Abstract
Acute megakaryoblastic leukemia (M7 AML) is a highly aggressive disease. We evaluated outcomes in 57 children (11 with Down syndrome) and 69 adults with M7 AML after first complete remission (CR1) following autologous or HLA-identical allogeneic transplantation. Characteristics of the recipients of autologous transplants (38 children, 37 adults) were, respectively: median age, 1.7 and 46 years; non-total body irradiation (non-TBI) conditioning regimen, 97% and 70%; bone marrow as stem cell source, 74% and 43%. Characteristics of the recipients of allogeneic transplants (19 children, 32 adults) were, respectively: median age, 2.8 and 37 years; non-TBI regimen, 63% and 42%; bone marrow as stem cell source, 95% and 69%. Autologous transplantation benefited children more; the relapse rate was high in adults. Results for autologous transplantation were (children and adults, respectively): engraftment, 90% and 100%; 3-year treatment-related mortality (TRM) rate, 3% and 8%; relapse rate, 45% and 64%; leukemia-free survival (LFS) rate, 52% and 27%; overall survival (OS) rate, 61% and 30%. After allogeneic transplantation, TRM was fairly low in children and adults, and relapse rates were lower than after autologous transplantation. Results for allogeneic transplantation were, respectively: engraftment, 95% and 90%; TRM, 0% and 26%; relapse rate, 34% and 28%; LFS, 66% and 46%; OS, 82% and 43%). We conclude that M7 AML patients in CR1 (except children with Down syndrome, who already have better outcomes) can benefit from transplantation. (Blood. 2005;105:405-409)
Introduction
Acute megakaryoblastic leukemia (M7 AML) is a rare subtype of acute myeloid leukemia (AML) that develops from primitive megakaryoblasts. It was first described by Van Boros and Korenyi in 1931, and it is the seventh AML subtype in the 1985 French-American-British (FAB) classification.1-3 Developments in cytochemistry and immunophenotyping have improved its diagnosis and differentiation from acute myelosclerosis.4-6 M7 AML has a bimodal age distribution that peaks in early childhood (younger than 3 years) and in adulthood.7 Approximately 1% of AML cases in adults and 5% to 10% in children are M7 AML.8,9 Its incidence is higher in children with Down syndrome.10,11
The prognosis for patients with M7 AML is poor. Although remission rates are not much lower than those for other myeloid leukemia subtypes, the relapse rate is high. Approximately half the patient population achieves complete remission (CR) with conventional chemotherapy, but few patients survive beyond 3 years.12-15 Median overall survival (OS) is estimated to be 40 weeks in adults,15 and the 2-year event-free survival is 14% in children.16 Outcomes are best in children with Down syndrome.16-18
Postremission therapy must be improved. It has been suggested that autologous and allogeneic bone marrow transplantation (BMT) might offer the best chance of cure for patients in remission, but available clinical data are scant. Published reports are often case reports19,20 or small clinical series with inconsistent results.15,16,21,22 The aim of this retrospective study is to evaluate outcomes after hematopoietic stem cell transplantation (HSCT) in patients with de novo M7 AML in first CR. The study covers autologous and HLA-identical sibling allogeneic transplantation.
Patients, materials, and methods
Patients
We reviewed patients with M7 AML who had received autologous or genoidentical allogeneic transplants from January 1986 to December 2002 in 77 European centers reporting to the Acute Leukemia (AL) registry of the European Group for Blood and Bone Marrow Transplantation (EBMT). Only patients with de novo M7 AML and in first CR (CR1) were included in the study. Patients on a nonmyeloablative regimen or receiving transplants from matched unrelated donors, haploidentical donors, or cord blood were excluded. Participating centers are listed in “Appendix.” Since 1996, annual clinical audits have been carried out in many EBMT centers.
All 77 EBMT centers were asked to confirm the diagnosis of M7 AML initially reported to the AL registry and to specify diagnostic methods and tools. Confirmation of each diagnosis was sought by a review of slides by a local expert panel and was obtained for 90% of patients. The remaining 10% of patients were excluded from the study. Criteria for M7 AML diagnosis were (1) cytology and classification as M7 AML (FAB classification) by the panel and (2) immunophenotyping of the cells showing positivity for CD42 and CD61, when available.
Hematopoietic recovery was defined as a neutrophil count of 0.5 × 109/L or more for 3 consecutive days and a platelet count of 50 × 109/L or more for 7 consecutive days without platelet support.
Statistical analysis
Results are reported as medians with ranges for discrete variables and as median cut-off points for continuous variables. The following were analyzed for their potential prognostic value: patient and donor characteristics (age, sex, and sex match) and transplantation-related factors (time from diagnosis to CR1, time from diagnosis to transplantation, source of stem cells, conditioning regimen including total body irradiation (TBI), and year of transplantation). Leukemia-free survival (LFS) was defined as survival without evidence of relapse; the event under study was death or relapse. Relapse was defined as hematologic recurrence at any site. Patients who died of procedure toxicity or of any other cause unrelated to leukemia were censored in the calculation of relapse rate. Transplant-related mortality (TRM) was defined as death while in CR.
Separate statistical analyses were performed for each end point (relapse rate, TRM, LFS, and OS) and for each subgroup (autograft and allograft, children and adults). The incidence of each event was estimated nonparametrically. Patients were censored at the time of relapse or last follow-up.23 LFS and OS probabilities were estimated by the product-limit method.24 The significance of differences between curves was estimated by the log-rank test (Mantel-Cox). All potential prognostic factors were included in a Cox proportional hazards model.25
Relapse mortality and nonrelapse mortality are competing events. Their incidence rates were, therefore, estimated using a nonparametric estimator of cumulative incidence curves. Predictive analyses were based on the proportional hazards model for subdistributions of competing risks.26 Analyses were performed using the cmprsk package (developed by Gray, June 2001), Splus 2000 software (Insightful, Seattle, WA), and SPSS software (SPSS, Chicago, IL).
Results
Patient characteristics
After confirmation of the diagnosis, the AL registry contained information on 324 patients (adults and children) with de novo M7 AML. Of these, 211 had received high-dose therapy followed by HSCT; 126 (57 children, 69 adults) were in CR1. Overall, 38 children and 37 adults in CR1 had undergone autologous HSCT; 19 children and 32 adults had undergone allogeneic HSCT from an HLA-identical sibling donor. Patient characteristics are given in Table 1. M7 AML occurred at early ages in children (median ages, 1.7 and 2.8 years after autologous and allogeneic HSCT, respectively) and at relatively young ages in adults, (45.5 and 37 years, respectively). Populations were almost equally divided between males and females. Most transplantations (except autologous transplantation in adults) used bone marrow rather than peripheral blood as a source of stem cells. Purging of the autologous graft was performed in some children. TBI was rarely used in the conditioning regimen (except allogeneic transplantation in adults). The combination of cyclosporin A and methotrexate was the most favored graft-versus-host disease (GVHD) prevention.
Patient characteristics
. | Autologous transplantation . | . | Allogeneic transplantation, HLA identical . | . | ||
|---|---|---|---|---|---|---|
. | Children N = 38 . | Adults N = 37 . | Children N = 19 . | Adults N = 32 . | ||
| Median age, y (range) | 1.7 (< 1-14) | 45.5 (16-71) | 2.8 (1-14) | 37 (18-66) | ||
| Sex | ||||||
| Male (%) | 22 (58) | 19 (51) | 10 (53) | 15 (47) | ||
| Female (%) | 16 (42) | 18 (49) | 9 (47) | 17 (53) | ||
| M/F ratio | 58:42 | 51:49 | 53:47 | 47:53 | ||
| Stem cell source | ||||||
| Bone marrow (%) | 28 (74) | 16 (43) | 18 (95) | 22 (69) | ||
| Peripheral blood (%) | 10 (26) | 21 (57) | 1 (5) | 10 (31) | ||
| Purge of stem cell source | ||||||
| No (%) | 19 (70) | 26 (90) | — | — | ||
| Yes (%) | 8 (30) | 3 (10) | — | — | ||
| Missing | 11 | 8 | — | — | ||
| GVHD prevention | ||||||
| CSA | — | — | 2 | 3 | ||
| MTX | — | — | 3 | 0 | ||
| CSA + MTX | — | — | 10 | 22 | ||
| Tdepl | — | — | 1 | 5 | ||
| TBI conditioning regimen | ||||||
| No (%) | 31 (97) | 24 (70) | 12 (63) | 14 (42) | ||
| Yes (%) | 1 (3) | 10 (29) | 7 (37) | 18 (58) | ||
| Missing | 6 | 3 | ||||
| Time from diagnosis to CRl, d (range) | 77 (28-184) | 49 (25-289) | 48 (29-145) | 69 (18-165) | ||
| Time from diagnosis to transplantation, d (range) | 187 (109-506) | 203 (104-796) | 146 (69-225) | 138 (63-817) | ||
| Median year of transplantation (range) | 1993 (1989-2002) | 1996 (1986-2002) | 1992 (1987-2001) | 1996 (1986-2002) | ||
. | Autologous transplantation . | . | Allogeneic transplantation, HLA identical . | . | ||
|---|---|---|---|---|---|---|
. | Children N = 38 . | Adults N = 37 . | Children N = 19 . | Adults N = 32 . | ||
| Median age, y (range) | 1.7 (< 1-14) | 45.5 (16-71) | 2.8 (1-14) | 37 (18-66) | ||
| Sex | ||||||
| Male (%) | 22 (58) | 19 (51) | 10 (53) | 15 (47) | ||
| Female (%) | 16 (42) | 18 (49) | 9 (47) | 17 (53) | ||
| M/F ratio | 58:42 | 51:49 | 53:47 | 47:53 | ||
| Stem cell source | ||||||
| Bone marrow (%) | 28 (74) | 16 (43) | 18 (95) | 22 (69) | ||
| Peripheral blood (%) | 10 (26) | 21 (57) | 1 (5) | 10 (31) | ||
| Purge of stem cell source | ||||||
| No (%) | 19 (70) | 26 (90) | — | — | ||
| Yes (%) | 8 (30) | 3 (10) | — | — | ||
| Missing | 11 | 8 | — | — | ||
| GVHD prevention | ||||||
| CSA | — | — | 2 | 3 | ||
| MTX | — | — | 3 | 0 | ||
| CSA + MTX | — | — | 10 | 22 | ||
| Tdepl | — | — | 1 | 5 | ||
| TBI conditioning regimen | ||||||
| No (%) | 31 (97) | 24 (70) | 12 (63) | 14 (42) | ||
| Yes (%) | 1 (3) | 10 (29) | 7 (37) | 18 (58) | ||
| Missing | 6 | 3 | ||||
| Time from diagnosis to CRl, d (range) | 77 (28-184) | 49 (25-289) | 48 (29-145) | 69 (18-165) | ||
| Time from diagnosis to transplantation, d (range) | 187 (109-506) | 203 (104-796) | 146 (69-225) | 138 (63-817) | ||
| Median year of transplantation (range) | 1993 (1989-2002) | 1996 (1986-2002) | 1992 (1987-2001) | 1996 (1986-2002) | ||
CsA indicates cyclosporin A; —, not applicable; MTX, methotrexate; and Tdepl, T-cell depletion.
Engraftment, GVHD, and treatment-related mortality
A good engraftment rate was observed (less than 10% failure rate) (Table 2). After autologous transplantation, all adults and 35 of the 38 children had successful engraftments. After allogeneic transplantation, 29 of the 32 adults and all children but 1 had successful engraftments. Median neutrophil recovery time was within the usual range (17-26 days), but platelet recovery was delayed in adult recipients of autologous transplants (median, 60 days).
Engraftment after transplantation
. | Autologous transplantation . | . | Allogeneic transplantation HLA identical . | . | ||
|---|---|---|---|---|---|---|
. | Children N = 38 . | Adults N = 37 . | Children N = 19 . | Adults N = 32 . | ||
| Engraftment | ||||||
| Yes | 35 | 37 | 18 | 29 | ||
| No | 3 | 0 | 1 | 3 | ||
| Neutrophil level at 0.5 × 109/L or more, d (range) | 26 (9-83) | 17 (7-103) | 19 (9-40) | 17 (11-26) | ||
| Platelet level at 50 × 109/L or more, d (range) | 41 (15-170) | 60 (13-108) | 27 (15-71) | 24 (12-179) | ||
. | Autologous transplantation . | . | Allogeneic transplantation HLA identical . | . | ||
|---|---|---|---|---|---|---|
. | Children N = 38 . | Adults N = 37 . | Children N = 19 . | Adults N = 32 . | ||
| Engraftment | ||||||
| Yes | 35 | 37 | 18 | 29 | ||
| No | 3 | 0 | 1 | 3 | ||
| Neutrophil level at 0.5 × 109/L or more, d (range) | 26 (9-83) | 17 (7-103) | 19 (9-40) | 17 (11-26) | ||
| Platelet level at 50 × 109/L or more, d (range) | 41 (15-170) | 60 (13-108) | 27 (15-71) | 24 (12-179) | ||
After allogeneic HLA-identical transplantation, acute GVHD of grade 2 or more developed in 32% (6 of 19) of children and in 28% (9 of 32) of adults. Of the 19 children and 24 adults alive and well 100 days after transplantation, chronic GVHD developed in 1 child and 8 adults.
After autologous transplantation, TRM was low in children and in adults (Table 3). After allogeneic transplantation, TRM was 0% for children but reached 26% in adults, partly because of GVHD.
Results at 3 years after transplantation
. | Autologous transplantation . | . | Allogeneic transplantation HLA identical . | . | ||
|---|---|---|---|---|---|---|
. | Children N = 38 . | Adults N = 37 . | Children N = 19 . | Adults N = 32 . | ||
| Median follow-up, mo (range) | 56 (5-162) | 13 (3-126) | 63 (4-166) | 47 (1-139) | ||
| TRM, % | 3 ± 5 | 8 ± 9 | 0 | 26 ± 15 | ||
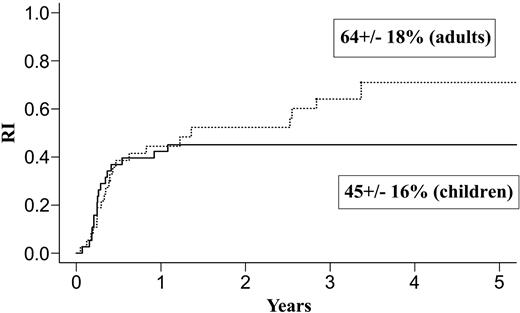
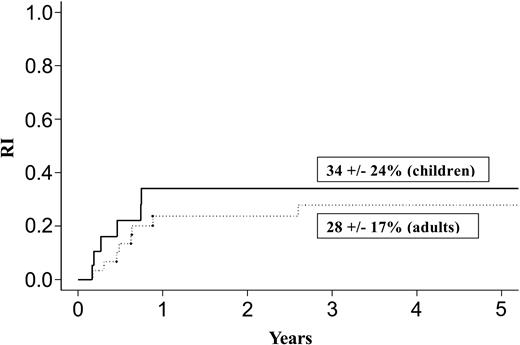
| Relapse rate, % | 45 ± 16 | 64 ± 18 | 34 ± 24 | 28 ± 17 | ||
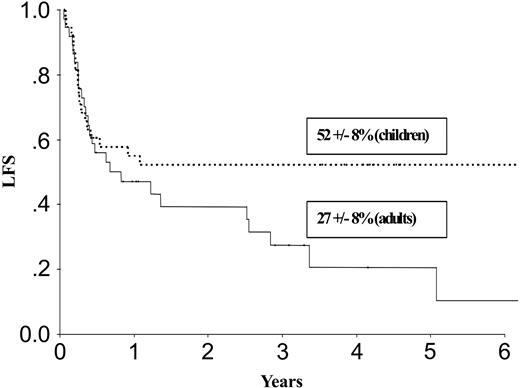
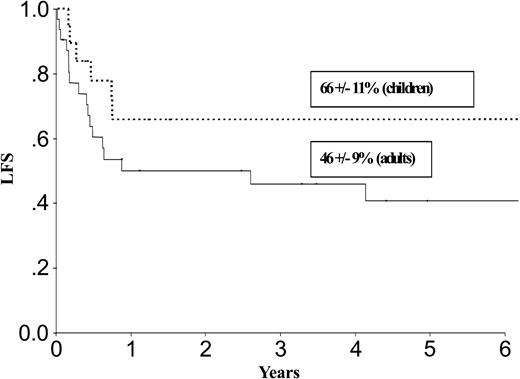
| LFS, % | 52 ± 8 | 27 ± 8 | 66 ± 11 | 46 ± 9 | ||
| OS, % | 61 ± 8 | 30 ± 9 | 82 ± 10 | 43 ± 10 | ||
. | Autologous transplantation . | . | Allogeneic transplantation HLA identical . | . | ||
|---|---|---|---|---|---|---|
. | Children N = 38 . | Adults N = 37 . | Children N = 19 . | Adults N = 32 . | ||
| Median follow-up, mo (range) | 56 (5-162) | 13 (3-126) | 63 (4-166) | 47 (1-139) | ||
| TRM, % | 3 ± 5 | 8 ± 9 | 0 | 26 ± 15 | ||
| Relapse rate, % | 45 ± 16 | 64 ± 18 | 34 ± 24 | 28 ± 17 | ||
| LFS, % | 52 ± 8 | 27 ± 8 | 66 ± 11 | 46 ± 9 | ||
| OS, % | 61 ± 8 | 30 ± 9 | 82 ± 10 | 43 ± 10 | ||
Results
Results at 3 years for children and adults after autologous or allogeneic transplantation are given in Table 3 and in Figures 1, 2, 3, and 4. The relapse rate was high, especially after autologous transplantation in adults (64% at 3 years, with no plateau). LFS and OS did not exceed 30% in adults. After allogeneic transplantation, all relapses in children and more than 90% of relapses in adults occurred within the first year. LFS and OS rates were higher than after autologous transplantation (greater than 40% in adults; 66% and 82%, respectively, in children).
Relapse after autologous transplantation in adults and children with de novo M7 AML in CR1.
Relapse after autologous transplantation in adults and children with de novo M7 AML in CR1.
LFS after autologous transplantation in adults and children with de novo M7 AML in CR1.
LFS after autologous transplantation in adults and children with de novo M7 AML in CR1.
Relapse after allogeneic transplantation in adults and children with de novo M7 AML in CR1.
Relapse after allogeneic transplantation in adults and children with de novo M7 AML in CR1.
LFS after allogeneic transplantation in adults and children with de novo M7 AML in CR1.
LFS after allogeneic transplantation in adults and children with de novo M7 AML in CR1.
Although we did collect cytogenetic data, our population was too small, and there were too many missing data to define subgroups as others have done.27
Down syndrome
Information on the presence or absence of Down syndrome was available for 100 of the 126 patients. Overall, 11 (23%) of 47 children (no adults) had Down syndrome. Of these 11 children, 5 received an allograft and 6 received an autograft. The median age at transplantation was 2.1 years (range, 1.3-4.3 years). In the allograft group, 3 are alive without disease 7, 10, and 11 years after transplantation, whereas 2 had relapses (2 and 3 months after transplantation) and died. In the autologous group, 3 are alive without disease 4.5, 4.5, and 8.5 years after transplantation, whereas 2 had relapses and died and 1 died of treatment toxicity.
Prognostic factors
In a univariate analysis, adult patients who received allografts after January 1996 had lower TRM rates (18% ± 18%) than those who received allografts before this date (38% ± 25%). This was confirmed by multivariate analysis; the TRM in adults was significantly lower after January 1996 (P = .03; RR = 0.11). No other factor (patient age, stem cell source, conditioning regimen including TBI, sex, and interval from diagnosis to transplantation) was significant.
Discussion
Our study with a median follow-up from 13 to 63 months, depending on patient age (adult or child) and transplantation modality, seems to confirm the results of preliminary observations indicating that transplantation might improve survival for patients with de novo M7 AML in CR1. OS rates at 3 years were 82% in children and 43% in adults after allogeneic transplantation and were lower (61% and 30%, respectively) after autologous transplantation. Published results for postremission transplantation are few. In an early study in 7 children undergoing BMT, 3 became long-term survivors.21 In a series of 29 patients with de novo M7 AML but without Down syndrome, the 2-year event-free survival rate was significantly higher after allogeneic BMT (26%) than after chemotherapy (0%; P = .019) and was significantly higher when performed during remission (46%) than during persistent disease (0%; P = .019).16 However, in another study, 6 of 7 patients who underwent transplantation during CR died within a few months, 3 from early toxicity and 3 from early relapse.15
Our data did not confirm concerns about long-lasting postchemotherapy aplasia15 and possible severe damage to the marrow stromal environment, as reported for some BMT recipients.28 After autologous transplantation, a good engraftment rate (less than 10% failure) was observed. Neutrophil recovery time was within the usual range, but platelet recovery was delayed in adults. TRM was low. After allogeneic transplantation, engraftment was highly satisfactory. TRM was low in children but reached 26% in adults, in part because of GVHD. Successful engraftment may be related to the reversal of bone marrow fibrosis by intensive chemotherapy or chemoradiotherapy followed by BMT.19,20
Our most striking observation was the high relapse rate. It was very high after autologous transplantation, especially in adults (64% at 3 years, with no plateau). This transplantation modality should, therefore, not be preferred for adults with M7 AML. A recent EBMT study found that outcome was also poorer after autologous transplantation in patients with M6 AML than in patients with other leukemia subtypes.29 In children, the relapse rate after autologous transplantation was also high (45%), but there was a plateau after the first year. Outcomes seemed better than they were with conventional chemotherapy. It should be borne in mind that our study experienced the usual selection biases. For instance, only those patients who reached remission and remained in remission long enough for stem cells collection actually underwent autografting. After allogeneic transplantation, all relapses in children and more than 90% of relapses in adults occurred within the first year, suggesting a graft-versus-leukemia effect.
M7 AML occurs at a very early age in children.30 In our study, the median ages at transplantation was 1.7 years and 2.8 years (for autologous and allogeneic transplantation, respectively), in line with the median age at diagnosis of 23.9 months given by Athale et al.16 This is probably because children with Down syndrome have many hematologic problems and are at high risk for acute leukemia, including M7 AML. In addition, in adults, the median age at transplantation in our study was relatively young (45.5 for autologous and 37 years for allogeneic transplantation) compared with ages for other AML subtypes (63 years). However, this comparison must be viewed with caution because the AL database of the EBMT is biased toward relatively young transplant recipients.
Time of transplantation was the only significant prognostic factor in a multivariate analysis of adults who underwent allogeneic transplantation; it was related to a lower TRM after January 1996. This indicates recent improvement in the management of adult patients. A similar improvement had previously been found in 1986 and was attributed to the combination of cyclosporin A and methotrexate.
M7 AML occurs approximately 400 to 500 times more often in children with Down syndrome than in other children.31,32 In our study, no adult had Down syndrome. The prevalence rate was 23% (11 of 47) in children, confirming rates (20%, 17%) reported in other studies 6,16 It is well known that patients with Down syndrome respond better to chemotherapy and have better survival. Intensification in these patients is more toxic, and the conditioning regimen should, therefore, be adjusted accordingly. In our study, intensification was well tolerated, and only 1 transplant-related death occurred. However, 36% of the children with Down syndrome had relapses, and there were no differences in outcomes after autologous and allogeneic transplantation.
In conclusion, the best treatment strategy for children with M7 AML in the absence of Down syndrome seems to be allogeneic transplantation if there is an HLA-identical sibling; otherwise, autologous transplantation would be the best strategy. The TRM rate is low, and, even though the relapse rate is approximately 50%, the LFS rate is higher than it is after conventional chemotherapy. In adults, a good option is HLA-identical allogeneic transplantation because of its 26% TRM rate and 46% LFS rate at 3 years. Autologous transplantation is not advisable because the relapse rate is too high. If transplantation is not possible, M7 AML will continue to be a very aggressive disease with poor prognosis in children (except those with Down syndrome) and in adults.
Appendix
Personnel and centers that registering patients with acute megakaryocytic leukemia in the AL registry of the EBMT: Manuel Abecassis, Portugues Oncologia, Lisboa, Portugal; Tevfik Akoglu, Marmara Universitesi Hastanesi, Istanbul, Turkey; Sergio Amadori, St Eugenio Hospital, Rome, Italy; Marino Andolino, Centro Trapianti Clinica Pediatrica, Trieste, Italy; M. Baccarani, Udine University Hospital, Italy; André Baruchel, Hopital Saint Louis, Paris, France; Y Beguin, Universite de Liege, Belgium; Didier Blaise, Institut Paoli Calmettes, Marseille, France; Marc Boasson, CHRU, Angers, France; Dominique Bron, Institut Jules Bordet, Brussels, Belgium; Donald Bunjes, Universitat Ulm, Germany; Victoria Castel, Hospital Infantil La Fe, Valencia, Spain; R.E. Clark, Royal Liverpool University Hospital, United Kingdom; Antonio De Laurenzi, S Camillo Hospital, Rome, Italy; J.L. Diez Martin, Hospital G U Gregorio Maranon, Madrid, Spain; Eric Dore, CHU Timone, Marseille, France; Athanasios Fassas, Hospital of Thessaloniki, Exokhi, Greece; Anders Fasth, Queen Silvia Children's Hospital, Goeteborg, Sweden; Leonardo Feldman, Antartida Hospital Privado, Buenos Aires, Argentina; Augustin Ferrant, Clinique Universitaire Saint Luc, Brussels, Belgium; Alain Fisher, Hopital Necker, Paris, France; Helmut Gadner, St Anna Kinderspital, Vienna, Austria; Eliane Gluckman, Hopital Saint Louis, Paris, France; Antony Goldstone, University College London Hospital, United Kingdon; Norbert Claude Gorin, Hopital Saint Antoine, Paris, France; Hildegard Greinix, Medical University, Vienna, Austria; Nicholas Harhalakis, Evangelismos Hospital, Athens, Greece; Jean Luc Harousseau, Nantes, France; R.P. Herrmann, Royal Perth Hospital, Melbourne, Australia; Christoph Huber, Johannes Gutenberg University, Mainz, Germany; A. Iriondo, Hospital Universitario, Santander, Spain; Teodosio Izzi, Spedali Civili, Brescia, Italy; Niels Jacobsen, Rigshospitalet, Copenhagen, Denmark; Dieter Korholz, Klinik fur Padiatrische Hamatologie, Dusseldorf, Germany; Veronique Leblond, Hopital la Pitie Salpetriere, Paris, France; Guy Leverger, Hopital Trousseau, Paris, France; Werner Linkesch, University of Graz, Austria; Franco Locatelli, Pediatric Clinic, University of Pavia, Italy; A.M. Martinez Rubio, Hospital Infantil La Paz, Madrid, Spain; Ospedale V Cervello, Palermo, Italy; Giovanna Meloni, Universita “La Sapienza,” Rome, Italy; Mauricette Michallet, Hopital E Herriot, Lyon, France; Emilio Montserrat, Hospital Clinic, Barcelona, Spain; Han Myint, Royal Bournemouth Hospital, United Kingdom; Juan Ortega, Hospital M. Infantil Vall d'Hebron, Barcelona, Spain; A.C. Parker, Western General Hospital, Edinburgh, United Kingdom; José Pico, Institut Gustave Roussy, Villejuif, France; Ray Powles, Royal Marsden Hospital, Sutton, United Kingdom; H.G. Prentice, Royal Free Hospital, London, United Kingdom; Manuel Ribas Mundo, Institut Dexeus, Barcelona, Spain; Bernard Rio, Hotel Dieu, Paris, France; Kenneth Romeril, Wellington Hospital, New Zealand; Jacob Rowe, Rambam Medical Center, Haifa, Israel; Bengt Sallerfors, University Hospital, Lund, Sweden; Miguel Sanz, Hospital Universitario La Fe, Valencia, Spain; W. Schneider, Heinrich Heine Universitat, Dusseldorf, Germany; Jorge Sierra, Hospital Santa Creu I Sant Pau, Barcelona, Spain; Shimon Slavin, Hadassah University Hospital, Jerusalem, Israel; A. Torrez Gomez, Cordoba Hospital, Spain; Cornelio Uderzo, Ospedale San Gerardo, Monza, Spain; Jaak Vossen, Leiden University Hospital, The Netherlands; Hannes Wandt, Klinikum Nurnberg, Germany; A.M. Will, Royal Manchester Children's Hospital, Pendlebury, United Kingdom; Atilla Yalcin, Gulhane Military Medical Academy, Ankara, Turkey; Isaac Yaniv, Schneider Children's Medical Center of Israel, Petach Tikva, Israel; Felix Zintl, University of Jena, Germany; Nicholas Zoumbos, Patras University Medical School, Greece.
Prepublished online as Blood First Edition Paper, June 10, 2004; DOI 10.1182/blood-2004-03-1103.
A complete list of the EMBT member centers that registered patients in this study of the Acute Leukemia Working Party and the Pediatric Working Party appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Tiiu Ojasoo for critical reading of the manuscript. Furthermore, we thank the personnel and centers listed in the “Appendix” for registering patients with acute megakaryocytic leukemia in the AL registry of the EBMT.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal