision software. □ indicates wt; ▪, CEACAM1-L–expressing cells. Results are presented as means ± SD of 2 independent experiments (n ≥ 10). *Decrease in protrusion length of CEACAM1-L–expressing cells by dominant-negative Rac1 expression represents significant difference, P < .001.
Rho- and Rac-dependent morphology of RBE cells during adhesion to laminin-1. (Ai) Serum-starved wild-type (wt) and CEACAM1-L–expressing cells were plated with or without manipulation on laminin-1 for 75 minutes, fixed, and stained for actin filaments with phalloidin-TRITC. During adhesion, cells were treated without (control, -FCS) or with 10% FCS (+FCS) or LPA (50 and 500 ng/mL) or Rho-kinase inhibitor Y27632 (10 μM) to activate or inhibit Rho-dependent signaling pathways. For demonstration of the reversibility of ROCK inhibition, cells were preincubated with Y27632 (10 μM) for one hour and plated in new serum-free medium without the inhibitor (wash-out). Original magnification, × 400. (ii) Protrusion length in RBE/wt (□) and CEACAM1-L–expressing (▪) cells after treatment with FCS, LPA, and Y27632 was measured with AxioVision software. Means ± SD of 2 independent experiments are shown (n ≥ 20). *Altered protrusion length of CEACAM1-L–expressing cells by the indicated treatment represents significant difference, P < .001. (Bi) Morphology of RBE cells overexpressing Rac1 wt, L61Rac1 (dominant active), or N17Rac1 (dominant negative) on laminin-1. Wild type (wt) and CEACAM1-L–expressing cells were transiently transfected with myc-tagged Rac1 constructs by nucleofection. Then 8 hours later, serum-starved cells were plated on laminin-1 for 75 minutes, fixed, and stained for actin filaments with phalloidin-TRITC (left panels) and with anti–myc-tag antibodies and anti–mouse Cy2-conjugated secondary antibodies (right panels) for visualization of Rac1-construct expression. Original magnification, × 400. (ii) Protrusion length of untransfected (control) or Rac1-construct–transfected RBE cells was measured with AxioVision software. □ indicates wt; ▪, CEACAM1-L–expressing cells. Results are presented as means ± SD of 2 independent experiments (n ≥ 10). *Decrease in protrusion length of CEACAM1-L–expressing cells by dominant-negative Rac1 expression represents significant difference, P < .001.
Finally, we analyzed the insolubility of the double-mutant Y448F/Y513F during adhesion on fibronectin. As shown in Figure 7D, a significant amount of CEACAM1-L/Y448F/Y513F was found in the Triton X-100–insoluble fraction, comparable with wild-type CEACAM1-L. Subsequently, cell lysates of CEACAM1-L/Y448F/Y513F cells adhering to fibronectin were immunoprecipitated with mAb Be9.2, and the resulting immunoprecipitates were immunoblotted with antitalin antibody 8d4. The results demonstrate that talin is coimmunoprecipitated with CEACAM1-L, independently of the tyrosine residues within the cytoplasmic tail.
Discussion
During vascular assembly, endothelial cells must respond to a variety of extracellular signals derived from soluble growth factors, cell–extracellular matrix interactions, and cell-cell interactions.20,21,29-32 Recently, soluble CEACAM1 was shown to exhibit angiogenic properties in vitro and in vivo.18 In addition to soluble CEACAM1 molecules, CEACAM1 is expressed as a transmembrane protein on the cell surface of capillary endothelial cells and angiogenic blood vessels. In the vascularizing central nervous system transmembrane, CEACAM1 was found in contact areas between capillary endothelial cells and pericytes, but no CEACAM1 was located in the contacts between adjacent endothelial cells.17 This indicates that endothelial CEACAM1 may participate in homophilic cell-cell adhesion in a strictly regulated manner during certain phases of vascularization or angiogenesis. However, our knowledge about the functional role of transmembrane endothelial CEACAM1 in vascularization/angiogenesis has so far been very limited. To date, an approved method for maintaining CEACAM1 expression on endothelial cells in cell culture is unknown, making it difficult to study CEACAM1-mediated effects in this cell type. Apart from the rat brain endothelial cell line, we recently generated endothelial cells from rat pancreas and rat fat tissue. Also in these cells, CEACAM1 expression was undetectable in vitro. In the present study, we circumvented variability of freshly prepared endothelial cells and the failure of CEACAM1 expression by stable transfection. By this procedure, we show for the first time that transmembrane CEACAM1-L has an important role in regulating network formation and extracellular matrix–specific morphology and migration of endothelial cells.
Our finding that the CEACAM1-L–triggered motile phenotype of endothelial cells occurred on laminin-1 but not on fibronectin is of particular importance, since laminins are integral parts of basement membranes. This suggests that CEACAM1-L might be implicated in the activation phase of angiogenesis, when endothelial cells penetrate the subendothelial basement membrane of the existing vessels, and migrate out to form new capillaries. The mechanism(s) of CEACAM1-L–induced cell dissociation and enhanced migration is still being defined, but agrees well with the observations that homophilic CEACAM1 binding in endothelial cells is a strictly regulated phenomenon and that cell surface expression of CEACAM1 does not automatically lead to cell-cell adhesion.17 Furthermore, the correlation between CEACAM1-L expression and increased motility is in line with our previous finding that CEACAM1-L is up-regulated in NBT-II cells undergoing epithelial-mesenchymal transition, which leads to increased cell motility and cell scattering.33 Thus, our present results are in excellent agreement with known properties of CEACAM1.
Actin-based cell motility is regulated by the Rho family of small GTPases.28 In human umbilical vein endothelial cells, the balance of RhoA/Rho-kinase and Rac1 activity regulates attachment, migration, and early network-formation.34,35 This proved to be true also for the CEACAM1-L–mediated regulation of rat endothelial cell spreading. The experimental data showed that the Rho pathway was inhibited and the Rac pathway was activated when the CEACAM1-L–expressing endothelial cells were plated on laminin-1. Thus, we conclude that CEACAM1 in collaboration with specific extracellular matrix components can affect cell signaling that influences Rho- and Rac-mediated effects on the actin filament system, leading to the observed motile phenotype with extended protrusions, leading lamellae, reduced number of stress fibers, and focal complexes.
Adhesion to and migration on extracellular matrices are critically dependent on integrin function, a fact that strongly suggests that the CEACAM1-L–mediated regulation of endothelial cell migration reflects functional interactions between integrins and CEACAM1. Indeed, there is accumulating evidence that CEACAM1 can interact with and modulate integrin signaling in several different cell systems. Anti-CEACAM1 antibodies affect the function of β1- and β2-integrins in neutrophils and B lymphocytes,6,8,36 and direct interactions between β3-integrins and CEACAM1-L have been observed in granulocytes, epithelial cells, and invading trophoblasts.37 Furthermore, paxillin has been reported to associate with CEACAM1-L in granulocytes, epithelial cells, and human umbilical vein endothelial cells.38 CEACAM1-L can also interact with the protein tyrosine phosphatases SHP-1 and SHP-2 and tyrosine kinases of the src-family,25,39,40 which participate in integrin signaling.41-45 In granulocytes and, as shown in this study, in endothelial cells also, talin associates with CEACAM1-L.38 Thus, CEACAM1-L is able to interact and interfere with integrins or integrin-regulated cellular functions. The presented data indicate that CEACAM1-L regulates laminin-binding integrins differently from fibronectin-binding integrins in endothelial cells.
Assembly of focal adhesions and FAK phosphorylation in RBE cells. (Ai) Representative images of serum-starved wild-type (wt) and CEACAM1-L–expressing cells plated on fibronectin (FN) or laminin-1 (LN) for 75 minutes. After removing nonadhering cells, adherent cells were fixed and stained for paxillin. Original magnification, × 400. (ii) Quantification of paxillin-containing dot-like structures per cell. □ indicates wt; ▪, CEACAM1-L–expressing cells. Means ± SD of 3 experiments are shown. *P < .001. (Bi) Serum-starved cells were kept in suspension (S) or attached to laminin-1 for the indicated times. Equal protein amounts were subjected to FAK immunoprecipitation, followed by immunoblotting with antiphosphotyrosine antibodies (PY99). Blots were stripped and reprobed with monoclonal anti-FAK antibodies. A representative immunoblot of 3 independent experiments is shown. (ii) Quantification of FAK-tyrosine phosphorylation during adhesion on laminin-1. ♦ indicates wt; ▪ indicates CEACAM-1–expressing cells. Fold induction represented as means ± SD from 3 independent experiments is shown. (C) Representative immunoblots showing expression levels of α6 and β1 integrins and the integrin-associated proteins talin, vinculin, FAK, and paxillin in RBE wt and CEACAM1-L–expressing cells. The results shown are representative of 2 independent experiments.
Assembly of focal adhesions and FAK phosphorylation in RBE cells. (Ai) Representative images of serum-starved wild-type (wt) and CEACAM1-L–expressing cells plated on fibronectin (FN) or laminin-1 (LN) for 75 minutes. After removing nonadhering cells, adherent cells were fixed and stained for paxillin. Original magnification, × 400. (ii) Quantification of paxillin-containing dot-like structures per cell. □ indicates wt; ▪, CEACAM1-L–expressing cells. Means ± SD of 3 experiments are shown. *P < .001. (Bi) Serum-starved cells were kept in suspension (S) or attached to laminin-1 for the indicated times. Equal protein amounts were subjected to FAK immunoprecipitation, followed by immunoblotting with antiphosphotyrosine antibodies (PY99). Blots were stripped and reprobed with monoclonal anti-FAK antibodies. A representative immunoblot of 3 independent experiments is shown. (ii) Quantification of FAK-tyrosine phosphorylation during adhesion on laminin-1. ♦ indicates wt; ▪ indicates CEACAM-1–expressing cells. Fold induction represented as means ± SD from 3 independent experiments is shown. (C) Representative immunoblots showing expression levels of α6 and β1 integrins and the integrin-associated proteins talin, vinculin, FAK, and paxillin in RBE wt and CEACAM1-L–expressing cells. The results shown are representative of 2 independent experiments.
Distribution and cytoskeletal association of CEACAM1-L in RBE cells. (A) Cell surface localization of CEACAM1-L during adhesion and spreading of serum-starved CEACAM1-L–transfected RBE cells on fibronectin (FN) and laminin-1 (LN) 75 minutes after plating. Unpermeabilized cells were stained with mAb Be9.2 and Cy-2–conjugated antimouse secondary antibody. Original magnification, × 400. (Bi) Translocation of CEACAM1-L into the Triton X-100–insoluble fraction during adhesion. The cells were solubilized with 0.5% Triton X-100 and insoluble CEACAM1-L was determined by immunoblotting of the Triton-insoluble fraction. β-Actin was used as a loading control. (ii) The amount of immunoblotted CEACAM1-L was quantified and is shown as the mean of 3 independent experiments ± SD. (C) Coimmunoprecipitation of talin with CEACAM1-L in spreading cells. Cells were kept in suspension (S) or were allowed to adhere for 75 minutes on fibronectin (FN) or laminin-1 (LN). Cells were solubilized and equal amounts of protein were immunoprecipitated with mAb Be9.2. Talin in complex with CEACAM1-L was detected by immunoblotting with mAb 8d4. Blots were reprobed with Be9.2 for detection of CEACAM1-L. A representative immunoblot of 3 independent experiments is shown. (D) Translocation of tyrosine double-mutant CEACAM1-L/Y448F/Y513F into the Triton X-100–insoluble fraction and coimmunoprecipitation of talin with CEACAM1-L/Y448F/Y513F during adhesion to fibronectin. Cells were solubilized 75 minutes after adhesion. CEACAM1-L/Y488513F in the pellet fraction was detected by immunoblotting with Be9.2. Talin in complex with the tyrosine double-mutant CEACAM1-L/Y488513F was detected in Be9.2-immunoprecipitated material with mAb 8d4. Representative immunoblots of 2 independent experiments are shown. IP indicates immunoprecipitation.
Distribution and cytoskeletal association of CEACAM1-L in RBE cells. (A) Cell surface localization of CEACAM1-L during adhesion and spreading of serum-starved CEACAM1-L–transfected RBE cells on fibronectin (FN) and laminin-1 (LN) 75 minutes after plating. Unpermeabilized cells were stained with mAb Be9.2 and Cy-2–conjugated antimouse secondary antibody. Original magnification, × 400. (Bi) Translocation of CEACAM1-L into the Triton X-100–insoluble fraction during adhesion. The cells were solubilized with 0.5% Triton X-100 and insoluble CEACAM1-L was determined by immunoblotting of the Triton-insoluble fraction. β-Actin was used as a loading control. (ii) The amount of immunoblotted CEACAM1-L was quantified and is shown as the mean of 3 independent experiments ± SD. (C) Coimmunoprecipitation of talin with CEACAM1-L in spreading cells. Cells were kept in suspension (S) or were allowed to adhere for 75 minutes on fibronectin (FN) or laminin-1 (LN). Cells were solubilized and equal amounts of protein were immunoprecipitated with mAb Be9.2. Talin in complex with CEACAM1-L was detected by immunoblotting with mAb 8d4. Blots were reprobed with Be9.2 for detection of CEACAM1-L. A representative immunoblot of 3 independent experiments is shown. (D) Translocation of tyrosine double-mutant CEACAM1-L/Y448F/Y513F into the Triton X-100–insoluble fraction and coimmunoprecipitation of talin with CEACAM1-L/Y448F/Y513F during adhesion to fibronectin. Cells were solubilized 75 minutes after adhesion. CEACAM1-L/Y488513F in the pellet fraction was detected by immunoblotting with Be9.2. Talin in complex with the tyrosine double-mutant CEACAM1-L/Y488513F was detected in Be9.2-immunoprecipitated material with mAb 8d4. Representative immunoblots of 2 independent experiments are shown. IP indicates immunoprecipitation.
The differences in cell morphology on laminin-containing matrices between the wt and the CEACAM1-L–expressing cells were sensitive to mutation of the tyrosine residues in the CEACAM1-L cytoplasmic domain with regard to the Rac-mediated formation of leading lamellae. A likely candidate for this tyrosine-dependent effect of CEACAM1-L might be the SH2-domain–containing tyrosine kinase c-src.46 However, these tyrosine residues were required neither for CEACAM1-L's cytoskeletal linkage nor for its association with talin on fibronectin. Furthermore, the tyrosine mutations did not affect protrusion formation controlled by Rho. Several examples of tyrosine-independent interactions between CEACAM1 and other proteins have previously been reported. It is, for example, well documented that the short isoform CEACAM1-S, lacking tyrosine residues, associates with the actin cytoskeleton and other cytoplasmic proteins such as annexin II, and both CEACAM1-L and CEACAM1-S can bind calmodulin in a tyrosine-independent manner.47-49 Further studies on the promorphogenic properties of CEACAM1-S50 should contribute to clarify the functional roles of the individual regions of the cytoplasmic domains of the CEACAM1 in cell matrix–regulated events.
The observed differences between the laminin-1–induced behavior of CEACAM1-L–expressing RBE cells on the one hand and wt-RBE cells/CEACAM1-L–expressing cells on fibronectin on the other hand can be put together in a tentative interaction scenario. On laminin-containing matrices, CEACAM1-L interferes with laminin-binding integrin signaling. FAK becomes transiently activated, followed by Rho inactivation, Rac activation, and assembly of actin filaments primarily into lamellipodia. CEACAM1-L becomes associated with the actin cytoskeleton, but not with talin. This may allow talin to activate integrins in an inside-out signaling,51 which leads to sustained network and protrusion formation and high motility. On fibronectin, CEACAM1-L does not interfere with integrin outside-in signaling. FAK becomes strongly activated, followed by Rho activation, Rac inactivation, and assembly of actin filaments primarily into stress fibers. CEACAM1-L becomes strongly associated with the actin cytoskeleton, and also binds a fraction of talin. This may lead to a lower amount of talin available for integrin activation, resulting in the less motile phenotype. The importance of talin during extracellular matrix–mediated signaling is supported by recent data, which indicate that talin plays a crucial role in the inside-out control of integrin activation by binding to different integrin beta-tails.52,53 In conclusion, our results describe a novel functional cooperation between CEACAM1-L and integrin signaling in endothelial cells and form the basis for analyzing the modes of interactions between CEACAM1, integrins, talin, and other integrin-associated proteins.
Prepublished online as Blood First Edition Paper, February 1, 2005; DOI 10.1182/blood-2004-09-3618.
Supported by the Deutsche Forschungsgemeinschaft (SFB 366, Zelluläre Signalerkennung und-umsetzung, TP C1), the Swedish Research Council (project 05200), and the Swedish Cancer Foundation (project 4720).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Iwona Chichocka for excellent technical assistance; Yihai Cao, Stockholm, Sweden, for his help in generating primary endothelial cells; Keith Burridge, Chapel Hill, NC, for providing us with Rac1 constructs; and Dr T. Scott, United Kingdom, for critically reading the manuscript.


![Figure 3. Morphology and cytoskeletal reorganization of RBE cells adhering on extracellular matrix proteins. Serum-starved wild-type (wt), mock-transfected (mock), or CEACAM1-L–expressing (CEACAM1-L) cells (3 × 104) were plated on fibronectin (FN), laminin-1 (LN), or Matrigel coatings (Matrigel, diluted 1:100) under serum-free conditions for 75 minutes. (Ai) Nonadherent cells were removed by washing, and adherent cells were fixed and visualized with crystal violet. Original magnification, × 100. (ii) For discrimination of differential cell morphologies, cells with or without at least one protrusion longer than the intersection of the cell body were counted using the AxioVision software. Relative numbers of cells with extended protrusions are presented as means ± SD of 2 independent experiments done in triplicate. *P < .001. (Bi) Visualization of the actin cytoskeleton by phalloidin-TRITC in wt (wt), mock-transfected (mock), tyrosine double-mutant (CEACAM1-L/Y448F/Y513F), or CEACAM1-L–expressing (CEACAM1-L) RBE cells during spreading on fibronectin (FN) and laminin-1 (LN). CEACAM1-L + α-CC1 pAb indicates treatment with polyclonal rabbit anti-CEACAM1 antiserum (50 μg/mL). Note the occurrence of small protrusions with lamellae after α-CC1 pAb treatment of CEACAM1-expressing cells on fibronectin (arrows). Original magnification, × 400. (ii) Protrusion length of adhering cells (distance in μm from cell body to the tip) was measured with AxioVision software and means ± SD of 2 experiments done in duplicate are shown (n = 20). Antibodies (mAb Be9.2 IgG and rabbit polyclonal anti-CEACAM1 IgG [α-CC1 pAb]) as well as control rabbit IgG were added at 50 μg/mL. *Increase in protrusion length of CEACAM1-L–expressing cells by α-CC1 treatment represents significant difference, P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/10/10.1182_blood-2004-09-3618/6/m_zh80100578660003.jpeg?Expires=1769119118&Signature=A~2FchZMDz6xdy9ntrpvsO~68caiGTgNU3JQ2HCe5WP4I4e4GX06bAhaGPiveXJTe9WUVby3GzSfQvnzAPmmI5zykz6sK0w-eDMwwZ61uCDaSPlkEZKUpxroyRTAarb9L4sBEc3oEFLozAbqTcmDJ9mc7SH6oI48uzFZp-mNNjyQUvOQGQlZHojD5RUJ~DGV9Shm9jGp91r2VQPjZ-mxlKg16e826z9-F99m9ED0TY6hvAxEjxbLm3~ta2gx25KqDJpnulBZqPE7Nuve-CQQVC7jBx2UY81jFRR~7biIgF~9Qt~lVq1gWnOQKBUBhuFqBLHV~vqLEK21YfyKREysrQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal