Abstract
Heparin-induced thrombocytopenia and thrombosis (HITT) is a severe complication of heparin therapy caused by antibodies to complexes between unfractionated heparin (UFH) and platelet factor 4 (PF4) that form over a narrow molar range of reactants and initiate antibody-induced platelet activation. We observed that UFH and tetrameric PF4 formed ultralarge (> 670 kDa) complexes (ULCs) only over a narrow molar range with an optimal ratio of PF4 to heparin of approximately 1:1. These ULCs were stable and visible by electron microscopy, but they could be dissociated into smaller complexes upon addition of heparin. ULCs formed inefficiently when PF4 was incubated with low-molecular-weight heparin, and none formed with the pentasaccharide fondaparinux sodium. In addition, mutation studies showed that formation of ULCs depended on the presence of PF4 tetramers. The ULCs were more reactive as determined by their capacity to bind to a HITT-like monoclonal antibody and showed greater capacity to promote platelet activation in an antibody- and FcγRIIA-dependent manner than were the smaller complexes. The capacity of PF4 to form ULCs composed of multiple PF4 tetramers arrayed in a lattice with several molecules of UFH may play a fundamental role in autoantibody formation, antibody-dependent platelet activation, and the propensity for thrombosis in patients with HITT.
Introduction
Heparin-induced thrombocytopenia and thrombosis (HITT) is a serious complication of heparin therapy. HITT develops in approximately 1% to 3% of patients treated with unfractionated heparin (UFH) for 5 to 10 days.1-3 HITT is a severe prothrombotic condition, with affected individuals having a 20% to 50% risk of developing new thromboembolic events4 with a mortality rate of approximately 20% and an additional approximate 10% of patients requiring amputations or suffering other major morbidity.5-8
It is generally accepted that the clinical manifestations of HITT are caused by antibodies that recognize a complex composed of heparin and platelet factor 4 (PF4),9 a platelet-specific chemokine released in large amounts by activated platelets that binds heparin with high affinity and that exists as a tetramer at concentrations found at sites of platelet activation.10 Our development of a transgenic mouse model of HITT conclusively demonstrated that heparin, PF4, anti–PF4-heparin antibody, and platelet FcγRIIA are necessary and sufficient to recapitulate the salient features of HITT in vivo.11 Plasma from more than 90% of patients with HITT contains anti–PF4-heparin antibodies.9,12,13 Although heparin binds to diverse other proteins, the reason why almost all cases of HITT are associated with antibodies to PF4-heparin complexes has not been explained. It is also unclear how antibodies to PF4-heparin complexes account for the almost exclusive propensity of patients with this particular drug-induced antibody to develop thrombosis, which involves platelet activation through occupation of FcγRIIA.14-16
Any theory of causation should also be able to account for the fact that the incidence of HITT is lower (0.2%-0.8%) in patients treated exclusively with low-molecular-weight heparin (LMWH)1,6,17 and that most patients with anti–PF4-heparin antibodies do not develop clinical manifestations.1,4,18-20 One clue to understanding these issues is the observation made by many investigators who have shown that HITT antibodies bind to complexes formed between UHF and PF4 tetramers only over a very narrow range of molar ratios of reactants, generally approximating 1 to 2 mol PF4 tetramer to 1 mol UFH.21 Both higher and lower concentrations of heparin relative to PF4 lead to a loss of antigenicity.22
We have previously identified 2 sites within PF4 that are required for recognition by most HITT antibodies and by a HITT-like monoclonal antibody, KKO.23,24 How heparin or other glycosaminoglycans induce PF4 to become antigenic is unknown. Greinacher et al25 showed that the antigenicity of the complex depended not only upon the molar ratio of the reactants but also upon the length, chemical composition, and structure of the glycosaminoglycan itself. These investigators have proposed that HITT antibodies recognize a multimeric complex composed of multiple PF4 tetramers assembled on glycosaminoglycans of the appropriate composition.26 However, it is important to understand additional structural details of the antigenic complexes to advance our knowledge of the pathophysiology of HITT.
The goals of this study were, therefore, several-fold: first, to characterize the biophysical structure of complexes formed between PF4 and heparin; second, to understand the relationship between the composition of these complexes and their recognition by antibody; and third, to gain insight into the mechanism by which antibodies directed toward PF4-heparin complexes activate platelets through FcγRIIA. We demonstrate that ultralarge complexes (ULCs; > 670 kDa) form between PF4 and UFH over a narrow ratio of molar concentrations, that these complexes are stable and recognized by a HITT-like antibody, and that they promote platelet activation. Our data both provide direct support for the concept that HITT antibodies recognize a multimeric complex of PF4 and heparin and provide insights into the relationship between the formation of PF4-heparin complexes and the pathogenesis of HITT.
Materials and methods
Materials
UFH was a sodium salt from porcine intestinal mucosa (H-3393; Sigma, St Louis, MO) had a molecular weight (MW) of 9 to 30 kDa with an average MW of 15 kDa. Enoxaparin sodium (Lovenox; Aventis Pharmaceutical Products, Bridgewater, NJ) had a MW of 4.5 kDa, while LMWH (Celsus Laboratories, Cincinnati, OH) had a MW of 3 to 4 kDa and fondaparinux sodium (Arixtra) pentasaccharides (Organon Sanofi-Synthelabo LLC, West Orange, NJ) had a MW of 1780 Da. HiTrap heparin affinity columns and Resource RPC chromatography columns used for protein purification were purchased from Amersham Biosciences (Uppsala, Sweden). Immunochemicals used included rabbit polyclonal anti–human PF4 antibody from PeproTech (Rocky Hill, NJ), horseradish peroxidase–conjugated swine antirabbit antibody from Dako (Carpinteria, CA), horseradish peroxidase–conjugated sheep polyclonal anti–human PF4 from Affinity Biologicals, (Hamilton, ON, Canada), and mouse immunoglobulin G 2bκ (IgG2bκ; MOPC 141) from Sigma. Murine monoclonal antibodies KKO (anti–human PF4-heparin complex),27 RTO (anti–human PF4), TRA (mIgG2bκ-isotype control) and IV.3 (FcγRIIA-blocking antibody)16 have been previously described. Monoclonal antibodies anti-CD41a peridinine chlorophyll protein cyanine 5 (PerCP-Cy5.5), anti-CD62P phycoerythrin (PE), and fluorescein isothiocyanate (FITC)–PAC-1 were purchased form BD Bioscience (San Jose, CA). Annexin-FITC was from BD Bioscience Pharmingen (San Diego, CA). Bicinchoninic acid (BCA) Protein Assay Reagent Kit and Bis(sulfosuccinimidyl)suberate (BS3) were obtained from Pierce (Rockford, IL). Maxisorp microtiter plates used for the enzyme-linked immunosorbent assay (ELISA) were from Nunc Brand Products (Roskilde, Denmark). Bovine serum albumin (BSA), prostaglandin E1 (PGE1), 3,3′,5,5′-Tetramethylbenzidine (TMB) liquid substrate system for ELISA, and SigmaMarker wide molecular weight range markers were purchased from Sigma. Molecular biologic reagents included pT7-7 vector from Novagen (Madison, WI), Escherichia coli strain BL21DE30 pLysS from Stratagene (La Jolla, CA), VENT polymerase from New England Biolabs (Beverly, MA), and Taq polymerase from Promega (Madison, WI).
Preparation of recombinant WT and mutant PF4
Wild-type (WT) human PF4 in the pT7-7 was expressed in BL21DE30 pLysS, and purified and characterized as described previously.28 Briefly, recombinant protein was isolated from the supernatant of the bacterial lysate by affinity chromatography using a HiTrap Heparin high-performance (HP) affinity column. Eluted proteins were purified further by fast protein liquid chromatography (FPLC) using a Resource RPC FPLC column. Protein purity was assessed by 15% (wt/vol) sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) followed by silver staining. Samples were subjected to immunoblotting after electrotransfer to polyvinylidenedifluoride membranes using rabbit anti-human PF4 polyclonal antibody, followed by swine antirabbit secondary antibody conjugated to horseradish peroxidase. Protein concentrations were determined according to the manufacturer's instructions using the bicinchoninic acid assay (Pierce) with BSA as standard.
Site-directed mutagenesis
Monomers within the PF4 tetramer were designated by the letters A to D used according to the nomenclature of the crystal structure.29 To disrupt the interface between the AB and CD dimers, and thereby prevent formation of tetramers, Glu28 and Lys50 were mutated to their analogous residues in interleukin 8 (IL-8; Arg and Glu, respectively). Mutagenesis was performed by extension overlap amplification30 using WT human PF4 pT7-7 construct as template and VENT polymerase enzyme. The sequences of the mutagenic primers used were as follows: WT PF4, 5′-GGA GAT ATA CAT ATG GAA GC-3′ (sense) and 5′-GGC AGC TGG ATC CTA ACT CTC CAA AAG TTT C-3′ (antisense); PF4 E28R, 5′-AGC CTG AGG GTG ATC AAG GCC-3′ (sense) and 5′ GAT CAC CCT CAG GCT GGT GAT-3′ (antisense); PF4 K50E, 5′GGA AGG GAA ATT TGC TTG GAC-3′ (sense) and GCA AAT TTC CCT TCC ATT CTT-3′ (antisense). The sequences of all mutant constructs were verified at the Nucleic Acid/Protein Research Core Facility at the Children's Hospital of Philadelphia. Mutated proteins were purified the same way as WT PF4. WT PF4 and PF4-E28R-K50E eluted from the heparin affinity column at 1.4 to 1.5M NaCl. PF4-K50E eluted from the heparin affinity column at 1.2 to 1.3M NaCl.
Complexes between PF4 and heparin
Radiolabeling of PF4 was performed with Na 125I (Amersham Life Sciences, Arlington Heights, IL) with immobilized chloramine T (Iodo-Beads; Pierce) according to the manufacturer's instructions. PF4-heparin complexes were prepared in a final volume of 150 μL in phosphate-buffered saline (PBS) by incubating 125I-PF4 (1.14 μM; 40 μg/mL) with varying amounts of heparin for 30 minutes at 37°C. PF4-heparin molar ratios were determined by calculating the number of moles of PF4 tetramers (for WT PF4 and PF4-E28R-K50E) or dimers (for PF4-K50E) relative to the number of moles of heparin using the average MW of the heparin preparation being studied. Samples were analyzed by size-exclusion high-performance liquid chromatography (SEC-HPLC) (HPLC-GOLD; Beckman-Coulter, Fullerton CA) using a Bio-Sil SEC 400-5300 × 75-mm columns operated at 0.5 mL/min at ambient temperature with 150-mM NaCl, 50 mM sodium phosphate, pH 7.2, as running buffer. The columns were calibrated for molecular weights of proteins using the Gel Filtration Standard Catalog No. 151-1901 (Bio-Rad Laboratories, Hercules, CA). 125I-PF4 alone or 125I-PF4/heparin mixtures were filtered through the column under identical conditions. The amount of radioactivity in successive 250-μL fractions was measured in a LKB Wallace (Gaithersburg, MD) CliniGamma gamma counter. To standardize experiments, the radioactivity in each fraction was expressed as the ratio of the counts in each fraction compared with the sum of the counts in all fractions (0–30 minutes retention times) × 100%.
Electron microscopy of rotary shadowed complexes of heparin and PF4
Complexes made with different ratios of PF4 and heparin were prepared as described in “Complexes between PF4 and heparin” and visualized by electron microscopy of rotary shadowed samples prepared by modifications of published methods.31-33 The samples were diluted into 50 mM ammonium formate containing 33% glycerol at pH 7.4 to give a final concentration of complex at approximately 30 μg/mL and sprayed onto pieces of freshly cleaved mica using an EFFA spray mount device (EF Fullam, Latham, NY). The sheets of mica were then placed in a Denton DV-502 vacuum evaporator (Denton Vacuum, Cherry Hill, NJ) and pumped until the vacuum was about 3 to 4 × 10–7 Torr. Tungsten was evaporated at an angle of about 4 to 7 degrees while the stage containing the mica was rotating and then carbon was evaporated on top of the tungsten as a support layer. The replicas were floated off the mica onto a water surface and picked up onto 400-mesh copper grids and examined with a Philips EM 400T transmission electron microscope (FEI, Hillsboro, OR) at 80 KV. Micrographs were taken from a variety of representative areas at a magnification of about 60 K.
Analysis of PF4 multimerization
The spontaneous formation of dimers and tetramers by WT and mutant PF4 was examined. Each form of PF4 was purified as described in “Preparation of recombinant WT and mutant PF4,” concentrated to 1, 3, and 10 μg/mL, and incubated with the cross-linking reagent BS3 (2 mM) for 30 minutes. The reaction was stopped by adding NuPAGE lithium dodecyl sulfate (LDS) sample buffer, and denatured by heating to 70°C for 10 minutes according to the manufacturer's instructions. Aliquots (15 μL) were analyzed electrophoretically on a 12% SDS–polyacrylamide gel under reducing conditions. Bands were quantified using the Kodak 1D Image Analysis system (Kodak, Rochester, NY). SigmaMarker served as the molecular weight standard.
Recognition of PF4-heparin complexes by anti-PF4 antibodies
ELISA plate wells were coated with the mouse monoclonal HITT-like antibody KKO,27 mouse monoclonal anti-PF4 antibody RTO,27 or polyclonal rabbit anti-PF4 antibody or an isotype control MOPC141 antibody (10 μg/mL each) in Sigma coating buffer, overnight at 4°C, and blocked with 1% BSA. PF4 (1.5 mg/mL; 85.5 μM) was incubated with equimolar amounts of UFH to form complexes, and the variously sized complexes were separated as described in “Complexes between PF4 and heparin.” Fractions containing either PF4 tetramer alone or variously sized PF4-heparin complex sizes were identified. A 100-μL aliquot was taken from each peak fraction, adjusted to the concentration of 10 μg/mL PF4 protein, and added to the respective wells for 2 hours at room temperature (RT). Bound PF4 was detected by adding peroxidase-conjugated polyclonal anti-PF4 antibody and developed with TMB substrate. After stopping the enzyme reaction with 1 M HCl, the optical density (OD) at 450 nm was measured in a Molecular Devices (Sunnyvale, CA) plate reader.
Platelet activation by KKO in the presence of PF4-heparin complexes
Whole blood was collected from healthy, aspirin-free volunteers in acid citrate dextrose (ACD; pH 4.5, 10:1, vol/vol) under a protocol approved by the Institutional Review Board for Studies involving Human Subjects of the University of Pennsylvania. The blood was centrifuged at 200g for 15 minutes at RT to generate platelet-rich plasma (PRP). PGE1 (final concentration, 1 μg/mL) was added to the PRP to help prevent spontaneous platelet activation. PRP was centrifuged at 2000g for 10 minutes at RT, and the pellet washed and resuspended in modified Tyrode buffer (134 mM NaCl, 3 mM KCl, 0.3 mM NaH2PO4, 2 mM MgCl2,5mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], 5 mM glucose, 12 mM NaHCO3, 0.1% BSA [Sigma A7030; fatty acid-free]). Washed platelets were used at a final concentration of 2 × 108/mL and incubated with PF4-heparin complexes as prepared in “Complexes between PF4 and heparin”(final concentration 20 μg/mL) for 15 minutes at RT. KKO or an isotype control (TRA) antibody was then added to each well for an additional 45 minutes. Unstimulated platelets and platelets stimulated by either adenosine diphosphate (ADP; 5 μM) or phorbol myristate acetate (PMA; 0.5 μM) served as negative and positive controls, respectively. In other experiments, platelets were incubated with the FcγRIIA blocking antibody mAb IV.3 (50 μg/mL) for 30 minutes at RT before adding complexes. Platelets were identified by immunofluorescence with anti-CD41a monoclonal antibody using flow cytometry (FACScan; Becton Dickinson). Activation of platelets was measured by expression of P-selectin, PAC-1 binding. and annexin V binding in the presence of 2 mM CaCl2.
Results
Heterogeneity of PF4-heparin complexes
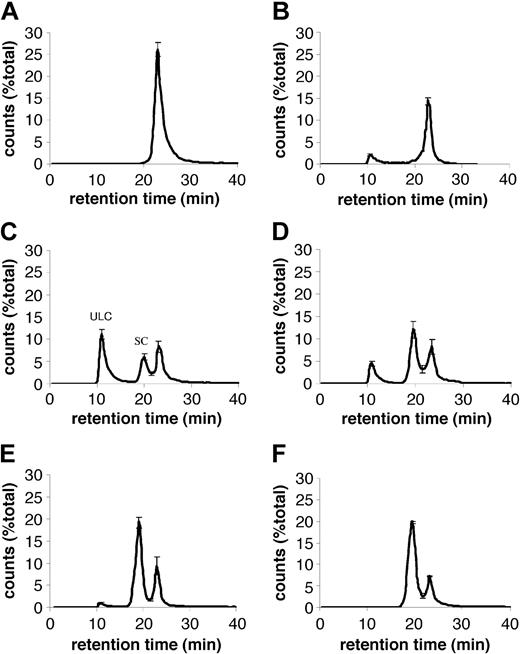
We studied the size distribution of complexes that form between PF4 and heparin at different molar ratios of reactants. In the absence of heparin, recombinant PF4 migrated on HPLC as a single peak at 23 minutes, corresponding to MW less than 44 kDa (Figure 1A), consistent with its predicted molecular weight of 35 kDa. When UFH (average MW, 15 kDa) was added to PF4, additional peaks with higher MW were identified. ULCs that migrated in the void volume of the column, corresponding to a MW greater than 670 kDa, were evident. ULCs formed at PF4-heparin ratios between 2:1 and 0.7:1 (Figure 1B-E). The total amount of ULCs formed was maximal at a PF4-heparin ratio of approximately 1:1. A broad peak of smaller complexes was noted over a retention time of 18 to 21 minutes with a calculated MW that ranged from 44 to 120 kDa. These smaller complexes predominated at PF4-heparin ratios below, but not above, 1:1.
Formation of PF4-heparin complexes. SEC-HPLC of PF4-heparin complexes formed at varying PF4-heparin ratios (PHRs). In the absence of heparin, PF4 migrates as a single peak at 23 minutes. In the presence of heparin, 2 populations of complexes are observed: ULCs and smaller complexes (SCs). A portion of the PF4 does not form complexes with heparin and elutes at 23 minutes. Data are expressed as the percentage of total counts of each experiment and represent the mean ± SEM of 3 independent experiments. (A) PF4; (B) PHR1.7:1; (C) PHR1.1:1; (D) PHR0.8:1; (E) PHR0.07:1; (F) PHR0.06:1.
Formation of PF4-heparin complexes. SEC-HPLC of PF4-heparin complexes formed at varying PF4-heparin ratios (PHRs). In the absence of heparin, PF4 migrates as a single peak at 23 minutes. In the presence of heparin, 2 populations of complexes are observed: ULCs and smaller complexes (SCs). A portion of the PF4 does not form complexes with heparin and elutes at 23 minutes. Data are expressed as the percentage of total counts of each experiment and represent the mean ± SEM of 3 independent experiments. (A) PF4; (B) PHR1.7:1; (C) PHR1.1:1; (D) PHR0.8:1; (E) PHR0.07:1; (F) PHR0.06:1.
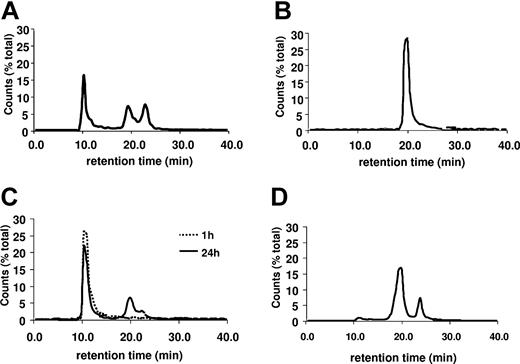
We next examined the stability of these 2 populations of PF4-heparin complexes. The chromatograph of PF4-heparin complexes formed at PF4-heparin ratio of 1:1 is shown in Figure 2A. The fraction containing small complexes was incubated for 24 hours at 37°C and then chromatography was repeated (Figure 2B). These small complexes eluted as a single peak at the original position (19.5 minutes; Figure 2B). The ULCs from the same experiment were studied in the same way. These ULCs also eluted as a single peak in the void volume after 1 hour of incubation at RT. Even when the complexes were incubated for 24 hours at 37°C, only 23% dissociated into smaller complexes and only 7% migrated as free PF4 tetramers (Figure 2C). However, addition of heparin to the ULC 30 minutes prior to incubation at 37°C (final PF4-heparin ratio = 1:2) resulted in almost their complete dissociation into small complexes and free PF4 (Figure 2D).
Stability of PF4-heparin complexes. SEC-HPLC of PF4-heparin complexes as in Figure 1. (A) Chromatograph of PF4-heparin complexes at a PHR of 1:1. (B) Rechromatograph of SC from (A) after 24 hours of incubation at 37°C. (C) Rechromatograph of ULCs from panel A after 1 or 24 hours of incubation at 37°C. (D) Rechromatograph of ULCs from panel A after incubation with excess heparin as described in “Results.”
Stability of PF4-heparin complexes. SEC-HPLC of PF4-heparin complexes as in Figure 1. (A) Chromatograph of PF4-heparin complexes at a PHR of 1:1. (B) Rechromatograph of SC from (A) after 24 hours of incubation at 37°C. (C) Rechromatograph of ULCs from panel A after 1 or 24 hours of incubation at 37°C. (D) Rechromatograph of ULCs from panel A after incubation with excess heparin as described in “Results.”
Visualization of PF4-heparin complexes by electron microscopy
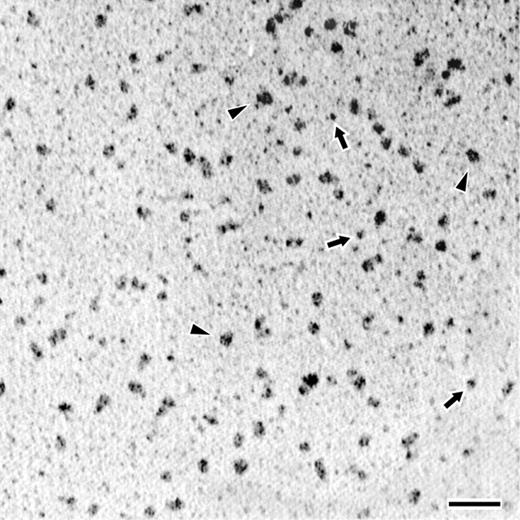
Next, PF4-heparin complexes were rotary shadowed and examined by electron microscopy. The complexes studied were prepared at a PF4-heparin ratio where only ULCs were expected to form (Figure 1C). As seen in Figure 3, electron microscopy showed an even distribution of particle size. The structures observed in these experiments were quite uniform in appearance and mostly globular in form, although they were commonly somewhat longer in 1 direction than in the other, with mean size of 7.5 ± 0.4 nm × 13.5 ± 2.0 nm. These dimensions are consistent with the SEC data and support a molecular weight of 650 kDa or greater. Although some of the particles had apparent substructure, the appearance was not consistent enough for more definitive identification.
Transmission electron microscopy of rotary shadowed PF4-heparin complexes. Fields demonstrating the appearance of PF4-heparin complexes at the optimal PF4-heparin ratio. Triangles (▸) indicate representative ULCs; arrows point to representative SCs. The coating of metal from the shadowing technique makes the extended structure of the IgG appear artificially larger in comparison to that of compact structure of the PF4-heparin complexes. Magnification bar = 50 nm.
Transmission electron microscopy of rotary shadowed PF4-heparin complexes. Fields demonstrating the appearance of PF4-heparin complexes at the optimal PF4-heparin ratio. Triangles (▸) indicate representative ULCs; arrows point to representative SCs. The coating of metal from the shadowing technique makes the extended structure of the IgG appear artificially larger in comparison to that of compact structure of the PF4-heparin complexes. Magnification bar = 50 nm.
Formation of ultralarge PF4-heparin complexes is dependent on heparin polymer length
The incidence of HITT is lower in patients treated exclusively with LMWH, although cross-reactivity of HITT antibodies with PF4-heparin complexes is well known. It has been proposed that shorter heparin chains are of insufficient length to efficiently bridge PF4 tetramers, but whether any ULCs form in the presence of LMWH or whether HITT antibodies can recognize complexes between 1 LMWH molecule and 1 PF4 tetramer is unknown.
To test these possibilities, we compared the ability of heparins of varying polymer length to form ULCs with PF4. The proportion of PF4 found in the form of ULCs at various PF4-heparin ratios for each form of heparin was determined (Table 1). There was a close relationship between the capacity of PF4 to form ULCs and the length of the heparin polymer. The greatest amount of PF4 in the form of ULCs was generated when PF4 was incubated with UFH at a PF4-heparin ratio of approximately 1:1. Less, but still significant amounts, of the ULCs formed between PF4 and enoxaparin (average molecular weight, 4.5 kDa), although in this case, complex formation was maximal at a PF4-heparin ratio of 1:2. Few ULCs were detected when PF4 was incubated with a smaller LMWH (average molecular weight, 3 kDa) even at a PF4-heparin ratio of 1:6, and no complexes were formed with the pentasaccharide fondaparinux sodium at any concentration tested (Table 1).
Ultralarge complexes formed with the various heparin formulations
PHR . | 1.7:1 . | 1.4:1 . | 1:1 . | 0.7:1 . | 1:2 . | 1:4 . | 1:6 . | 1:9 . |
|---|---|---|---|---|---|---|---|---|
| UHF | 14.8 | 23.0 | 29.5 | 10.1 | 0 | 0 | 0 | — |
| Enoxaprin 4.5 kDa | — | — | 3.3 | 14.6 | 18.4 | 4.0 | — | — |
| LMWH 3-4 kDa | — | 3.4 | 3.7 | 3.3 | 3.7 | 5.3 | 7.5 | 4.3 |
| Fondaparinux sodium pentasaccharide | — | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
PHR . | 1.7:1 . | 1.4:1 . | 1:1 . | 0.7:1 . | 1:2 . | 1:4 . | 1:6 . | 1:9 . |
|---|---|---|---|---|---|---|---|---|
| UHF | 14.8 | 23.0 | 29.5 | 10.1 | 0 | 0 | 0 | — |
| Enoxaprin 4.5 kDa | — | — | 3.3 | 14.6 | 18.4 | 4.0 | — | — |
| LMWH 3-4 kDa | — | 3.4 | 3.7 | 3.3 | 3.7 | 5.3 | 7.5 | 4.3 |
| Fondaparinux sodium pentasaccharide | — | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Proportion of PF4 found as ULCs was calculated as the area below the ULC peak (9-13 min) divided by area below whole curve × 100.
— indicates not tested.
Tetrameric structure of PF4 is critical to form ULCs
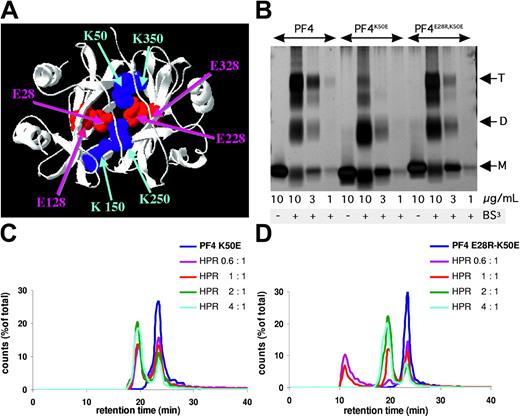
On the basis of these observations, we inferred that mutations that disrupt the capacity of PF4 monomers to form tetramers would preclude formation of these larger heparin-dependent complexes. The PF4 tetramer has a ring of positive charges, which most likely comprises the heparin-binding site.29,34 The most important forces stabilizing the PF4 tetramer involve 2 pairs of salt bridges29 (Glu128/Lys350, Glu328/Lys150, and Glu228/Lys450, Glu428/Lys250) (Figure 4A). Therefore, to disrupt formation of the PF4 tetramer, Lys50 was replaced with the analogous residues in IL-8 (Glu), creating PF4-K50E. We hypothesized that this substitution would disrupt/preclude formation of ionic bonds between position 28 and position 50. This substitution was chosen because IL-8, a homologous member of CXC chemokine family, forms dimers but is not known to form tetramers or higher-ordered structures.35 Additionally, a double mutation was constructed in which both the Glu28 and Lys50 residues in PF4 were replaced with those of IL-8, ie, PF4-E28R-K50E, that should theoretically restore the ability to form an ionic bond and thereby a PF4 tetramer.
Disruption of PF4 tetramers. (A) Cartoon based on the crystal structure of PF4, illustrating the salt bridges that may be important in tetramer formation. (B) SDS-PAGE demonstrating the equilibrium of PF4 monomer (M), dimers (D), and tetramers (T) in WT PF4 and PF4 with induced mutations after BS3 crosslinking. This gel is representative of 3 independent experiments. (C-D) Chromatographs from SEC-HPLC of PF4-K50E (C) and PF4-E28R-K50E (D), as in Figure 1.
Disruption of PF4 tetramers. (A) Cartoon based on the crystal structure of PF4, illustrating the salt bridges that may be important in tetramer formation. (B) SDS-PAGE demonstrating the equilibrium of PF4 monomer (M), dimers (D), and tetramers (T) in WT PF4 and PF4 with induced mutations after BS3 crosslinking. This gel is representative of 3 independent experiments. (C-D) Chromatographs from SEC-HPLC of PF4-K50E (C) and PF4-E28R-K50E (D), as in Figure 1.
Both PF4-K50E and PF4-E28R-K50E bound heparin as well as WT PF4 as judged by the requirement to use more than 1.0 M NaCl to elute the protein from a heparin column (data not shown). The ability of PF4-K50E and PF4-E28R-K50E to spontaneously form oligomers was examined after chemical cross-linking with BS3. Cross-linked oligomers were separated by SDS-PAGE. As predicted, the PF4-K50E variant existed in the form of monomers and dimers at higher concentrations, with little tetramer detected at any concentration tested (Figure 4B). Introduction of a second, complementary mutation in PF4-E28R-K50E restored tetramer formation to almost the same extent as were formed by WT PF4.
We next asked whether the capacity of PF4 to form tetramers is required to form ULCs in the presence of UFH, as assessed by SEC-HPLC. PF4-K50E, which did not form tetramers, also failed to form ULCs when incubated with UFH at any ratio of PF4-heparin tested (5:1-1:2), whereas it retained the ability to form smaller complexes (Figure 4C). In contrast, the double mutant PF4-E28R-K50E, which restored capacity to form tetramers, formed ULCs to nearly the same extent as WT PF4 (Figure 4D). In view of the fact that PF4-K50E retained its capacity to bind heparin, but is unable to form tetramers, these data support the concept that oligomerization involves the assembly of preformed PF4 tetramers on a heparin lattice.
Ultralarge PF4-heparin complexes are preferentially recognized by the HITT-like monoclonal antibody KKO.
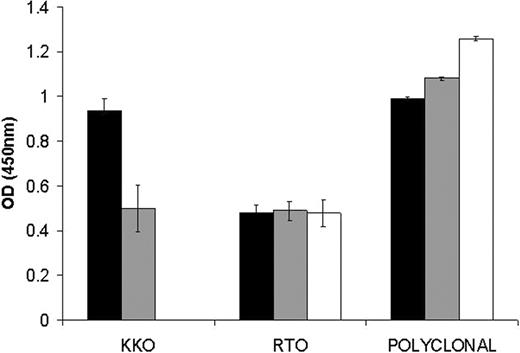
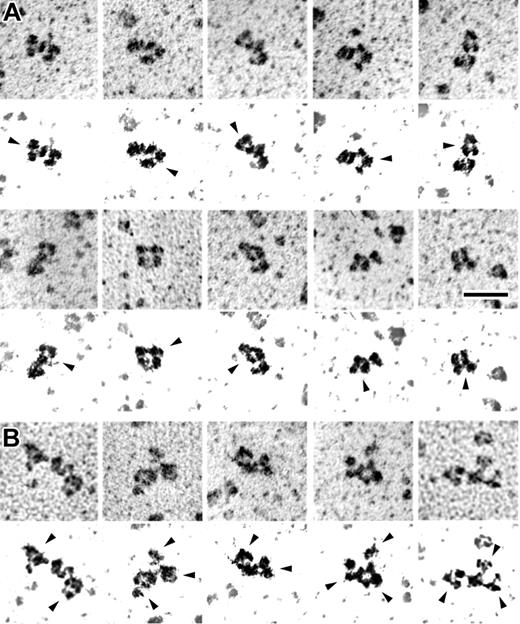
KKO is a monoclonal antibody that mimics a subset of HITT antibodies27 and that causes heparin-induced thrombocytopenia and thrombosis in a murine model.11 The ability of KKO to recognize the various PF4-heparin complexes was tested by using a capture ELISA. Wells were precoated with KKO or with monoclonal or polyclonal anti-PF4 and equal amounts by weight of PF4 from each of the 3 major fractions were added. As shown in Figure 5, KKO preferentially captures ULCs. Binding of the antibody to ULCs was verified by immunoelectron microscopy (Figure 6). Although antibodies are flexible structures, their 3-lobed structures were often seen binding to the PF4-heparin complexes (Figure 6A). In addition, numerous examples were visualized where there were 2 or more antibodies binding to 1 PF4-heparin complex (Figure 6B).
Recognition of complexes by KKO. Equal amounts of PF4 (□), ULCs (▪), or SCs (▦) were captured on immobilized KKO, RTO, or polyclonal antibody. The amount captured was measured with a secondary anti-PF4 antibody. Data are the mean ± SD of 3 independent experiments performed in triplicate.
Recognition of complexes by KKO. Equal amounts of PF4 (□), ULCs (▪), or SCs (▦) were captured on immobilized KKO, RTO, or polyclonal antibody. The amount captured was measured with a secondary anti-PF4 antibody. Data are the mean ± SD of 3 independent experiments performed in triplicate.
Transmission electron microscopy of rotary shadowed PF4-heparin complexes with KKO antibody bound. Galleries showing the appearance of PF4-heparin complexes with KKO antibody bound. IgG molecules have a typical 3-lobed structure, but their appearance can vary widely because they are quite flexible molecules. (A) Examples of PF4-heparin complexes with a single KKO bound. (B) Examples of PF4-heparin complexes with multiple KKO antibodies bound to each. Below each frame is very high contrast image to highlight the shadowed molecules and eliminate the background. Arrows point to the antibodies. Magnification bar = 50 nm. Adobe Photoshop 7.0 (Adobe, San Jose, CA) was used to produce high-contrast images.
Transmission electron microscopy of rotary shadowed PF4-heparin complexes with KKO antibody bound. Galleries showing the appearance of PF4-heparin complexes with KKO antibody bound. IgG molecules have a typical 3-lobed structure, but their appearance can vary widely because they are quite flexible molecules. (A) Examples of PF4-heparin complexes with a single KKO bound. (B) Examples of PF4-heparin complexes with multiple KKO antibodies bound to each. Below each frame is very high contrast image to highlight the shadowed molecules and eliminate the background. Arrows point to the antibodies. Magnification bar = 50 nm. Adobe Photoshop 7.0 (Adobe, San Jose, CA) was used to produce high-contrast images.
Sensitization of platelets by PF4-heparin complexes to antibody-mediated activation
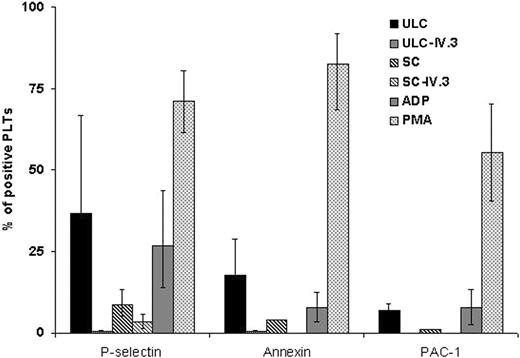
We next compared the capacity of the ultralarge and smaller PF4-heparin complexes to sensitize platelets for antibody-induced activation. Platelets were incubated with equal amounts of PF4 by weight in the form of ultralarge or smaller complexes, KKO was added, and platelet activation was examined by flow cytometry using 3 markers of platelet activation: P-selectin surface expression, annexin V binding, and binding of PAC-1 to activated GPIIb/IIIa. Platelets incubated with ULCs were activated upon addition of KKO (Figure 7), but not an isotype control (data not shown). The extent of platelet activation was greater in the presence of ULCs than when the same amount of small complexes was added (Figure 7). Platelet activation by KKO in the presence of both ultralarge and smaller complexes was abrogated by the FcγRIIA blocking antibody IV.3, demonstrating that the platelet activation was dependent on the formation of immune complexes.
Activation of human platelets by KKO and PF4-heparin complexes. Platelet activation as measured by flow cytometry examining P-selectin expression, PAC-1 binding, and annexin V binding. Platelet agonists, ADP and PMA, were included as positive controls. Data are presented as the percentage of activated platelets after each treatment and are the mean ± SD of 3 independent experiments.
Activation of human platelets by KKO and PF4-heparin complexes. Platelet activation as measured by flow cytometry examining P-selectin expression, PAC-1 binding, and annexin V binding. Platelet agonists, ADP and PMA, were included as positive controls. Data are presented as the percentage of activated platelets after each treatment and are the mean ± SD of 3 independent experiments.
Discussion
Our studies show that incubation of PF4 with heparin results in the formation of 2 general classes of complexes that differ with respect to size, composition, antigenicity, and capacity to promote antibody-induced platelet activation. These biophysical and functional parameters reflect differences in the content of PF4 and heparin. ULCs (> 670 kDa) that are readily visible by electron microscopy form when PF4 tetramers are present in approximately equimolar ratios with UFH. Decreasing the molar ratio of PF4 to heparin by as little as 40% reduces the proportion of ULCs that is formed with a concomitant increase in the proportion of smaller complexes.
Formation of ULCs was most efficient when PF4 was incubated with UFH. Far fewer ULCs were formed when small-intermediate molecular weight heparins were used (mean molecular weight, 3-4.5 kDa), but higher concentrations of heparin were required. No complexes formed when PF4 was incubated with the pentasaccharide fondaparinux sodium. These studies support the concept that the ULCs are composed of multiple PF4 tetramers held together in a lattice through their ability to bind several heparin molecules simultaneously, consistent with previously published studies.25 It is likely that an equilibrium exists between the ultralarge and intermediate-sized complexes. The proportion of these 2 forms varies depending, in part, on the length of the heparin polymer, which confers it with the capacity to bridge multiple tetramers. Formation of ULCs in the presence of LMWHs may be due to the presence of contaminating larger heparin chains, for example, as stated in the product information provided by the manufacturer, as is the case for enoxaprin. Thus, our data are consistent with the notion that heparin chains larger than 12 saccharide units are required to form antigenic PF4-heparin complexes or strongly promote their formation.36
The fact that small changes in heparin concentration caused a dramatic and precise shift in the size of the complex, as opposed to generating a series of complexes of intermediate molecular weights, lead us to estimate that the ULCs are composed of at least 16 PF4 tetramers linked in a lattice to several heparin molecules, as originally proposed by Greinacher et al37 based on the antigenicity of PF4 induced by diverse synthetic glycosaminoglycans, as well as fluoresce anisotropy data suggesting the capacity of PF4 and heparin to form very-high-molecular-weight PF4-heparin complexes.38
The PF4 variants we generated provide additional insight into the complexes formed between PF4 and heparin. PF4-K50E, which disrupts the salt bridge at the Glu28/Lys50 interface without affecting heparin binding, neither formed tetramers nor formed ULCs in the presence of heparin. PF4-K50E formed smaller complexes with UFH. Since PF4-K50E does not form tetramers, we hypothesize that these small complexes are composed of heparin chains partially wrapped around the cationic surface of PF4-K50E dimers. Restoring the salt bridge by introducing a complementary mutation (PF4-E28R-K50E) reconstituted both formation of tetramers and formation of the ULCs. Together, these studies provide indirect evidence that PF4 must form a tetramer, and the lengths of the heparin polymers must suffice to link these tetramers to optimize antigenicity.
Additional analytic approaches will be needed to determine the exact weight and composition of these complexes. Until such studies are performed, the precise molar concentration of complexes we studied cannot be determined. We have, therefore, controlled the present studies by equalizing the mass of PF4 in the complexes. Whatever their exact composition or compositions, it is clear that ULCs are stable for at least 24 hours. However, they can be dissociated by addition of excess heparin. These complexes are preferentially recognized by KKO, a HITT-like monoclonal antibody, in comparison to the smaller complexes, whereas free PF4 is not recognized at these concentrations. Moreover, these ULCs preferentially sensitize platelets to antibody and FcγRIIA-mediated activation compared with smaller complexes.
These results may have important implications for understanding the pathogenesis of HITT and the biology of PF4. We demonstrate that maximal amounts of ULCs form at a PF4-heparin ratio of 1:1, and exclusively at a ratio of 2:1, at least in solution. Formation of ultralarge complex formation is maximal when PF4 is incubated with UFH, while less ULCs are formed when PF4 is incubated with LMWH, again coincident with the observed difference in their antigenicity in the clinical setting.1,6,17 Indeed, we show that a HITT-like antibody binds with highest avidity to isolated ULCs, and that ULCs are more potent than smaller complexes in terms of sensitizing platelets to activation by a HITT-like antibody.
When PF4 and heparin were present in a 2:1 ratio, ULCs were formed exclusively (Figure 1). At a 1:1 ratio, more ULCs were formed, but smaller complexes were present as well. Several groups have reported that HIT antibodies show maximal binding to immobilized antigen when PF4 and heparin are incubated at a 2:1 molar ratio.26,39 There are several possible explanations for this seeming discrepancy. First, we estimated the mass of heparin used in our experiments based on the mean chain length of the UFH preparation used throughout our studies. This assumption was supported by experiments in which we found that PF4 depleted heparins of all chain lengths from UFH (data not shown). Nevertheless, these ratios should be considered as estimates. Second, our measurements of complex formation were made in solution rather than using measuring binding to PF4 immobilized on plastic surfaces, which might alter their composition and antigenicity. Third, it is possible that complexes formed at these ratios differ in their biologic activity. Small complexes bound the HIT-like antibody KKO less avidly than large complexes and were less capable of promoting platelet activation. It is possible that the smaller complexes may divert antibody from the ULCs, which are spatially better suited to occupy the limited number of FcγRIIA receptors available on the platelet surface. Studies are in progress to address this possibility. Of interest, this is consistent with the findings of Horne et al21,39 who observed that both PF4 and IgG binding to platelets was maximal at a 1:1 ratio of PF4-heparin.
The capacity of PF4 and heparin to form ULCs is an attractive mechanism for understanding the antigenic response in patients. It is known that repetitively arranged epitopes with the paracrystalline structure of a viral envelope cross-link B-cell receptors efficiently and induce the production of antibodies that recognize strictly ordered antigens.40 A similar role for repetitive self-antigens such as DNA or collagen has been implicated in various autoimmune diseases.41 Thus, the striking propensity for PF4, in complex with heparin, to become the target of auto-antibodies may relate to its capacity to oligomerize relative to other heparin-binding proteins.
A remarkable clinical feature of HITT is that, unlike most other drug-induced or idiopathic autoantibody conditions, anti–PF4-heparin antibodies predispose to thrombosis. Anti-PF4-heparin antibodies activate platelets in vitro and in vivo by causing signal transduction through FcγRIIA.16,42-44 Why most other platelet-reactive antibodies fail to activate platelets to a similar extent is unclear. We propose that the formation of ULCs by heparin and PF4 on the surface of platelets provides a dynamic structure that facilitates formation of an array of multiple IgG antibodies, as observed here by electron microscopy (Figure 6). This lattice affords the antibodies a greater opportunity to occupy and potentially to cross-link FcγRIIA receptors.45 Although we studied the effect of adding complexes exogenously, it is more likely that such complexes form on the platelet involving endogenous proteoglycans as well as PF4 and heparin.37,46 Studies are in progress to examine the size, composition, and antigenicity of complexes formed by the addition of PF4 to platelets and endothelial cells.
These studies may provide insight into several clinical observations as well. First, they may help explain the lower incidence of HITT in patients treated with LMWH. LMWH forms ULCs less efficiently than UFH and do so only at much lower PF4-heparin ratios. Therefore, LMWH would be predicted to be less likely to provoke antibody formation. However, once such antibodies are formed, they will recognize the smaller complexes formed by LMWH, and such complexes will activate platelets in the presence of HIT antibodies, albeit less efficiently than the ULCs. The fact that we were unable to detect the formation of complexes between PF4 and the heparin pentasaccharide fondaparinux sodium is consistent with the absence of antibody cross-reactivity reported by others,36,47 and provides a rationale for the use of fondaparinux sodium, or other small anionic or cationic molecules that may prevent or disrupt the formation of ULCs between PF4 and heparin in patients with HITT.
Prepublished online as Blood First Edition Paper, August 10, 2004; DOI 10.1182/blood-2004-04-1544.
Supported by grants from the American Heart Association (160508U, B.S.S.; 0325659U, L.R.; HL54500, M.P. and D.B.C.; HL69471, D.B.C., S.E.M., and M.P.R.; and HL68631, M.P. and D.B.C.) and the National Institutes of Health (1 K08 HL04245, B.S.S.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal