Abstract
Fludarabine and rituximab combination therapies in chronic lymphocytic leukemia (CLL) have yielded promising early results, but no comparative efficacy data relative to standard fludarabine treatment regimens have been reported. To assess the effect of the addition of rituximab to fludarabine therapy, we retrospectively compared the treatment outcome of patients with similar clinical characteristics enrolled on 2 multicenter clinical trials performed by the Cancer and Leukemia Group B and the US Intergroup that used fludarabine and rituximab (CALGB 9712, n = 104) or fludarabine (CALGB 9011, n = 178). In multivariate analyses controlling for pretreatment characteristics, the patients receiving fludarabine and rituximab had a significantly better progression-free survival (PFS; P < .0001) and overall survival (OS; P = .0006) than patients receiving fludarabine therapy. Two-year PFS probabilities were 0.67 versus 0.45, and 2-year OS probabilities were 0.93 versus 0.81. Infectious toxicity was similar between the 2 treatment approaches. These comparative data are retrospective and could be confounded by differences in supportive care or dissimilar enrollment of genetic subsets on each trial. Confirmation of these findings will require a prospective randomized trial comparing fludarabine and rituximab to fludarabine.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in the western hemisphere. The natural history of CLL is varied, with median survival exceeding 10 years in early-stage patients.1 Because of this long survival and a host of studies demonstrating no benefit to early intervention,2 CLL is treated only when patients become symptomatic from the disease. At this time, accepted initial treatment for CLL includes alkylator therapy (chlorambucil or cyclophosphamide) or fludarabine.3 The latter agent is more frequently chosen for younger patients, based on data from 3 randomized studies4-6 demonstrating a significantly higher overall response rate, higher complete response (CR) rate, and longer progression-free survival (PFS) with fludarabine-based therapy as compared with alkylator-based therapy. Despite the longer PFS in the fludarabine arm of all 3 studies,4-6 statistically significant improved survival with fludarabine therapy has not been observed to date in CLL. This may be reflective of the ability of patients initially receiving alkylator-based therapy to later receive treatment with fludarabine at the time of disease progression.
Rituximab is a chimeric monoclonal antibody directed against the cell surface antigen CD20. Previous studies have demonstrated that rituximab is active in a variety of different B-cell lymphomas and enhances the response to chemotherapy. Rituximab also has single-agent activity in CLL.7-9 Additionally, several large phase 2 studies combining rituximab with fludarabine10,11 or the combination of fludarabine and cyclophosphamide12 have demonstrated a much higher complete response rate than previously observed with any other therapeutic approaches used in CLL before this time. To date, comparative studies of rituximab and fludarabine combinations to fludarabine monotherapy have not been performed. Herein, we perform a retrospective comparison of the outcome of patients enrolled on CALGB 9712,11 a randomized phase 2 study of 2 different schedules of rituximab combined with fludarabine, to patients with similar pretreatment characteristics enrolled on the fludarabine arm of a randomized phase 3 CALGB trial.4
Patients, materials, and methods
Patients
Patients were enrolled on 2 clinical trials4,11 following written informed consent. The eligibility criteria of the 2 studies were virtually identical, with all patients required to have histologically and immunophenotypically documented CLL as defined by the National Cancer Institute (NCI) 1996 guidelines.13 Neither trial permitted viral or bacterial prophylaxis, nor was there prospective monitoring for cytomegalovirus infection. All patients were either Rai stage III/IV or Rai stage I/II with evidence of disease activity as defined by the NCI 1996 guidelines13 and had received no prior therapy. Interphase cytogenetics and molecular studies are currently being examined for patients enrolled on CALGB 9712, but they were not performed as part of CALGB 9011.
Comparison groups used in this analysis
CALGB 9712, a randomized phase 2 trial open to accrual from 1997 to 1999, examined administration of rituximab either concurrently (n = 51) or sequentially (n = 53) with fludarabine therapy.11 At a median follow-up of 43 months among patients still being followed for progression, the PFS and overall survival (OS) of the concurrent and sequential arms were found to be similar (data not shown). On the basis of this finding, all 104 patients enrolled on this trial were combined into 1 comparison group for the analyses reported in this paper. CALGB 9011, open to accrual from 1990 to 1994, randomly assigned patients to either fludarabine, chlorambucil, or their combination.4 The fludarabine-only arm of that trial, with a sample size of 178, was used in the present paper as the second comparison group. Criteria for response in each trial were those specified in the 1996 NCI-sponsored Working Group Guidelines.13 As specified by these guidelines, a response had to be maintained for a period of 2 months. PFS was defined from the date of randomization to date of progression or death (events) or last follow-up (censored), whichever came first. Survival time was measured from the date of randomization to date of death (event) or date last seen alive (censored). The data for each study were up to date as of July 2003.
Statistical analysis
Pretreatment demographic and clinical characteristics of the 2 treatment groups were compared with the chi-square test of proportions or the 2-sample Wilcoxon test. The Kaplan-Meier method was used to estimate the distribution of PFS and OS within each treatment group. Two-year PFS and OS probabilities with 95% confidence intervals (CIs) are presented; these probabilities will change minimally with further follow-up since there are only 4 censored values on PFS and OS before 2 years. Response rates of the treatment groups were statistically compared with logistic regression; PFS and OS of the treatment groups were compared with the proportional hazards model. Each treatment comparison was tested both uncontrolled and controlled for sex, age at time of randomization, and pretreatment values of white blood count (WBC), lactate dehydrogenase (LDH), stage (I/II versus III/IV), and presence/absence of splenomegaly. Age, WBC, and LDH were modeled as continuous variables. P values less than .05 were considered statistically significant. Analyses were performed by a CALGB statistician.
Results
The demographics of the 104 patients on CALGB 9712 who were assigned to receive fludarabine and rituximab and 178 patients enrolled on CALGB 9011 who were assigned to receive fludarabine are shown in Table 1. The median age of patients enrolled on CALGB 9712 was 63 years (range, 38-88 years) and on CALGB 9011 64 years (range, 37-87 years). The median WBC on CALGB 9712 was 83 × 109/L (range, 9-436 × 109/L), whereas on CALGB 9011 it was 77 × 109/L (range, 9-709 × 109/L). Splenomegaly was observed in 61% of patients enrolled on CALGB 9712 versus 68% on CALGB 9011. Forty percent of the patients enrolled on CALGB 9712 had high-risk (Rai stage III or IV) disease as compared with 39% on CALGB 9011. At the time of this analysis, 60 of the 104 patients assigned to fludarabine and rituximab had not yet progressed (median follow-up of these 60 patients is 43 months), and 16 of the 178 patients receiving fludarabine only had not yet progressed (median follow-up of these 16 patients is 95 months). The follow-up for CALGB 9712 is shorter than CALGB 9011, reflecting the time of initiation of enrollment of these 2 trials (1998 versus 1990). However, actual follow-up data for longer than 2 years were available for all but 4 patients included in this analysis.
Demographic and clinical characteristics of patients according to treatment assignment
. | CALGB 9712 . | CALGB 9011 . | P . |
|---|---|---|---|
| No. of patients | 104 | 178 | |
| Median age, y (range) | 63 (38-88) | 64 (37-87) | .75 |
| Patients younger than 50 y, % | 14 | 14 | .93 |
| Patients aged 70 y or older, % | 23 | 25 | .88 |
| Rai stage I, % | 2 | 2 | |
| Rai stage II, % | 57 | 55 | |
| Rai stage III, % | 14 | 41 | < .0001 |
| Rai stage IV, % | 26 | 2 | |
| Enlarged liver, % | 15 | 20 | .43 |
| Enlarged spleen, % | 61 | 68 | .19 |
| Median WBC, 109/L (range) | 83 (9-436) | 77 (9-709) | .39 |
| Median Hgb, g/dL (range) | 12.3 (1.3-16.1) | 12.0 (4.6-16.6) | .26 |
| Median platelets, × 109/L (range) | 158 (33-316) | 155 (12-451) | .57 |
| Median, LDH, IU (range) | 212 (92-950) | 198 (39-1389) | .20 |
. | CALGB 9712 . | CALGB 9011 . | P . |
|---|---|---|---|
| No. of patients | 104 | 178 | |
| Median age, y (range) | 63 (38-88) | 64 (37-87) | .75 |
| Patients younger than 50 y, % | 14 | 14 | .93 |
| Patients aged 70 y or older, % | 23 | 25 | .88 |
| Rai stage I, % | 2 | 2 | |
| Rai stage II, % | 57 | 55 | |
| Rai stage III, % | 14 | 41 | < .0001 |
| Rai stage IV, % | 26 | 2 | |
| Enlarged liver, % | 15 | 20 | .43 |
| Enlarged spleen, % | 61 | 68 | .19 |
| Median WBC, 109/L (range) | 83 (9-436) | 77 (9-709) | .39 |
| Median Hgb, g/dL (range) | 12.3 (1.3-16.1) | 12.0 (4.6-16.6) | .26 |
| Median platelets, × 109/L (range) | 158 (33-316) | 155 (12-451) | .57 |
| Median, LDH, IU (range) | 212 (92-950) | 198 (39-1389) | .20 |
Hgb indicates hemoglobin.
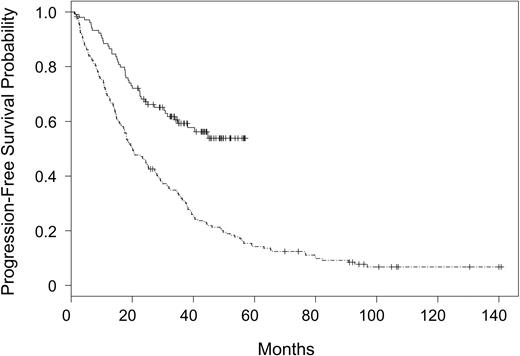
Patients assigned to receive fludarabine and rituximab had a higher incidence of complete response (0.38 versus 0.20, P = .002) and overall response (0.84 versus 0.63, P = .0003) as compared with fludarabine alone. Multivariate analyses of the effect of treatment assignment on response, controlling for sex, age, WBC, LDH, and stage, showed that the treatment effect remained essentially unchanged after controlling for these covariates (data not shown). In parallel with response outcome, patients assigned to receive fludarabine and rituximab had a significantly improved PFS (P < .0001) as compared with those patients assigned to receive fludarabine alone. These results are summarized in Figure 1. The 2-year PFS probability was 0.67 (CI, 0.58-0.76) in patients receiving fludarabine and rituximab versus 0.45 (CI, 0.37-0.52) in patients receiving fludarabine alone. The treatment effect was unchanged in a multivariate analysis controlling for sex, age, WBC, LDH, stage, and splenomegaly. Fludarabine alone treatment assignment (P < .0001), high WBC (P = .0003), and high LDH (P = .01) were significantly associated with early progression (Table 2).
Progression-free survival for patients assigned to rituximab and fludarabine on CALGB 9712 versus fludarabine on CALGB 9011. CALGB 9712 (solid line) and CALGB 9011 (divided line).
Progression-free survival for patients assigned to rituximab and fludarabine on CALGB 9712 versus fludarabine on CALGB 9011. CALGB 9712 (solid line) and CALGB 9011 (divided line).
Hazard ratios from multivariate analysis of progression-free survival
Clinical predictor (hazard ratio comparison) . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Treatment, CALGB 9712 vs CALGB 9011 | 2.89 (2.02-4.14) | < .0001 |
| WBC, 75th vs 25th percentile | 1.25 (1.11-1.42) | .0003 |
| Age, 75th vs 25th percentile | 1.19 (0.96-1.47) | .11 |
| LDH, 75th vs 25th percentile | 1.15 (1.03-1.29) | .01 |
| Sex, male vs female | 1.09 (0.79-1.49) | .61 |
| Rai stage, high vs intermediate | 0.93 (0.70-1.24) | .61 |
| Splenomegaly, present vs absent | 1.08 (0.79-1.48) | .61 |
Clinical predictor (hazard ratio comparison) . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Treatment, CALGB 9712 vs CALGB 9011 | 2.89 (2.02-4.14) | < .0001 |
| WBC, 75th vs 25th percentile | 1.25 (1.11-1.42) | .0003 |
| Age, 75th vs 25th percentile | 1.19 (0.96-1.47) | .11 |
| LDH, 75th vs 25th percentile | 1.15 (1.03-1.29) | .01 |
| Sex, male vs female | 1.09 (0.79-1.49) | .61 |
| Rai stage, high vs intermediate | 0.93 (0.70-1.24) | .61 |
| Splenomegaly, present vs absent | 1.08 (0.79-1.48) | .61 |
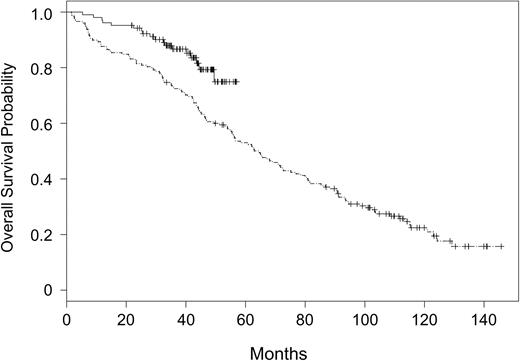
Overall survival outcome according to treatment assignment to fludarabine and rituximab (CALGB 9712) or fludarabine alone (CALGB 9011) is summarized in Figure 2. Patients assigned to receive fludarabine and rituximab had significantly improved OS (P = .003) as compared with those patients assigned to receive fludarabine alone. The 2-year OS probability was 0.93 (CI, 0.88-0.98) in patients receiving fludarabine and rituximab versus 0.81 (CI, 0.75-0.87) in patients receiving fludarabine alone. The treatment effect was unchanged in a multivariate analysis controlling for the covariates listed in Table 1. Fludarabine-alone treatment assignment (P = .0006), high WBC (P = .009), older age (P = .001), and high LDH (P = .02) were significantly associated with shortened survival (Table 3).
Overall survival for patients assigned to rituximab and fludarabine on CALGB 9712 versus fludarabine on CALGB 9011. CALGB 9712 (solid line) and CALGB 9011 (divided line).
Overall survival for patients assigned to rituximab and fludarabine on CALGB 9712 versus fludarabine on CALGB 9011. CALGB 9712 (solid line) and CALGB 9011 (divided line).
Hazard ratios from multivariate analysis of overall survival
Clinical predictor (hazard ratio comparison) . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Treatment, CALGB 9712 vs CALGB 9011 | 2.59 (1.50-4.46) | .0006 |
| WBC, 75th vs 25th percentile | 1.23 (1.05-1.44) | .009 |
| Age, 75th vs 25th percentile | 1.59 (1.18-1.93) | .001 |
| LDH, 75th vs 25th percentile | 1.18 (1.03-1.36) | .02 |
| Sex, male vs female | 1.25 (0.86-1.81) | .24 |
| Rai stage, high vs intermediate | 1.22 (0.88-1.69) | .23 |
| Splenomegaly, present vs absent | 1.20 (0.84-1.71) | .33 |
Clinical predictor (hazard ratio comparison) . | Hazard ratio (95% CI) . | P . |
|---|---|---|
| Treatment, CALGB 9712 vs CALGB 9011 | 2.59 (1.50-4.46) | .0006 |
| WBC, 75th vs 25th percentile | 1.23 (1.05-1.44) | .009 |
| Age, 75th vs 25th percentile | 1.59 (1.18-1.93) | .001 |
| LDH, 75th vs 25th percentile | 1.18 (1.03-1.36) | .02 |
| Sex, male vs female | 1.25 (0.86-1.81) | .24 |
| Rai stage, high vs intermediate | 1.22 (0.88-1.69) | .23 |
| Splenomegaly, present vs absent | 1.20 (0.84-1.71) | .33 |
Examination of the toxicity observed in the 2 treatment groups is summarized in Table 4. This demonstrated that neutropenia, hypotension, and dyspnea were more commonly noted in the fludarabine and rituximab treatment group, but no difference in other hematologic toxicity (anemia or thrombocytopenia) or infection was noted.
Grade 3 or 4 Toxicity in CALGB 9712 and CALGB 9011
Type . | 9712 toxicity rate . | 9011 toxicity rate . | P . |
|---|---|---|---|
| Platelets | 0.16 | 0.11 | > .2 |
| Hgb | 0.07 | 0.10 | > .2 |
| Granulocytes | 0.61 | 0.21 | < .001 |
| Infection | 0.26 | 0.23 | > .2 |
| Herpes virus | 0.12 | 0.09 | > .2 |
| Dyspnea | 0.14 | 0.03 | .002 |
| Hypotension | 0.05 | 0.01 | .03 |
Type . | 9712 toxicity rate . | 9011 toxicity rate . | P . |
|---|---|---|---|
| Platelets | 0.16 | 0.11 | > .2 |
| Hgb | 0.07 | 0.10 | > .2 |
| Granulocytes | 0.61 | 0.21 | < .001 |
| Infection | 0.26 | 0.23 | > .2 |
| Herpes virus | 0.12 | 0.09 | > .2 |
| Dyspnea | 0.14 | 0.03 | .002 |
| Hypotension | 0.05 | 0.01 | .03 |
Discussion
In this report, we provide retrospective comparative data of CLL patient outcome enrolled on 2 multicenter cooperative group trials where treatment fludarabine and rituximab or fludarabine alone was used for initial treatment of this disease. The eligibility criteria for these 2 clinical trials were virtually identical. The pretreatment demographics of patients enrolled and subsequently compared in this analysis are either similar with exception of a significantly higher frequency of thrombocytopenia in the fludarabine and rituximab treatment group. Despite the marked similarities between patient groups, our data demonstrate that those assigned to receive fludarabine and rituximab on CALGB 9712 had a significantly improved PFS and OS as compared with those assigned to receive fludarabine alone as part of CALGB 9011. Because this analysis focuses predominately on the 2-year time period after initiation of treatment, none of the patients enrolled on CALGB 9011 received rituximab therapy that was not available during that period. The addition of rituximab to fludarabine did not appear to increase the risk of grade III or IV infections or herpes virus infections. Furthermore, a multivariate analysis examining treatment (CALGB 9712 versus CALGB 9011) and other pretreatment clinical and laboratory features demonstrated that treatment assignment was as good as or better than WBC and age at predicting PFS and OS. Similar data using the fludarabine, cyclophosphamide, and rituximab regimen have been reported for previously treated patients with CLL by the MD Anderson Cancer Center CLL group where PFS and OS were significantly prolonged as compared with those receiving other non–rituximab-containing fludarabine combinations.15-17 These data suggest that the addition of rituximab to fludarabine therapy in symptomatic CLL has great promise for the treatment of this disease and should be explored further in larger randomized phase 3 trials.
Limitations of this analysis include the retrospective nature of the comparison and potential different patient molecular characteristics not compared that could explain the discordant outcome independent of treatment that was observed in this study. While the eligibility criteria of CALGB 9712 and CALGB 9011 are similar with exception to the former study allowing performance status 0 to 3 patients (versus 0-2 on CALGB 9011) and excluding those with need for chronic corticosteroid administration, the analysis reported herein was not planned at the time of initiation of these 2 studies. In addition, schedule of rituximab administration used in the 2 treatment arms of CALGB 9712 was not identical. Further, since few of the patients who failed fludarabine therapy on CALGB 9011 would have received rituximab as salvage therapy, it is possible that later administration of rituximab could have resulted in diminishment of the survival advantage observed. The current comparison does not address whether concurrent treatment with fludarabine and rituximab is superior to sequential therapy. Indeed, progression-free survival and overall survival between these 2 groups of patients remains similar at this time, suggesting that rituximab either concurrently or sequentially may yield similar outcomes for patients with CLL. While the efficacy of rituximab monotherapy in relapsed CLL is limited, with virtually no complete responses, the finding of similar outcome in the concurrent and sequential treatment arm raises the possibility that attaining an early CR (ie, 2 months after treatment) may not be important to predicting long-term outcome.8,9,18 As responses to rituximab can often improve following completion of therapy, future studies examining the bone marrow later in treatment will be required to accurately estimate the importance of CR as a surrogate end point to prolonged PFS and OS. All of these limitations mandate caution in overinterpreting the results reported herein beyond strong consideration of fludarabine and rituximab–based combinations in future randomized phase 3 trials. Thus, future efforts should focus on confirming these results and clinical importance of minimal residual disease at completion of therapy. In addition, it will be important to determine whether select molecular risk factors such as having VH (heavy chain variable region) un-mutated status,16,19 ZAP-70 (zeta-associated protein 70) protein overexpression,20 or p53 dysfunction21,22 adversely influence outcome of symptomatic patients with CLL receiving fludarabine and rituximab–based combination therapies.
Appendix
The following institutions participated in this study: Brooklyn CCOP, Methodist Hospital, Brooklyn, NY–Sameer Rafla, MD; CALGB Statistical Office, Durham, NC–Stephen George, PhD, supported by CA33601; Case Western-MetroHealth Med Center, Cleveland, OH–Edward Mansour, MD, supported by CA14548; Christiana Care Health Services, CCOP, Wilmington, DE–Stephen Grubbs, MD, supported by CA45418; Community Hospital-Syracuse CCOP, Syracuse, NY–Jeffrey Kirshner, MD, supported by CA45389; Dana-Farber Cancer Institute, Boston, MA–George P. Canellos, MD, supported by CA32291; Dartmouth Medical School, Lebanon, NH–Marc Ernstoff, MD, supported by CA04326; Duke University Medical Center, Durham, NC–Jeffrey Crawford, MD, supported by CA47577; Fox Chase Cancer Center, Philadelphia, PA–Peter O'Dwyer, MD, supported by CA27525; Georgetown University Medical Center, Washington, DC–Edward Gelmann, MD, supported by CA77597; Grady Memorial Hospital CCOP, Atlanta, GA–J. William Eley, MD, MPH; Illinois Oncology Research Associates, Peoria–John W. Kugler, MD; Indiana University Medical Center, Indianapolis–Patrick Loehrer, Sr, MD. supported by CA49883; Johns Hopkins University, Baltimore, MD–Arlene Forastiere, MD, supported by CA16116; Kaiser Permanente CCOP, San Diego, CA–JonathanA. Polikoff, MD, supported by CA45374; Massachusetts General Hospital, Boston, MA–Michael L. Grossbard, MD, supported by CA12449; Mayo Clinic, Rochester, MN–Thomas Habermann, MD, supported by CA13650; McGill Department of Oncology, Montreal, QC, Canada–Brian Leyland-Jones, MD, supported by CA31809; Medical College of Wisconsin, Milwaukee–Tom Anderson, MD; Medical University of South Carolina, Charleston–Mark Green, MD, supported by CA03927; Memorial Sloan-Kettering Cancer Center, New York, NY–Clifford Hudis, MD, supported by CA77651; Mercy Hospital CCOP, Scranton, Scranton, PA–William Heim, MD; Metro-Minnesota CCOP, Abbott-NW, Minneapolis–Patrick Flynn, MD; Milwaukee CCOP, Milwaukee, WI–Ronald Hart, MD, supported by CA45400; Mount Sinai Medical Center CCOP-Miami, Miami Beach, FL–Rogerio Lilenbaum, MD, supported by CA45564; Mount Sinai School of Medicine, New York, NY–Lewis Silverman, MD, supported by CA04457; New York University Medical Center, New York, NY–Ronald Blum, MD; North Shore-Long Island Jewish Medical Center, Manhasset, NY–Daniel R. Budman, MD, supported by CA35279; Rhode Island Hospital, Providence–Louis A. Leone, MD, supported by CA08025; Roswell Park Cancer Institute, Buffalo, NY–Ellis Levine, MD, supported by CA02599; Rush Presbyterian, St Luke's Medical Center, Chicago, IL–Jules Harris, MD; South New Jersey CCOP, Camden–Jack Goldberg, MD, supported by CA54697; Southeast Cancer Control Consortium Inc CCOP, Goldsboro, NC–James N. Atkins, MD, supported by CA45808; Southern Nevada Cancer Research Foundation CCOP, Las Vegas–John Ellerton, MD, supported by CA35421; St Francis Hospital, Oklahoma CCOP, Tulsa–Alan Keller, MD, FACP; St Michael's Medical Center Tri-County CCOP, Paterson, NJ–Arnold D, Rubin, supported by CA60247; SUNY Upstate Medical University, Syracuse, NY–Stephen L. Graziano, MD, supported by CA21060; The Ohio State University Medical Center, Columbus–Clara D. Bloomfield, MD, supported by CA77658; Tufts/New England Medical Center, Boston, MA–Daniel Karp, MD, supported by CA07190; University of Alabama Birmingham, Birmingham–Robert Diasio, MD, supported by CA47545; University of California at San Diego, San Diego–Stephen L. Seagren, MD, supported by CA11789; University of Chicago Medical Center, Chicago, IL–Gini Fleming, MD, supported by CA41287; University of Illinois at Chicago, Chicago–Lawrence E. Feldman, MD, supported by CA74811; University of Iowa Hospitals, Iowa City–Gerald H. Clamon, MD, supported by CA47642; University of Maryland Cancer Center, Baltimore–Martin Edelman, MD, supported by CA31983; University of Massachusetts Medical Center, Worcester–F. Pankaj Bhargava, MD, supported by CA37135; University of Miami, Miami, FL–Peter Cassileth, MD; University of Minnesota, Minneapolis–Bruce A. Peterson, MD, supported by CA16450; University of Missouri/Ellis Fischel Cancer Center, Columbia–Michael C. Perry, MD, supported by CA12046; University of Nebraska Medical Center, Omaha–Anne Kessinger, MD, supported by CA77298; University of North Carolina at Chapel Hill, Chapel Hill–Thomas C. Shea, MD, supported by CA47559; University of Pennsylvania, Philadelphia–John Glick, MD, supported by CA15488; University of Pittsburgh, Pittsburgh, PA–John Kirkwood, MD, supported by CA39229; University of Pretoria, Pretoria, South Africa–Geoffrey Falkson, MD, supported by CA59307; University of Rochester, Rochester, NY–John Bennett, MD, supported by CA11083; University of Tennessee Memphis, Memphis–Harvey B. Niell, MD, supported by CA47555; University of Wisconsin, Madison–James Stewart, MD, supported by CA21076; Vermont Cancer Center, Burlington–Hyman B. Muss, MD, supported by CA77406; Virginia Commonwealth University MB CCOP, Richmond–John D. Roberts, MD, supported by CA52784; Wake Forest University School of Medicine, Winston-Salem, NC–David D. Hurd, MD, supported by CA03927; Walter Reed Army Medical Center, Washington, DC–John C. Byrd, MD, supported by CA26806; Walter Reed Army Medical Center, Washington, DC–Joseph J. Drabeck, MD, supported by CA26806; Washington University School of Medicine, St Louis, MO–Nancy L. Bartlett, MD, supported by CA77440; Weill Medical College of Cornell University, New York, NY–Scott Wadler, MD, supported by CA07968.
Prepublished online as Blood First Edition Paper, May 11, 2004; DOI 10.1182/blood-2004-03-0796.
The research for CALGB 9011 and 9712 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (R.L. Schilsky, MD, Chairman), and by grants to the Southwest Oncology Group (CA32102) and Eastern Cooperative Oncology Group (CA21115). Support was also from the Sidney Kimmel Cancer Research Foundation (J.C.B.), Leukemia and Lymphoma Society of America (J.C.B.), and D. Warren Brown Foundation (J.C.B.), Peter Jay Sharp Foundation (K.R.), and Joel Finkelstein Cancer Foundation (K.R.). J.C.B. is a clinical scholar of the Leukemia and Lymphoma Society of America.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal