Abstract
Bone marrow-derived endothelial precursor cells incorporate into neovasculature and have been successfully used as vehicles for gene delivery to brain tumors. To determine whether systemically administered Sca1+ bone marrow cells labeled with superparamagnetic iron oxide nanoparticles can be detected by in vivo magnetic resonance imaging in a mouse brain tumor model, mouse Sca1+ cells were labeled in vitro with ferumoxides-poly-l-lysine complexes. Labeled or control cells were administered intravenously to glioma-bearing severe combined immunodeficient (SCID) mice. Magnetic resonance imaging (MRI) was performed during tumor growth. Mice that received labeled cells demonstrated hypointense regions within the tumor that evolved over time and developed a continuous dark hypointense ring at a consistent time point. This effect was not cleared by administration of a gadolinium contrast agent. Histology showed iron-labeled cells around the tumor rim in labeled mice, which expressed CD31 and von Willebrand factor, indicating the transplanted cells detected in the tumor have differentiated into endothelial-like cells. These results demonstrate that MRI can detect the incorporation of magnetically labeled bone marrow-derived precursor cells into tumor vasculature as part of ongoing angiogenesis and neovascularization. This technique can be used to directly identify neovasculature in vivo and to facilitate gene therapy by noninvasively monitoring these cells as gene delivery vectors. (Blood. 2005;105:420-425)
Introduction
Angiogenesis is essential for tumor growth and the development of metastasis.1 Migration and proliferation of endothelial cells originating from local capillaries are thought to be the primary mechanism for angiogenesis in the adult in the presence of proangiogenic stimuli. Evidence demonstrates that bone marrow-derived endothelial precursor cells (EPCs) also contribute to the endothelial lining of adult neovasculature in both physiologic and pathologic angiogenesis.2,3 Lyden et al4 have demonstrated that recruitment of bone marrow-derived endothelial or hematopoietic precursors are necessary for early angiogenesis and tumor growth. The population of Sca1+ mouse bone marrow-derived cells is enriched for EPCs,5 and these cells have been shown to incorporate and differentiate into endothelial-like cells in tumor vasculature following infusion into the circulation in a mouse glioma model.6 Secondary to their ability to home to sites of angiogenesis while contributing little if at all to quiescent endothelium, bone marrow-derived stem cells that are endothelial precursors, including mouse Sca1+ cells, are being investigated as angiogenesis-selective gene-targeting vectors.6,7
Vector toxicity, poor in vivo transduction efficiency, and lack of tumor specificity are major limitations to the successful clinical application of cancer gene therapy. EPCs represent a potential vector system that may selectively target a pathophysiologic process intrinsic to tumor biology, thereby limiting systemic toxicity. Recently, it has been shown that genetically modified EPCs can selectively deliver a cytotoxic gene to tumor-induced neovasculature.6,7
It is important to determine the distribution of cells containing a toxic or therapeutic gene to optimize delivery and measure potential adverse effects. Our approach is to magnetically label Sca1+ stem cells, making them detectable by magnetic resonance imaging (MRI), and to serially image tumor-bearing animals to determine the temporal-spatial distribution of those labeled cells that migrate to the brain during tumor growth. We labeled cells using a clinically approved, dextran-coated superparamagnetic iron oxide (SPIO) nanoparticle contrast agent (ferumoxides, FE) with poly-L-lysine (PLL), forming a complex through electrostatic interactions known as FE-PLL.8 Previous studies have shown this cell labeling technique sequesters SPIO nanoparticles in endosomes in the cytoplasm and is nontoxic to a variety of stem cells and immune cells.8-11 We have shown in several animal models that this methodology can be used to track cell transplants by MRI.12-14 FE-PLL-labeled cells appear hypointense (dark) on MRI as a result of significant T2 and T2* relaxation time shortening by SPIO nanoparticles. Grouping or clumping of these SPIO nanoparticles within endosomes enhances the decrease in signal intensity on MRI localized at the labeled cell or cell groups.11 Endosomal incorporation of the SPIO nanoparticles following incubation with FE-PLL has been demonstrated by transmission electron microscopy.15
Numerous methods have been applied to imaging tumor angiogenesis and neovascularization by many imaging modalities, including MRI.16 However, direct and specific noninvasive high-resolution imaging of tumor neovasculture remains a challenge.17 Angiogenesis is assessed by MRI using either indirect methods of detecting tumor perfusion and microvascular density such as dynamic contrast-enhanced scanning or via detection of antibody-targeted contrast agents to endothelial cell antigens.16-19 Dynamic contrast MRI (DCE) methods usually require complex modeling of changes in signal intensity over time, to determine an estimate of the vascular leakage space, permeability surface area, and tumor blood volume and flow. These have been correlated to microvascular density18,19 and are indirect indicators of areas undergoing angiogenesis. Intravascular agents that are larger molecules also allow for the enhancement and detection of tumor vessels transporting the agent. They do not provide a means to image cellular incorporation into tumor vessels. Contrast agents targeted to specific cell receptors with antibodies17 can potentially identify new vessels or endothelial cells. Monoclonal antibody-based methods depend on the selectivity and specificity of binding to target endothelium surface antigens. All the above approaches have been used to evaluate tumor angiogenesis and, in some cases (ie, perfusion imaging), response to antiangiogenic agents. However, they are not informative in detecting the incorporation of genetically altered cells into tumor vasculature.
In vitro labeling of Sca1+ bone marrow cells with SPIO nanoparticles has the potential to detect the migration of these cells toward angiogenic stimuli and their incorporation within developing tumor neovasculature directly by MRI. The purpose of this study was to determine whether in vivo MRI could detect the incorporation of FE-PLL-labeled Sca1+ cells into angiogenic vasculature in a mouse glioma model.
Materials and methods
Animal model
Animal experiments were performed in accordance with National Institutes of Health (NIH) guidelines for animal care and use. Severe combined immunodeficient (SCID) mice with implanted gliomas were administered intravenous Sca1+ bone marrow cells, either FE-PLL-labeled or control cells. A total of 18 glioma-bearing mice with Sca1+ cell transplants were imaged, which included 12 injected with labeled cells, 4 with unlabeled cells, and 2 receiving killed labeled cells, in 3 experimental groups. In group 1, mice were infused with FE-PLL-labeled (n = 2) or control unlabeled cells (n = 2) 5 to 6 days after tumor implantation into the brain, and MRI was performed once. In group 2, mice were infused with FE-PLL-labeled (n = 8) or control killed labeled cells (n = 2) 2 days after tumor implantation into the brain. Serial MRI was performed up to 13 days from infusion of cells. In group 3, sublethally irradiated mice were infused with FE-PLL-labeled (n = 2) or control unlabeled cells (n = 2) 2 days before tumor implantation. Serial MRI was performed up to 12 days from infusion of cells.
For tumor implant 5 × 105 RT2 rat glioma cells (American Type Culture Collection, Manassas, VA) were implanted by intracranial injection using stereotactic guidance into 5-week-old SCID mice (National Cancer Institute [NCI], Frederick, MD). Briefly, mice were anesthetized with xylazine (10 mg/kg) and ketamine (90 mg/kg) intraperitoneally and placed in a mouse stereotactic frame. The skull was exposed, and a burr hole was drilled through the skull 2 mm lateral and 1 mm anterior to the bregma. Under stereotactic guidance a Hamilton syringe with a 27-gauge needle was inserted 2.5 mm into the brain parenchyma, and the tumor cells were injected over 5 minutes in a volume of 1 μL. The burr hole was then filled with bone wax and the scalp sutured. Mice in group 3 received whole-body irradiation of 3.5 Gy using a Gamma irradiator (JL Shepherd, San Fernando, CA) 4 days prior to tumor implant.
Cell labeling
Sca1+ cells were isolated from the marrow of Swiss Webster mice (NCI). Briefly, mice were killed using bottled CO2. Femurs were removed and flushed with phosphate-buffered saline to remove the marrow. The Sca1+ cells were then isolated using anti-Sca1 magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and put in culture with StemSpan medium (StemCell Technologies, Vancouver, BC) containing thrombopoietin, Flt3 ligand, and stem cell factor for 3 to 7 days. Cells were labeled with FE-PLL by incubating with a complex of SPIO nanoparticles (Ferumoxides, Feridex IV; Berlex Laboratories, Wayne, NJ) and poly-l-lysine (388 kDa; Sigma, St Louis, MO), using procedures previously described.8,9,11 Iron concentration for mouse bone marrow cells is 1 to 5 pg iron per cell as determined by nuclear magnetic resonance relaxometry.11 All cells were washed in phosphate-buffered saline to remove any excess iron particles from the cell surface. Labeling efficiency of FE-PLL into Sca1+ cells was approximately 100%. Controls were unlabeled (groups 1, 3) or FE-PLL-labeled Sca1+ cells (group 2) killed by incubation with 4% paraformaldehyde for 15 minutes.
Cell transplants
Sca1+ cells were infused by intravenous injection of 5 × 105 cells (either FE-PLL-labeled or control) via tail vein. Mice received labeled or control cells at different time points: either 5 to 6 days after implantation of tumor cells (group1), 2 days after tumor cells (group 2), or 2 days before (group 3); in these mice sublethal irradiation was followed by cell transplantation 2 days later and then tumor implant 4 days later. Mice receiving either FE-PLL or control Sca1+ cells had a similar clinical course and met predefined euthanasia criteria by 14 to 16 days after tumor implant.
Magnetic resonance imaging
Mice were imaged during the 2-week period of tumor growth at 7 Teslas (T) using a 21-cm horizontal-bore MR unit (Bruker, Billerica, MA) with 39 G/cm gradients and a 35-mm transmit-receive birdcage volume coil. Mice were imaged in a head holder with 1.5% to 2% isoflurane anesthesia by nosecone and physiologic monitoring. MRI was performed using 2-dimensional (2D) T2-star-weighted (T2*)-weighted gradient echo (TR/TE = 400-450/3.8-4.2 ms, number of excitations [nex] = 10), 2D T2 weighted spin echoes (TR/TE = 2200/7.8 ms; 3 echoes, nex = 2), and/or 3-dimensional (3D) T2-weighted RARE imaging (TR/TE = 1300/7.1 ms; rare factor 8, nex = 1). Resolution in 2D images was 500 × 80 × 70 μm and in 3D was 106 × 93 × 93 μm to 117 μm isotropic.
To determine whether slow venous blood flow was a source of hypointense regions within or demarcating the edge of the tumor, or both, a paramagnetic contrast agent was used on 2 mice from group 2. Postcontrast-enhanced 2D gradient echo images were obtained 20 minutes following a subcutaneous injection of 0.4 mmol/kg Gadopentetate dimeglumine (Gd-DTPA, Magnevist; Berlex Laboratories, Wayne, NJ).
Mice were killed at selected time points for histologic and ex vivo MRI analysis of the tumors. The mice were deeply anesthetized using xylazine (10 mg/kg) intraperitoneally and ketamine (90 mg/kg) intraperitoneally. The mice were then killed by exsanguination and perfused with 20 mL phosphate-buffered saline (PBS) followed by 20 mL 4% paraformaldehyde (PFA). Brains were removed immediately and fixed overnight at 4°C in 4% PFA followed by dehydration in 30% sucrose in PFA for 24 hours. Fixed brains were imaged in a 7-Tesla vertical bore microimaging system (Bruker) with 95 G/cm gradients, using T2*W 3D gradient echoes, at 45 to 50 μm isotropic resolution (TR/TE = 280/6.0 ms).
Histopathology
Tissue preparation was performed following in vivo MRI analysis. Histopathology was performed on 6-μm to 10-μm sections from paraformaldehyde-fixed, paraffin-embedded brains. Both Prussian blue staining (ferric hexacyanoferrate and hydrochloric acid; Sigma, St. Louis, MO) and immunohistochemistry were performed following deparaffinization and rehydration of the slides. Prussian blue was performed by incubating sections for 30 minutes with 2% potassium ferrocyanide (Perl Prussian blue reagent) in 3.7% hydrochloric acid, washed again, and counterstained with nuclear fast red on sections from the FE-PLL-labeled groups and controls. Staining for CD31 was performed by Pathology/Histotechnology Laboratory at NCI using standard immunohistochemistry methods. For the expression of activated endothelial cell marker, immunochemical staining for von Willebrand factor (VWF) was performed on 6-μm deparaffinized, rehydrated, and endogenous peroxidase-blocked sections. After blocking the nonspecific binding sites with serum, sections were flooded with anti-VWF antibody (polyclonal anti-human VWF antibody; Dako Cytomation, Carpenteria, CA) at 1:100 dilution and incubated at room temperature for 1 hour. After washing thoroughly the sections were flooded with secondary antibody conjugated with peroxidase (Dako Cytomation) and incubated for 30 minutes, washed, and then incubated with activated diaminobenzidine (DAB) solution for 3 to 5 minutes. After DAB enhancement, slides were washed, stained for Prussian blue, counter-stained with nuclear fast red, dehydrated, and photographed. Microscopy was performed using a Zeiss Axioplan 2 imaging microscope with AxioVision 4.1 imaging software, AxioCam HRc camera, and Zeiss objectives Fluar 10×/0.50, Plan-Apochrome 20×/0.75, and Plan-Neofluar 100×/1.30 oil (Carl Zeiss Vision, Oberkochen, Germany).
Results
MRI findings varied depending on whether mice received FE-PLL-labeled, unlabeled, or labeled killed Sca1+ cells. The RT2 glioma reaches an advanced stage and is usually fatal to mice within 3 weeks after implantation into the brain. All animals tolerated infusion of FE-PLL-labeled and unlabeled EPCs, and no difference in clinical course was observed between animals with implanted tumor receiving labeled cells and the controls receiving unlabeled or killed labeled cells.
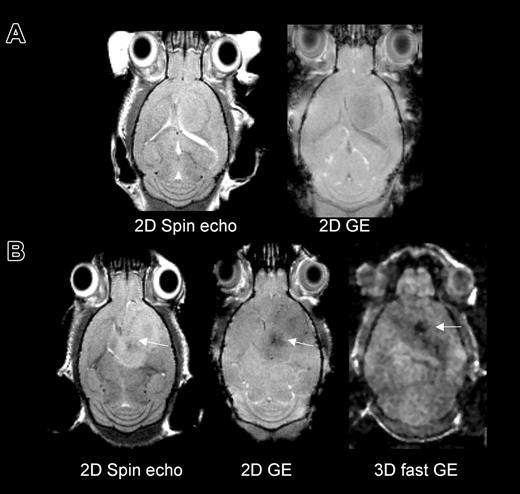
MRI was first performed in group 1 to assess the detectability of cells on different MRI pulse sequences. For this group, MRI was performed on day 12 following tumor implantation mice with magnetically labeled or unlabeled cells infused on day 5. A dark (hypointense) region as compared with contralateral “normal” brain was detected in the area within and surrounding the tumor on all sequences. The susceptibility effect progressively increased in intensity from a spin-echo to a fast gradient-echo sequence (Figure 1). Serial MRI studies to determine the time course were performed on groups 2 and 3. These revealed that in mice that received Sca1+ FE-PLL-labeled cells within 2 days of tumor implant, hypointense regions in the tumor initially appear at about 9 days from transplantation and ultimately appear to circumscribe the tumor rim evolving into a continuous dark ring. Figure 2 shows time course MRI performed from day 4 to 11 of a mouse that received labeled cells 2 days after tumor implant. At day 4 the needle tract from the tumor implantation was evident on MRI; there were no other hypointense effects. At 9 days, a slight hyperintense area around the original needle tract is observed which is indicative of edema from growing tumor. Hypointense areas compared with contralateral hemisphere were also observed at this time point in and around the tumor. At day 11, a ring around the tumor was observed (Figure 2), on both in vivo and ex vivo MRI. Linear projections into the center of this tumor were also observed in vivo and were clearly delineated at higher resolution on ex vivo MR. Five of 6 mice that were infused with FE-PLL-labeled cells and imaged 10 or more days later exhibited a hypointense ring surrounding the tumor.
MRI in mice that received either no cell transplant or magnetically labeled Sca1+ bone marrow cells at day 5 of tumor growth and were imaged 7 days later to investigate detection of labeled cells by several MRI techniques. (A) Mouse with 12-day tumor and no cell transplant on 2D spin echo and gradient echo (GE) images. (B) Mouse with 12-day tumor and 7 days from labeled cell transplant on spin echo, GE, and fast GE images. Hypointense areas in the tumor were detectable with all sequences in the labeled mouse. The fast gradient echo had the greatest sensitivity to iron and the least anatomic definition.
MRI in mice that received either no cell transplant or magnetically labeled Sca1+ bone marrow cells at day 5 of tumor growth and were imaged 7 days later to investigate detection of labeled cells by several MRI techniques. (A) Mouse with 12-day tumor and no cell transplant on 2D spin echo and gradient echo (GE) images. (B) Mouse with 12-day tumor and 7 days from labeled cell transplant on spin echo, GE, and fast GE images. Hypointense areas in the tumor were detectable with all sequences in the labeled mouse. The fast gradient echo had the greatest sensitivity to iron and the least anatomic definition.
Serial MRI in mice that received magnetically labeled Sca1+ bone marrow cells, group 2. (A) 3D RARE images showing evolution of hypointense regions within and around tumor, due to incorporation of labeled cells into vascular structures or within parenchyma of tumor. Arrowheads indicate needle tract on day 4 after tumor implantation and growing tumor area on days 9 and 11. Hypointense areas in and around the tumor are evident at day 9, and a hypointense rim surrounding the tumor is observed on day 11. (B) Ex vivo gradient echo MRI of the same mouse at day 11.
Serial MRI in mice that received magnetically labeled Sca1+ bone marrow cells, group 2. (A) 3D RARE images showing evolution of hypointense regions within and around tumor, due to incorporation of labeled cells into vascular structures or within parenchyma of tumor. Arrowheads indicate needle tract on day 4 after tumor implantation and growing tumor area on days 9 and 11. Hypointense areas in and around the tumor are evident at day 9, and a hypointense rim surrounding the tumor is observed on day 11. (B) Ex vivo gradient echo MRI of the same mouse at day 11.
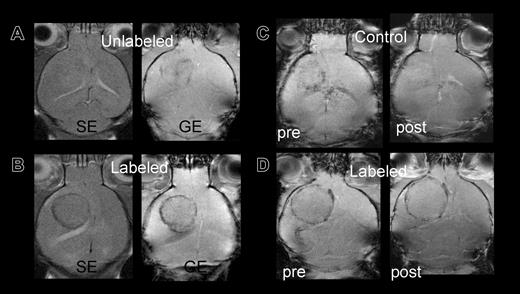
Control mice infused with unlabeled or killed FE-PLL-labeled cells did not develop a hypointense ring surrounding the tumor performed 10 or more days following RT2 cell implantation. Control versus labeled mice are shown in Figure 3A-B. One mouse that received killed labeled cells on day 3 following RT2 cell implantation had slight decrease in signal intensity around the tumor on day 12 after infusion of Sca1+ cells that was thought to be due to blood flow effects. To discriminate the signal intensity decrease because of slow venous blood flow from the susceptibility artifact for FE-PLL-labeled cells, Gd-DTPA was administered. An increase in signal intensity and disappearance of hypointense regions occurred following the infusion of Gd-DTPA in the mouse that received killed labeled Sca1+ cells, whereas the hypointense area remained in a mouse with FE-PLL-labeled viable cells (Figure 3C-D).
Control versus labeled mice: group 3 mice imaged at day 10 after cell transplantation. (A) Signal intensity in tumor of control mouse with unlabeled cells on either spin echo (SE) or gradient echo (GE) sequences are almost isointense with contralateral hemisphere. (B) Mouse receiving FE-PLL-labeled cells demonstrates clear ring at the tumor periphery on both SE and GE. (C-D) Post-Gd-DTPA contrast-enhanced MRIs to differentiate between slow blood flow in patent vessels at the tumors' edge and the presence of magnetically labeled cells. The hypointense area in a group 2 control mouse (C) that received killed labeled cells becomes isointense with surrounding brain after contrast, indicating the Gd-DTPA in the area increased the signal intensity, whereas the dark ring persists in the mouse with labeled cells (D).
Control versus labeled mice: group 3 mice imaged at day 10 after cell transplantation. (A) Signal intensity in tumor of control mouse with unlabeled cells on either spin echo (SE) or gradient echo (GE) sequences are almost isointense with contralateral hemisphere. (B) Mouse receiving FE-PLL-labeled cells demonstrates clear ring at the tumor periphery on both SE and GE. (C-D) Post-Gd-DTPA contrast-enhanced MRIs to differentiate between slow blood flow in patent vessels at the tumors' edge and the presence of magnetically labeled cells. The hypointense area in a group 2 control mouse (C) that received killed labeled cells becomes isointense with surrounding brain after contrast, indicating the Gd-DTPA in the area increased the signal intensity, whereas the dark ring persists in the mouse with labeled cells (D).
Group 3 mice were sublethally irradiated in an effort to increase the number of infused EPCs that would respond to angiogenic stimuli within the developing tumor vasculature by decreasing the contribution of endogenous EPCs. MR images performed early (day 4) in the time course of tumor growth demonstrated compact hypointense regions along or adjacent to the tumor implant needle tract in both mice receiving FE-PLL-labeled or unlabeled Sca1+ cells (data not shown). This is presumed to be clinically insignificant hemorrhage secondary to whole body irradiation-induced thrombocytopenia and possibly more friable intracerebral blood vessels in the irradiated animals. By 10 days following tumor implantation the labeled animals developed a hypointense ring and were distinguishable from controls. Other than the tendency to see more early hemorrhage the effects in irradiated mice were analogous to the effects seen in the nonirradiated mice (group 2).
Histology with vascular marker CD31 and Prussian blue (PB) staining shows iron-positive cells at the tumor rim (Figure 4B) and does not show thrombi within the tumor vasculature. The control mouse (Figure 4A) is Prussian blue-negative and shows the distribution of CD31+ areas (brown stain). The CD31 stain was not intense enough to be apparent simultaneously in areas where there is PB staining of intracellular iron. A section stained for vascular marker CD31 shown before and after staining for iron with Prussian blue (Figure 4C-D) demonstrates that a large proportion of iron-labeled cells on the tumor periphery are CD31+. A section stained for both the vascular marker von Willebrand factor and Prussian blue show cells that are both VWF positive and PB positive (Figure 4E).
Histopathology of brain tumor 7 to 11 days after systemic cell transplant. (A) CD31 (platelet endothelial cell adhesion molecule 1 [PECAM-1]) and Prussian blue stains of fixed brain in a mouse receiving unlabeled cells. Slide is positive for CD31 (brown) and negative for iron (Prussian blue). (B) CD31 and Prussian blue stain of fixed brain in a mouse receiving FE-PLL-labeled cells. Iron-labeled (blue) cells are distributed around the tumor periphery. The CD31 stain could not be seen simultaneously with PB except in iron-negative areas. (C) Fixed 6-μm section from a labeled mouse stained for CD31 only. (D) Same CD31 slide after staining for Prussian blue. Cells that are both CD31+ and PB+ are circled. (E) Fixed 6-μm section stained with VWF and Prussian blue. Arrows indicate cells that are both VWF+ and PB+.
Histopathology of brain tumor 7 to 11 days after systemic cell transplant. (A) CD31 (platelet endothelial cell adhesion molecule 1 [PECAM-1]) and Prussian blue stains of fixed brain in a mouse receiving unlabeled cells. Slide is positive for CD31 (brown) and negative for iron (Prussian blue). (B) CD31 and Prussian blue stain of fixed brain in a mouse receiving FE-PLL-labeled cells. Iron-labeled (blue) cells are distributed around the tumor periphery. The CD31 stain could not be seen simultaneously with PB except in iron-negative areas. (C) Fixed 6-μm section from a labeled mouse stained for CD31 only. (D) Same CD31 slide after staining for Prussian blue. Cells that are both CD31+ and PB+ are circled. (E) Fixed 6-μm section stained with VWF and Prussian blue. Arrows indicate cells that are both VWF+ and PB+.
Discussion
We hypothesized that magnetic cell labeling methodology would be useful in following the migration of bone marrow-derived stem cells into areas of new vessel growth and hence provide important information about the time course of their incorporation into tumor vasculature. Depending on the stimulus in vivo, there is probably more than 1 source of endothelial precursors originating from the bone marrow, and because interactions between cell types in the marrow may be important to their contribution to angiogenesis, we used in the current study a broader population of cells (Sca1+). Sca1+ cells have been shown to have phenotypic characteristics of both hematopoietic and endothelial precursors and have the ability to repopulate bone marrow.4-6 Labeled Sca1+ cells were detected in vivo and showed a change in distribution over time with distinct spatial details, whereas unlabeled or killed labeled cells were not detected. MR evidence of labeled cell incorporation demonstrates that neovascularization occurs primarily at the tumor periphery and that it occurs in the later stages of the development of the tumor. In all mice in which a ring effect was observed, it occurred from 10 to 13 days from transplantation (up to experimental end point) and was not observed earlier by MRI. The results of this study demonstrate the ability to image temporal-spatial migration of magnetically labeled Sca1+ cells into developing functional tumor vasculature using in vivo MRI.
Because the ability to detect labeled cells on MRI will depend on the iron concentration in the tissue, it is likely that low concentrations of iron-labeled cells may have been present before 9 days but were not detected; labeled cells in tissue are not unequivocally detectable on MRI in tissue until they reach a density of at least 10 cells per pixel (or about a 100 μm3 area). Of note, ability to detect magnetically labeled cells in vivo is highly dependent on the iron concentration in the cells, cell density in a voxel (ie, volume element), as well as MRI system characteristics such as field strength, resolution, and imaging coils used, and thus can be expected to vary.
The consistent timing observed supports the onset of signaling to attract cells to the tumor is not continuous but occurs at a certain point in tumor growth. This correlates well to other studies of the onset of angiogenesis in gliomas.20-22 Four stages of development were observed on confocal microscopy using fluorescently labeled glioma: perivascular organization of tumor cells around existing vessels, proliferation of tumor cells, apoptosis and involution of the preexisting vascular cells, followed by angiogenesis.20 Contrast-enhanced MRI of the same model21 demonstrated high signal intensity within the tumor due to breakdown of the blood-brain barrier prior to evidence of angiogenesis. Enhancement by Gd-DTPA flowing through dense blood vessels around the tumor developed in a later stage of tumor growth. Only once ring enhancement of the tumor was observed on MRI was there also histologic evidence of angiogenesis.
In the current study the increase in labeled cell incorporation at a specific stage of tumor development (primarily located at the periphery), was evident on MRI as a hypointense region. Because the labeled cells home specifically to new or developing vasculature, the pattern observed on MRI represents a specific, noninvasive indicator of angiogenesis around the tumor. One labeled mouse was negative. This may have been caused by an insufficient number of cells or nonviable cells being administered due to techniques in transplantation, or there may have been slower tumor growth accounting for less angiogenic signaling from the tumor at the stage it was imaged. We have shown by histology at 6 to 13 days after transplantation that once the labeled cells are incorporated into the tumor tissue vasculature, many express CD31 and von Willebrand factor, suggesting the iron-labeled cells imaged around the tumor have differentiated toward an endothelial phenotype.
In the SCID mouse RT2 glioma model, Ferrari et al6 showed using green fluorescent protein-labeled cells and immunohistochemistry for Flk-1, Flt-1/VEGFR1 (vascular endothelial growth factor receptor-1), and Tie-2 endothelial-specific markers, that transplanted Sca1+ bone marrow cells migrate to the tumor and differentiate into endothelial-like cells. Transduction of the Sca1+ cells with a suicide gene (HSV-tk) resulted in significant reduction in tumor volume and increased apoptosis, demonstrating that cells had migrated to and were significant to the viability of the tumor. Had these cells migrated to the tumor without participating in the neovasculature or angiogenic process, for example, as primarily macrophages, their death on activation of the suicide gene would not be expected to affect the viability of the tumor and prolong survival.6 Although the current study was not designed to count the distribution of the total number of Sca1+ cells infused, prior biodistribution studies of Sca1+ cells in this model6 noted few cells in the spleen and liver, with occasional cells in lung and normal brain occurring only if the mice had been irradiated. Recently, supporting evidence22 shows CD34+ EPCs homed to developing angiogenesis in implanted brain tumor after a period of approximately 7 to 14 days from transplantation, and that the biodistribution of these cells show the majority have migrated to the brain (∼88% of detected cells) with approximately 6% of detected cells found in the spleen and significantly fewer in lung, liver, and kidney. The cells did not migrate to brain in the absence of tumor. Of note, the bone marrow was not examined in this study.
In previous work with transplanted human CD34+ EPCs in this glioma model6 it was hypothesized that sublethal irradiation would serve to reduce competition from native EPCs and optimize the incorporation of donor cells. The proportion of endothelial cells (ECs) of donor origin in the tumor vasculature was reported to be about 10% to 25% of the total ECs. We have imaged both irradiated and nonirradiated mice and found the conspicuity of the hypointense effect on MRI was not different from that in nonirradiated mice. Overall, irradiation did not have an advantage in terms of the MRI technique. We noted an increased tendency to hemorrhage early in the course of tumor growth in irradiated mice that resolved over the time period the mice were imaged. Because of the potential to obscure detection of labeled cells, hemorrhage is a disadvantage for MRI, though the late-stage effect on MRI was analogous to nonirradiated mice. Sublethal irradiation may be useful in optimizing cell delivery for therapeutic purposes, and our results indicate it is compatible with MRI tracking of the cells.
The administration of a paramagnetic contrast agent may be helpful in differentiating areas of slow blood flow from vessels incorporating iron-labeled cells. Vasculature with labeled cell incorporation was observed to remain hypointense in the presence of a paramagnetic contrast agent, whereas the darkening near the tumor rim in a control mouse became isointense following the administration of Gd-DTPA (Figure 3C-D). The disappearance of the rim is an indication that the decrease in signal intensity observed in the control animal with killed labeled cells on precontrast images was due to slow blood flow. Of note, infusion of killed labeled cells should have been cleared from circulation shortly after they were administered early in tumor development (day 2) and thus could not respond to the chemotactic stimuli that induce the migration of cells to sites of ongoing angiogenesis. This result also indicates the susceptibility effect from iron-containing cells in the vasculature has a greater net effect on signal intensity than does the paramagnetic T1 and T2 shortening in the blood; thus, it is possible to use these in conjunction when indicated to detect areas of blood-brain barrier disruption or to measure tumor relative cerebral blood volume.18,19
The findings in the current study indicate that MRI can directly image tumor neovasculature through the incorporation of magnetically labeled bone marrow-derived cells. To our knowledge this is the first report to demonstrate the noninvasive imaging of endothelial precursor cells incorporation into tumor neovasculature by in vivo MRI. These findings are significant for future noninvasive, in vivo clinical monitoring of gene therapy for tumors as well as therapeutic transplantations of EPCs for neovascularization in cerebral infarction or myocardial infarction. In vivo monitoring is important for clinical trials that will require a means to determine dosage, frequency, and timing of cell transplantations to optimize therapeutic effects and predict potential adverse effects. Our cell labeling method uses a Food and Drug Administration (FDA)-approved contrast agent (ferumoxides), complexed to PLL, has been shown to be nontoxic to cells and does not interfere with the function of immune cells14 or differentiation of several mammalian stem cell and precursor cells.8 . We recently reported the first cell labeling method incorporating 2 FDA-approved agents, ferumoxides with protamine sulfate,23 which used for cell labeling analogously to FE-PLL, opens the possibility of translating this method into a clinical setting.
Prepublished online as Blood First Edition Paper, August 26, 2004; DOI 10.1182/blood-2004-06-2222.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 4. Histopathology of brain tumor 7 to 11 days after systemic cell transplant. (A) CD31 (platelet endothelial cell adhesion molecule 1 [PECAM-1]) and Prussian blue stains of fixed brain in a mouse receiving unlabeled cells. Slide is positive for CD31 (brown) and negative for iron (Prussian blue). (B) CD31 and Prussian blue stain of fixed brain in a mouse receiving FE-PLL-labeled cells. Iron-labeled (blue) cells are distributed around the tumor periphery. The CD31 stain could not be seen simultaneously with PB except in iron-negative areas. (C) Fixed 6-μm section from a labeled mouse stained for CD31 only. (D) Same CD31 slide after staining for Prussian blue. Cells that are both CD31+ and PB+ are circled. (E) Fixed 6-μm section stained with VWF and Prussian blue. Arrows indicate cells that are both VWF+ and PB+.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/1/10.1182_blood-2004-06-2222/6/m_zh80010571450004.jpeg?Expires=1767698086&Signature=AmhkfQ2Brof0AJIBQ3LTNGYywJwI4GqOXR7yolmelVC2Mj4oIBPMTrEeTt7t9N-ytstfqqd0432AMNzgBPqSQPy4I6hdhD9GHIVS-szspE6R47DoJ3Gcx7ulg34pR8d~v75zE9mKuxlCLtbmGxs1mbVsFqABOB0zTiIDcRRI6tMUgoIIca726u18OPPDgBVfTkL5mpZiChGMMkk1D41Qv4rOF0OJ9KwBJ5qGpT7M-ikner1FZWsja7~fFy4rQST7682~v8TMOZdBuOETdQsmk5YYdsZmL-V1ySkr2bzen-MIdYBxcLwJ0pTm6ectr8xjasFh6ZBT~sGLDXMBJm03fQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal