Abstract
We took advantage of the proliferative and permissive environment of the developing preimmune fetus to develop a noninjury large animal model in sheep, in which the transplantation of defined populations of human hematopoietic stem cells resulted in the establishment of human hematopoiesis and led to the formation of significant numbers of long-lasting, functional human liver cells, with some animals exhibiting levels as high as 20% of donor (human) hepatocytes 11 months after transplantation. A direct correlation was found between hepatocyte activity and phenotype of transplanted cells, cell dose administered, source of cells used on a cell-per-cell basis (bone marrow, cord blood, mobilized peripheral blood), and time after transplantation. Human hepatocytes generated in this model retained functional properties of normal hepatocytes, constituted hepatic functional units with the presence of human endothelial and biliary duct cells, and secreted human albumin that was detected in circulation. Transplanting populations of hematopoietic stem cells can efficiently generate significant numbers of functional hepatic cells in this noninjury large animal model and thus could be a means of ameliorating or curing genetic diseases in which a deficiency of liver cells or their products threatens the life of the fetus or newborn.
Introduction
Several authors have demonstrated the generation of donor-derived liver cells after bone marrow (BM) transplantation, providing optimistic evidence that stem cells within the adult hematopoietic system could potentially be of clinical usefulness to generate hepatocytes for replacing damaged or deficient liver tissue. Peterson et al1 first reported that rodent BM cells were able to give rise to oval cells and hepatocytes on transplantation into lethally irradiated rats. Since then, several other authors have reported similar results in murine recipients of BM transplantation.2-4 In this regard, in the most convincing study, Lagasse et al3 were able to rescue the hematopoietic system and to correct the liver defect in lethally irradiated fumarylacetoacetate hydrolase (FAH)–deficient mice by transplanting highly defined populations of mouse hematopoietic stem cells (HSCs). That HSCs may have the potential to give rise to hepatic elements was also suggested by the findings of Krause et al4 showing the presence of donor-derived hepatocytes in lethally irradiated mice rescued by the transplantation of a single mouse HSC. Although the study performed by Wagers et al,5 in which only a single adult HSC was transplanted, failed to show HSC contribution to the liver, direct and indirect studies in animals and humans strongly suggest that cells within the hematopoietic compartment are capable of generating hepatocytic cells under appropriate conditions, such as lethal irradiation or liver injury, and that, in the absence of selective pressure, the differentiation of BM cells into mature hepatocytes is highly inefficient.6,7 Wang et al8 showed that in the presence of hepatic injury, human HSC and progenitor cell populations had the capacity to generate cells within the recipient liver that synthesized and secreted human albumin into the sera of mice after transplantation. In humans, indirect observations have shown that donor-derived hepatocytes arise after HSC transplantation.9-11 However, the specific cell population that harbors hepatocytic potential has not yet been identified, and studies performed in animal models have shown that human mesenchymal cells and HSCs have the ability to generate hepatocyte-like cells in vivo.7,12-14 Whether the formation of liver cells under the different experimental conditions is a reflection of stem cell plasticity or is the result of donor and recipient cell fusion is not fully understood.15,16 This is especially true of the role of human HSCs and progenitor cells in liver formation. An experimental model system that would allow the formation of relatively large numbers of human-derived hepatic cells under near physiologic conditions would provide a valuable tool for the study of the mechanisms underlying the involvement of cells of human hematopoietic compartments in hepatopoiesis.
In the studies described here, we took advantage of the highly proliferative and permissive environment of the developing preimmune fetus to create a large animal model in sheep in which the transplantation of defined populations of human hematopoietic cells results not only in the establishment of multilineage human hematopoiesis14,17-23 but also in the formation of relatively large numbers of functional liver cells. In this human/sheep xenograft model, donor human hepatopoiesis persists long term and is associated with the synthesis and secretion of human albumin into sheep circulation.
Materials and methods
Isolation of human donor cells
Heparinized human adult BM and mobilized PB were obtained from healthy donors selected using the standard criterion of the American Association of Blood Banks (AABB) for blood donors. All donors signed informed consent according to guidelines from the Office of Human Research Protection at the University of Nevada, Reno. To obtain mobilized PB, granulocyte colony-stimulating factor (G-CSF) was administered, as previously described, subcutaneously for 5 days with a daily dose of 10 μg/kg, after which donors underwent apheresis. Umbilical cord blood (UCB) was collected by standard procedures used for UCB banking.24
Low-density mononuclear cells (MNCs) were separated using a Ficoll density gradient (1.077 g/mL; Sigma, St Louis, MO) and were washed twice in Iscove modified Dulbecco media (IMDM; Gibco Laboratories, Grand Island, NY). MNCs from each source were either enriched for CD34 (Miltenyi Biotec, Auburn, CA) or were depleted of lineage (Lin)–committed cells (StemSep column; StemCell Technologies, Vancouver, BC, Canada) according to the supplier's directions after staining the cells with the following cocktail of lineage monoclonal antibodies (mAbs): CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, and glycophorin A.
Immunofluorescence staining for flow cytometric analysis and selection of cells by cell sorting
Sorting of the Lin–, CD34+, and CD– populations and sorting for other additional different phenotypes used in these studies (CD38, CD133) were performed on a FACS Vantage (Becton Dickinson, Mountain View, CA) after labeling with the respective antibodies to various cluster designations directly conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) according to the manufacturer's recommendation. Flow cytometric analysis of the cell populations after sorting was performed on a FACScan (Becton Dickinson) to ensure the purity of the sorted populations.
Creation of the human sheep chimeras
Preimmune fetal sheep underwent transplantation at 55 to 60 days of gestation following the procedure described in detail previously.17,18 Cell phenotypes tested from human CB, adult BM, or mobilized PB were injected in a 0.5-mL volume by intraperitoneal injection. These populations were used because we have demonstrated in previous studies the ability of these cells to engraft and differentiate into all blood cell lineages in this human/sheep xenograft model.18-23 Numbers of sheep used in each experiment, cell phenotypes, and sources are described in detail in “Results” for each specific approach. All time points given refer to the length of time after transplantation. Because the gestation length in sheep is 145 days, some of the samples were collected before birth.
Assessment of human donor hematopoietic cell engraftment
Sheep that underwent transplantation were analyzed for donor (human) cell engraftment in BM and PB by flow cytometry at the posttransplantation intervals indicated for each experiment in “Results.” Monoclonal antibodies to various cluster designations (CDs) directly conjugated with FITC or PE were used according to the manufacturer's recommendation. These included CD3, CD7, CD10, CD13, CD20, CD34, CD45, CD33 (BDIS), and glycophorin A (Coulter Immunotech, Miami, FL).
Detection of human cells by immunohistochemistry
Paraffin sections 3- to 4-μm thick were prepared on poly-l-lysine–coated slides from formalin-fixed, paraffin-embedded tissues. Slides were incubated at 60° C for 12 to 16 hours and then were deparaffinated by two 10-minute incubations in xylene followed by rehydration through a graded ethanol series to deionized water. To facilitate antigen detection, slides were incubated at 90° C for 10 minutes in tissue-unmasking fluid (TUF; Signet Laboratories, Dedham, MA). Human cells were then detected with antibodies to human CD45 (DAKO, Carpinteria, CA) (to exclude hematopoietic contamination), human albumin (Sigma), and human hepatocytes OCH1E5 (DAKO) using a previously published methodology.16,24 OCH1E5 is an antihuman hepatocyte-specific antibody, and the antigen recognized by the antibody is present in normal human hepatocytes. The antibody reacts with human liver tissue producing a distinct, granular, cytoplasmic staining. We analyzed 6 different liver sections for each sheep that underwent transplantation and each age-matched control sheep. To exclude variability between lobes in some of the experiments, and as mentioned in “Results,” we analyzed 4 different sections from each of the 4 lobes of the liver of sheep that underwent transplantation and control sheep.
In situ hybridization for detection of human cells
After deparaffinization and hydration of paraffin-embedded sections from livers, slides were incubated for 5 minutes with proteinase K (100 μg/mL) and were postfixed in 1% formalin in phosphate-buffered saline (PBS) as previously described.24 A fluorescein-conjugated human ALU probe (Innogenex, San Ramon, CA) was applied, and denaturation was performed at 80° C for 5 minutes, followed by hybridization at 37° C for 2 hours. Slides were developed using the Innogenex in situ hybridization kit for fluorescein-labeled probes (Innogenex) according to the manufacturer's instructions.
ELISA for identification of human albumin in serum of chimeric sheep
To determine whether human hepatocytes within the sheep liver were functionally active, we examined the sera of sheep after transplantation for the presence of human albumin. We used an immunoenzymatic assay with an amplified biotin/streptavidin detection system that allows detection of human albumin in serum at less than 200 pg/mL (Cygnus Technologies, Plainville, MA). Sera were collected and analyzed according to the manufacturer's instructions immediately after collection or were frozen until enough samples were collected to perform the assay. All samples were run in triplicate. We included in the enzyme-linked immunosorbent assay (ELISA) 6 different concentrations of human albumin to create a standard curve from which to extrapolate the absorbance reading for each test sample. A triplicate sample of the diluent alone (no human albumin was included) was used to set the zero point for the ELISA. Sera from 5 control sheep were also included in the assay. The final absorbance obtained in our test samples was calculated after subtracting the zero point absorbance. Sheep control samples gave absorbance levels equal to or lower than those of the zero points.
In situ hybridization with sheep-specific and human-specific probes to assess the occurrence of cell fusion
Livers were harvested from sheep that received transplantations of different populations of human stem cells in utero and were then paraffin embedded and sectioned according to standard procedures. To perform in situ hybridization, sections were first deparaffinized by heating for 15 minutes on a 60° C hot plate until the paraffin melted and evaporated. Slides were then washed in xylene for 10 minutes, air dried, and fixed in methanol/acetic acid (3:1) for 45 seconds. Slides were allowed to air dry. Before hybridization, slides were treated with Proteinase K (10 μg/mL) for 1 hour at 37° C in 10 mM Tris/0.1% sodium dodecyl sulfate (SDS), washed twice in 2 × SSC for 5 minutes each, and then incubated in RNase A (100 μg/mL) diluted in 2 × SSC for 1 hour at 37° C. Slides were rinsed twice in diH2O, dehydrated through a graded ethanol series, and air dried. For hybridization, 12.5 μL human pericentromeric Texas red probe25 and 12.5 μL ovine pericentromeric FITC probe26 were premixed in an Eppendorf tube and pipetted onto each slide. A coverslip was then applied and sealed with rubber cement.
Probe and target DNA sequences were co-denatured on a 90° C hot plate as follows: 6 minutes on, 2 minutes off, 5 minutes on, 1 minute off, 4 minutes on. Slides were incubated overnight at 37° C in a humidified chamber. To generate the Texas red–labeled human pericentromeric probe, we performed 40 cycles of polymerase chain reaction (PCR) on human genomic DNA using standard PCR conditions with the addition of Texas red-12-dUTP (Molecular Probes, Eugene, OR) at a ratio of 3:1 dTTP/dUTP-Texas red. After PCR, identity or purity of the product was confirmed by gel electrophoresis, and the product was purified by passage through a QIAquick DNA Cleanup spin column (Qiagen, Valencia, CA). The FITC-labeled ovine pericentromeric probe was generated using a commercially available PCR-based FITC-dUTP labeling system (Roche Applied Science, Indianapolis, IN) and was also purified by passage through a QIAquick column after product identity/purity assessment by gel electrophoresis.
After overnight hybridization, the rubber cement was removed and the coverslips were floated off in Tris saline. Slides were washed 3 times for 5 minutes each at 37° C in 50% formamide/2 × SSC with agitation and 3 times for 5 minutes each at room temperature in 0.1 × SSC. Slides were subsequently counterstained with DAPI-Vectashield (Vector Laboratories, Burlingame, CA) and were viewed through an Olympus BX-60 fluorescent compound microscope equipped with the following objective lenses: 10 × (f 0.25), 40 × (f 0.75), and 60 × oil (f 1.4) all from Olympus (Olympus America, Melville, NY). Images were acquired with a Q-Color 5 cooled charge-coupled device (CCD) camera system using Q-Capture Pro software (Olympus America). Images were then processed using Adobe Photoshop (Adobe Systems, San Jose, CA).
Results
Adult human hematopoietic stem cells generate significant numbers of hepatocytes in a noninjury fetal model
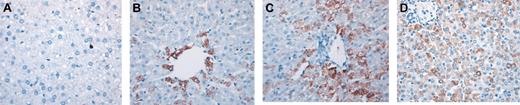
To begin addressing whether populations of human hematopoietic cells could give rise to hepatocytes in vivo in the absence of liver injury, we performed a retrospective study in which we analyzed sheep that underwent transplantation in utero with various doses of human CD34+Lin– cells or CD34–Lin– cells from adult BM or CB. All recipients exhibited significant human hematopoietic activity (including the presence of human CD34+ cells) at the time points analyzed. Table 1 summarizes the phenotypes and doses of human cells transplanted in these studies and the times after transplantation at which livers were collected and evaluated for the presence of donor (human)–derived hepatocytes. To identify human hepatocytes in sheep liver sections, we performed immunohistochemistry in 6 different sections from each sheep that underwent transplantation and each control sheep using a monoclonal antihuman hepatocyte antibody (clone OCH1E5) that did not recognize sheep hepatocytes (Figure 1A). Furthermore, analysis of liver sections with an antibody specific for CD45 did not show the presence of human hematopoietic cells in the parenchyma of the liver at any time point (data not shown). For each sheep analyzed, the variability of the percentage of human/donor hepatocytes between sections was not significant. Results obtained were then confirmed by in situ hybridization with a human Alu-specific probe. CD34+Lin– cells from human adult BM and from CD34+Lin– cells and CD34–Lin– cells from human CB generated hepatocytes in vivo. In sheep that received transplanted BM-derived CD34+Lin– cells, 1.8% and 3% of the hepatocytes present in the liver sections analyzed at 3 weeks after transplantation were of human origin (Figure 1B). At 4 months after transplantation, significantly higher numbers of human hepatocytes were present in numerous sites throughout the host liver, often in close association with portal spaces (Figure 1C). Within each site, donor hepatocytes represented more than 80% of total hepatic cells. Overall, in these animals, human hepatocytes comprised approximately 5.5% and 10% of the total liver cellularity in each histologic section. There was minimal variability in the percentage of human cells seen in different sections from the same sheep, as can be seen by the small standard deviation seen for each sheep. In the animal that received transplanted CB-derived CD34–Lin– cells and was analyzed 11 months after transplantation, donor hepatocytes were more uniformly distributed throughout the sections and represented 15% to 20% of the total cells in liver sections of that animal (Figure 1D).
Human HSCs generate significant numbers of hepatocytes in a noninjury fetal model
Phenotype . | Source . | Cell dose . | No. animals . | Time after transplantation . | Human hepatocytes, % . |
|---|---|---|---|---|---|
| CD34+Lin- | BM | 5.0 × 106 | 2 | 3 wk | 1.8 ± 0.1 |
| 3.0 ± 0.05 | |||||
| CD34+Lin- | BM | 1.3 × 105 | 2 | 5 wk | 2.0 ± 0.08 |
| 3.2 ± 0.1 | |||||
| CD34+Lin- | BM | 6.7 × 104 | 2 | 4 mo | 5.5 ± 0.2 |
| 10 ± 0.8 | |||||
| CD34+Lin- | CB | 3.8 × 105 | 2 | 2 mo | 1.0 ± 0.01 |
| 2.0 ± 0.05 | |||||
| CD34-Lin- | CB | 1.1 × 106 | 1 | 2 mo | 2.0 ± 1.0 |
| CD34-Lin- | CB | 5.0 × 105 | 1 | 11 mo | 17.0 ± 2.0 |
Phenotype . | Source . | Cell dose . | No. animals . | Time after transplantation . | Human hepatocytes, % . |
|---|---|---|---|---|---|
| CD34+Lin- | BM | 5.0 × 106 | 2 | 3 wk | 1.8 ± 0.1 |
| 3.0 ± 0.05 | |||||
| CD34+Lin- | BM | 1.3 × 105 | 2 | 5 wk | 2.0 ± 0.08 |
| 3.2 ± 0.1 | |||||
| CD34+Lin- | BM | 6.7 × 104 | 2 | 4 mo | 5.5 ± 0.2 |
| 10 ± 0.8 | |||||
| CD34+Lin- | CB | 3.8 × 105 | 2 | 2 mo | 1.0 ± 0.01 |
| 2.0 ± 0.05 | |||||
| CD34-Lin- | CB | 1.1 × 106 | 1 | 2 mo | 2.0 ± 1.0 |
| CD34-Lin- | CB | 5.0 × 105 | 1 | 11 mo | 17.0 ± 2.0 |
Human HSCs were isolated by cell sorting and were transplanted intraperitoneally into preimmune sheep fetuses at 55 to 65 days of gestation. Values for human hepatocytes represent the average ± SD of hepatocytes found in each of the animals analyzed at a given time point.
Adult human HSCs generate significant numbers of hepatocytes in a noninjury fetal model. (A) Control sheep liver section (nontransplanted) stained with an antibody antihuman hepatocyte (clone OCH1E5) as described in “Materials and methods.” (B-C) Liver sections obtained at 3 weeks (B) and 4 months (C) after transplantation from sheep that received transplanted human BM CD34+Lin– cells, stained with the same antibody showing a higher number of human hepatocytes in the latter (C). (D) Liver section obtained at 11 months after transplantation from sheep that received transplanted CB-derived CD34–Lin– cells, stained with antihuman hepatocyte antibody. Human hepatocytes in all sections can be identified by the dark brown coloration.
Adult human HSCs generate significant numbers of hepatocytes in a noninjury fetal model. (A) Control sheep liver section (nontransplanted) stained with an antibody antihuman hepatocyte (clone OCH1E5) as described in “Materials and methods.” (B-C) Liver sections obtained at 3 weeks (B) and 4 months (C) after transplantation from sheep that received transplanted human BM CD34+Lin– cells, stained with the same antibody showing a higher number of human hepatocytes in the latter (C). (D) Liver section obtained at 11 months after transplantation from sheep that received transplanted CB-derived CD34–Lin– cells, stained with antihuman hepatocyte antibody. Human hepatocytes in all sections can be identified by the dark brown coloration.
Donor hepatocyte activity correlates with donor hematopoietic engraftment and time after transplantation
To find a plausible explanation for the different numbers of human hepatocytes generated by the same source of cells in the previous studies, we hypothesized that the generation of hepatocytes in vivo correlated with the levels of hematopoietic engraftment and the length of time after transplantation. Because we believe that different sources and cell phenotypes had impacts on the outcomes of donor-derived HSC and liver cell production in these studies, we investigated whether the generation of hepatocytes from BM CD34+Lin– cells in vivo correlated with the levels of hematopoietic engraftment. To this end, we compared the percentages of donor-derived hepatocytes with the levels of human hematopoietic engraftment at 3 weeks, 5 weeks, and 4 months after transplantation (Table 2). For the same time point after transplantation, a direct correlation was found between hepatocyte formation and hematopoietic cell engraftment. This was evident from the increased percentage of donor-derived hepatocytes in animals exhibiting higher levels of human hematopoiesis. Three weeks after transplantation, the percentage of human blood cells in circulation as assessed by CD45 positivity varied from 0.8% to 1.1%, and the percentages of human hepatocytes found in the liver section of these sheep fetuses were correspondingly 1.8% and 3%. Five weeks after transplantation, levels of hematopoietic cell engraftment of 0.62% and 1.5% corresponded to 2% and 3.2% of human hepatic cells, respectively. Four months after transplantation, a similar effect was found, with 0.87% of hematopoietic engraftment corresponding to 5.5% of human hepatic cells and 6.4% of human blood production to 10% of human hepatopoiesis. We also compared the percentages of human hepatocytes in sheep that had similar levels of hematopoietic engraftment at different time points after transplantation to investigate the possibility that the number of hepatocytes increased over time. We compared the percentage of human hepatocytes in the sheep that had 1.1% of human cells in circulation 3 weeks after transplantation with that of the sheep that had 0.87% of human hematopoietic chimerism 4 months after transplantation. As can be seen in Table 2, a relative increase in the percentage of human hepatocytes was found 4 months after transplantation (5.5%) when compared with the 3-week time point (3%). Of note is that because of the differences in liver sizes at 3 weeks (approximately 45-60 g) and 4 months (approximately 150-250 g), after transplantation, the difference between 3% and 5.5% is highly significant. Furthermore, the data in Table 3 concurs with the data in Table 2. At 8 weeks after transplantation, for instance, looking at adult BM, 1.9% of human CD45 corresponded to 0.6% human hepatocytes and 3.3% corresponded to 1.2% human hepatocytes. With CB the same effect is seen; 3% CD45 corresponds to 0.18%, whereas 4% CD45+ corresponds to 0.2%. The same is seen in mobilized PB. We do not, however, think there is a general broad correlation between hematopoietic engraftment and liver production. We realize that we cannot compare different phenotypes or different sources of stem cells because we know that different sources and phenotypes do have different potentials. For this reason, our findings and analyses are restricted to exactly the same source of cells; in this case, the results suggest that there is a correlation between hematopoietic engraftment and liver formation for the same type of cells.
Donor hepatocyte activity correlates with cell dose, donor hematopoietic engraftment, and time after transplantation
Cells injected . | No. animals . | Time after transplantation . | Human hepatocytes, % . | Human CD45+ cells, % . |
|---|---|---|---|---|
| BM 34+Lin- | 2 | 3 wk | 1.8 ± 0.05 | 0.8 |
| 3 ± 0.1 | 1.1 | |||
| BM 34+Lin- | 2 | 5 wk | 2 ± 0.03 | 0.62 |
| 3.2 ± 0.01 | 1.5 | |||
| BM 34+Lin- | 2 | 4 mo | 5.5 ± 0.2 | 0.87 |
| 10 ± 0.08 | 6.4 |
Cells injected . | No. animals . | Time after transplantation . | Human hepatocytes, % . | Human CD45+ cells, % . |
|---|---|---|---|---|
| BM 34+Lin- | 2 | 3 wk | 1.8 ± 0.05 | 0.8 |
| 3 ± 0.1 | 1.1 | |||
| BM 34+Lin- | 2 | 5 wk | 2 ± 0.03 | 0.62 |
| 3.2 ± 0.01 | 1.5 | |||
| BM 34+Lin- | 2 | 4 mo | 5.5 ± 0.2 | 0.87 |
| 10 ± 0.08 | 6.4 |
Values for human hepatocytes represent the average of hepatocytes found in 6 different liver sections from each of the animals analyzed at a given time point ± SD.
As it can be seen at each time point, a correlation exists between the percentage of human hepatocytes and the percentage of human CD45+ cells. Correlation coefficient was calculated according to the formula:
Levels of CD45+ cells of hematopoietic engraftment reflect only the levels of human CD45+ cells in PB and BM.
Comparison of the hepatocytic potential of transplanted cells from different sources
. | CD34+Lin- . | . | . | ||
|---|---|---|---|---|---|
| Time after transplantation . | CB . | Adult BM . | Mobilized PB . | ||
| 8 wk | |||||
| Human CD45, % | |||||
| Sheep 1 | 3.0 | 1.9 | 3.0 | ||
| Sheep 2 | 4.0 | 3.3 | 6.0 | ||
| Human hepatocytes, % | |||||
| Sheep 1 | 0.18 ± 0.01 | 0.6 ± 0.1 | 0.05 ± 0.01 | ||
| Sheep 2 | 0.22 ± 0.02 | 1.2 ± 0.05 | 0.08 ± 0.01 | ||
| 10 wk | |||||
| Human CD45, % | |||||
| Sheep 1 | 2.0 | 2.2 | 1.46 | ||
| Sheep 2 | 3.2 | — | 2.7 | ||
| Human hepatocytes, % | |||||
| Sheep 1 | 1.0 ± 0.01 | 3.0 ± 0.04 | 0.1 ± 0.0 | ||
| Sheep 2 | 2.0 ± 0.01 | — | 1.0 ± 0.01 | ||
| Human albumin, μg/mL | |||||
| Sheep 1 | 0.18 | 0.6 | 0.0 | ||
| Sheep 2 | 0.2 | 1.2 | 0.01 | ||
. | CD34+Lin- . | . | . | ||
|---|---|---|---|---|---|
| Time after transplantation . | CB . | Adult BM . | Mobilized PB . | ||
| 8 wk | |||||
| Human CD45, % | |||||
| Sheep 1 | 3.0 | 1.9 | 3.0 | ||
| Sheep 2 | 4.0 | 3.3 | 6.0 | ||
| Human hepatocytes, % | |||||
| Sheep 1 | 0.18 ± 0.01 | 0.6 ± 0.1 | 0.05 ± 0.01 | ||
| Sheep 2 | 0.22 ± 0.02 | 1.2 ± 0.05 | 0.08 ± 0.01 | ||
| 10 wk | |||||
| Human CD45, % | |||||
| Sheep 1 | 2.0 | 2.2 | 1.46 | ||
| Sheep 2 | 3.2 | — | 2.7 | ||
| Human hepatocytes, % | |||||
| Sheep 1 | 1.0 ± 0.01 | 3.0 ± 0.04 | 0.1 ± 0.0 | ||
| Sheep 2 | 2.0 ± 0.01 | — | 1.0 ± 0.01 | ||
| Human albumin, μg/mL | |||||
| Sheep 1 | 0.18 | 0.6 | 0.0 | ||
| Sheep 2 | 0.2 | 1.2 | 0.01 | ||
Data represent donor (human) hematopoietic activity and hepatocytic production in primary sheep recipients 8 and 10 weeks after transplantation. For each time point, 2 fetal sheep underwent transplantation with 2 × 104 CD34+Lin- cells from each of these sources.
Human hepatocyte values represent ranges of percentages found in 4 different sections from 4 different lobes of livers of the 2 animals per group to undergo transplantation. — indicates not done.
Comparison of the hepatocytic potential of HSC populations from different sources
After determining that time after transplantation and levels of hematopoietic engraftment correlated with human-derived hepatocytic activity, we compared the ability of identical doses of human CD34+Lin– cells isolated from adult human BM, CB, or mobilized PB to generate functional human hepatocytes after in utero transplantation. To this end, fetal sheep underwent transplantation with 2 × 104 CD34+Lin– cells from each of these sources (n = 6). A small number of cells were used in these studies to facilitate quantitative comparison of the hepatocytic potential of the different populations. Eight weeks after transplantation, recipients were analyzed for the presence of human hematopoietic cells in BM and PB as an indicator of successful transplantation. Although multilineage engraftment was obtained with all sources tested, the highest level of human blood cell production was achieved with mobilized PB as assessed by flow cytometry for the presence of human CD45+ cells (Table 3). Immunohistochemistry was performed on 4 sections prepared from each of the 4 lobes of the liver of sheep after transplantation using a monoclonal antibody specific for human hepatocytes. In addition, in situ hybridization was performed using a human Alu-specific probe. Donor-derived hepatocytes were detected in livers of all animals after transplantation, regardless of the source of donor cells. Although some lobes exhibited greater positivity than others, overall there did not appear to be a preferred distribution of human hepatocytes in one lobe versus another. Human adult BM-derived CD34+Lin– cells generated hepatocytes with the highest efficiency (0.6%-1.2% human hepatocytes), whereas CB cells with the same phenotype only generated a maximum of 0.2% human hepatocytes at this same time point. Few human hepatocytes were produced by the CD34+Lin– fraction from mobilized PB (Table 3).
Expansion of donor hepatocytes over time
We were unable to detect human hepatocytes in any of the sheep recipients before 3 weeks after transplantation at the dose of 2 × 104 CD34+Lin– cells/fetus from CB, adult BM, or mobilized PB. To evaluate the expansion of donor hepatocytes over time, 6 more sheep underwent transplantation with 2 × 104 CD34+Lin– cells (2 fetus/group) from each source and were analyzed at 10 weeks after transplantation, 2 weeks later than in the previous study. For each given population, we observed a higher number of human hepatocytes within the sheep analyzed at 10 weeks after transplantation compared with those analyzed at 8 weeks, suggesting that the hepatocytes generated from the hematopoietic graft expanded over time, gradually comprising a larger percentage of the total hepatic mass. As we had seen at 8 weeks after transplantation, sheep that received transplanted BM CD34+Lin– cells had higher levels of human hepatocytes than sheep that received transplanted CB or mobilized PB 10 weeks after transplantation (Table 3).
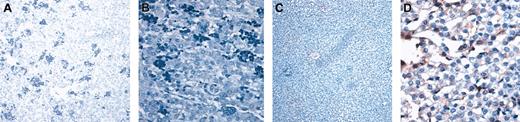
Human hepatocytes generated in this in vivo model display the functional properties of normal human hepatocytes and constitute hepatic functional units. To determine whether the human hepatocytes generated in this large animal model displayed the functional properties of human hepatocytes, we performed immunohistochemistry using an antibody specific for human albumin. As can be seen in Figure 2, sections of liver tissue that contained human hepatocytes also contained human albumin-producing cells, demonstrating that the human hepatocytes generated in vivo from the human hematopoietic graft were functional. Significantly, the percentage of human hepatocytes identified by OCH1E5 correlated well with the percentage of human hepatocytes detected using the antialbumin antibody. Furthermore, the average levels of human albumin found in the sera of sheep that underwent transplantation with BM, CB, and mobilized PB were 0.6 and 1.2 ng/mL, 0.18 and 0.2 ng/mL, and 0 and 0.01 ng/mL, respectively (Table 3), correlating well with the differences we saw in the numbers of hepatocytes generated by the stem cells from each of these sources.
Human hepatocytes generated in vivo from the human hematopoietic stem cells are functional. (A-B) Low (A) and high (B) magnification of sections of livers from control (no transplantation) sheep stained with an antibody specific for human albumin. (C-D) Low (C) and high (D) magnification of a section from sheep liver given transplanted BM CD34+Lin– cells that had the highest amount of human albumin in serum. Human hepatocytes can be identified by the light brown coloration.
Human hepatocytes generated in vivo from the human hematopoietic stem cells are functional. (A-B) Low (A) and high (B) magnification of sections of livers from control (no transplantation) sheep stained with an antibody specific for human albumin. (C-D) Low (C) and high (D) magnification of a section from sheep liver given transplanted BM CD34+Lin– cells that had the highest amount of human albumin in serum. Human hepatocytes can be identified by the light brown coloration.
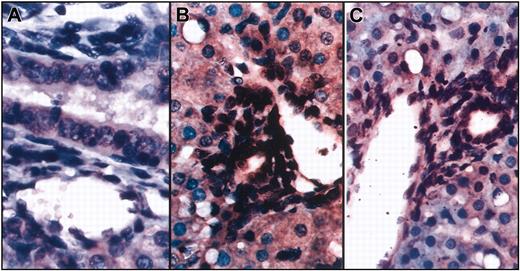
To further evaluate the differentiation potential of transplanted human cells in vivo, we performed in situ hybridization on the same liver sections of the sheep that underwent transplantation using a probe specific for the human Alu sequence and an antibody specific for human CD45. As can be seen in Figure 3, numerous cell nuclei within the vessel walls and biliary ducts were of human origin and were negative for CD45, excluding the possibility that these cells were hematopoietic in nature. These findings are of particular importance because they demonstrate that the in utero approach to human cell transplantation is capable of producing human portal triads within the human hepatic parenchyma.
In situ hybridization shows that cells within the vessel walls and biliary ducts are of human origin. Combined in situ hybridization and immunohistochemistry were performed on liver sections from sheep that underwent transplantation using a probe specific for the human Alu sequence and an antibody specific for human CD45. (A) Control (no transplantation) sheep. (B-C) Two different sections show numerous cell nuclei within the vessel walls and biliary ducts stained dark brown, demonstrating that they are of human origin. None of these cells exhibit red staining for CD45.
In situ hybridization shows that cells within the vessel walls and biliary ducts are of human origin. Combined in situ hybridization and immunohistochemistry were performed on liver sections from sheep that underwent transplantation using a probe specific for the human Alu sequence and an antibody specific for human CD45. (A) Control (no transplantation) sheep. (B-C) Two different sections show numerous cell nuclei within the vessel walls and biliary ducts stained dark brown, demonstrating that they are of human origin. None of these cells exhibit red staining for CD45.
Human BM CD34+Lin–CD38– cells have higher hepatocytic potential
To begin defining which specific subsets of human BM hematopoietic cells harbor hepatocytic potential, we assessed the hematopoietic and hepatopoietic activities of phenotypically defined populations of BM-derived cells. Thus, we performed transplantation on 10 fetal sheep to compare the hepatocytic potentials among CD34+Lin–CD38– cells, CD34–Lin–CD38– cells, CD34+CD133+ cells, CD34+CD133– cells, and CD34+Lin– cells. Sections prepared from the livers of sheep that underwent transplantation with the different populations were analyzed by immunohistochemistry with antihuman hepatocyte and antihuman albumin antibodies. CD34+Lin–CD38– cells were able to generate more hepatocytes in vivo (8%-10%) than any other phenotype tested, suggesting that the CD34+Lin–CD38– fraction of cells from adult human BM are enriched not only for HSCs but also for cells with hepatocytic potential. By contrast, the CD34–Lin–CD38– cell population was only able to generate 0.9% to 2% hepatocytes, and CD34+CD133+ (0.1%-0.5%) and CD34+CD133– (0.3%-0.6%) cells gave rise to fewer donor hepatocytes than either the CD34+Lin– (3%-5%) or the CD34+Lin–CD38– cell population (8%-10%).
Some populations of human HSCs maintain their ability to produce human hepatocytes in secondary sheep recipients
We used serial transplantation to investigate whether different phenotypes of human HSCs that had migrated to and engrafted within the BM retained their ability to produce hepatocytes. To this end, we transplanted human CD45+ cells isolated from the BM of chimeric primary sheep recipients that had received CD34+Lin–CD133+, CD34+Lin–CD38–, or CD34–Lin–CD38– cells into secondary 55- to 60-day-old fetal sheep recipients. Three months after transplantation, we humanely killed the secondary sheep recipients and evaluated the presence of hematopoietic chimerism and human hepatocytes in the BM and liver, respectively. All secondary sheep recipients exhibited human hematopoietic chimerism as determined by flow cytometric analysis using a human-specific CD45 antibody. Secondary recipients (n = 3) of the transplanted CD34+Lin–CD38– exhibited 3.3% to 4% human cells in BM and 0.44% to 6.54% in PB. Secondary recipients (n = 3) of the transplanted CD34–Lin–CD38– had donor cell levels of 0.06% to 2.3% in BM and 0.42% to 8% in PB. CD34+Lin–CD133+ cells generated approximately 0.5% human cells in the BM and 0.21% in the PB in secondary recipients after transplantation (n = 2).
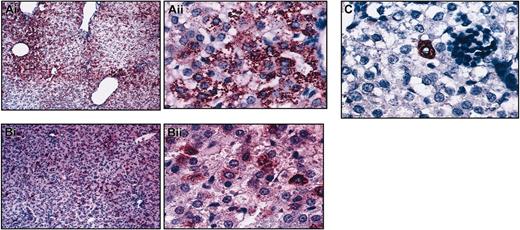
Secondary recipients of CD34+Lin–CD38– (n = 3) and CD34–CD38–Lin– (n = 3) displayed multiple hepatocytic foci throughout the hepatic parenchyma at levels similar to those generated in the primary recipients, demonstrating that cells present within these phenotypically defined populations that had migrated to the marrow at primary transplantation retained hepatocytic potential. Despite the hematopoietic engraftment in secondary recipients of transplanted CD34+CD133+Lin– cells, few hepatocytes were found in the liver sections of these sheep, suggesting once more that different phenotypically defined populations of HSCs have different characteristics regarding hepatocyte production (Figure 4).
Different phenotypes of HSC have different characteristics regarding hepatocyte production. Low and high magnification of sections of livers from secondary sheep that received transplanted human cells from (A) CD34+Lin–CD38–, (B) CD34–CD38–Lin–, or (C) CD34+CD133+ Lin– primary recipients (high magnification Ai, Bii, C). Liver sections were stained with an antibody antihuman hepatocyte (clone OCH1E5), as described in “Materials and methods.” Animals that received transplanted CD38– populations displayed multiple hepatocytic foci throughout the hepatic parenchyma at levels similar to those generated in the primary recipients (A-B). Few hepatocytes were found in the liver sections of CD34+CD133+Lin– secondary recipients (C).
Different phenotypes of HSC have different characteristics regarding hepatocyte production. Low and high magnification of sections of livers from secondary sheep that received transplanted human cells from (A) CD34+Lin–CD38–, (B) CD34–CD38–Lin–, or (C) CD34+CD133+ Lin– primary recipients (high magnification Ai, Bii, C). Liver sections were stained with an antibody antihuman hepatocyte (clone OCH1E5), as described in “Materials and methods.” Animals that received transplanted CD38– populations displayed multiple hepatocytic foci throughout the hepatic parenchyma at levels similar to those generated in the primary recipients (A-B). Few hepatocytes were found in the liver sections of CD34+CD133+Lin– secondary recipients (C).
Formation of human hepatocytes in chimeric sheep does not appear to be caused by fusion
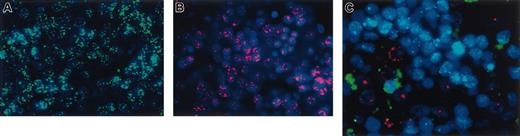
We next used in situ hybridization with sheep-specific and human-specific probes to examine whether cell fusion played a role in the mechanism whereby the transplanted human cells produced hepatocytes. As can be seen in Figure 5A-B, the probes were completely species specific, with a control sheep displaying only the green signal from the sheep probe (Figure 5A), whereas the control human liver exhibited only red fluorescence from the human probe (Figure 5B). When we examined the livers from chimeric sheep, we observed that the human hepatocytes present within the sections exhibited pure red fluorescence, demonstrating that they contained only human DNA and not sheep DNA. A representative section from one of the chimeric sheep can be seen in Figure 5C. To date, we have examined multiple sections (n = 4) and have screened from 150 to 500 human cells in each section from 6 different chimeric sheep that underwent transplantation with CD34+38–Lin– or CD34+Lin– cells. We chose these 2 groups because these donor populations consistently produced the highest levels of donor-derived hepatopoiesis, with levels of human hepatocytes ranging from 3% to 10%, as seen in the results described above. Thus far, all the human hepatocytes examined have exhibited exclusively human genetic material, providing evidence that fusion does not likely play a major role in the generation of hepatocytes from transplanted human cells in this model system. However, because these sections were analyzed at a time during gestation when liver cells did not display a high degree of polyploidy, it is possible that later in life, if circulating human cells lodge within the livers of these animals, the mechanism to generate new human hepatocytes would involve cell fusion. In the liver sections analyzed at this point, the main mechanism seemed to be independent of cell fusion.
Human hepatocyte generation in the fetal sheep model is not caused by cell fusion. In situ hybridization of liver sections of (A) a control sheep hybridized with human pericentromeric repeat (red) and sheep pericentromeric repeat (green) probes, and (B) control human liver also hybridized with both probes. As can be seen, the probes were completely species specific. Control sheep displayed only the green signal from the sheep probe, whereas the control human liver exhibited only red fluorescence from the human probe. (C) In situ hybridization of a representative liver section of a chimeric sheep. Human hepatocytes within the sections exhibited pure red fluorescence, demonstrating that they contain only human DNA and not sheep DNA, whereas the sheep hepatocytes exhibit only green fluorescence.
Human hepatocyte generation in the fetal sheep model is not caused by cell fusion. In situ hybridization of liver sections of (A) a control sheep hybridized with human pericentromeric repeat (red) and sheep pericentromeric repeat (green) probes, and (B) control human liver also hybridized with both probes. As can be seen, the probes were completely species specific. Control sheep displayed only the green signal from the sheep probe, whereas the control human liver exhibited only red fluorescence from the human probe. (C) In situ hybridization of a representative liver section of a chimeric sheep. Human hepatocytes within the sections exhibited pure red fluorescence, demonstrating that they contain only human DNA and not sheep DNA, whereas the sheep hepatocytes exhibit only green fluorescence.
Discussion
The human liver is an organ with immense regenerative capacity. Although normally quiescent, the liver maintains a balance between cell gain and loss. Partial hepatectomy and viral infections or inflammatory responses that distress the liver parenchyma invoke a rapid regenerative response to restore hepatocytic function. Most liver recovery is achieved through hepatocyte proliferation. However, more severe injury can activate a putative stem cell compartment that can give rise to hepatocytes and biliary epithelial cells.1 Several investigators have raised the possibility that there is another population of stem cells within the body that is capable of contributing to liver regeneration, namely, the HSC cell compartment within the BM.1-13,27-29 Although it remains controversial whether an HSC exists that is able to give rise to blood cells and liver cells, evidence is accumulating that a population of cells within the BM-derived HSC compartment can make a significant contribution to liver regeneration. To date, most of the in vivo model systems that have been used to demonstrate the versatility of stem cells have made use of external stress, such as radiation- or chemical-induced injury or an experimentally created shortage of a specific cell type, in the recipient to induce the transplanted cells to differentiate into the specific missing or injured organ cells.1-3,7 In the tyrosinemia type 1 murine model, it was reported that fusion was the principal mechanism responsible for HSCs becoming hepatocytes.15,16 However, these studies have, by virtue of the model used, also biased the transplanted cells to a particular fate by providing environmental cues specific to a particular mechanism of cell regeneration. It is possible that in a more physiologic setting, the mechanism that leads to the contribution of the hematopoietic system to hepatocytes is distinct from that of cell fusion. In the present studies, we used an in utero transplantation model to examine the ability of human HSCs to generate functional human hepatocytes in the absence of injury or external stimuli. Using this in utero xenotransplantation system, we show in the present studies that human HSC populations have the ability to generate significant numbers of hepatocytes on transplantation to fetal sheep in the absence of cell fusion. Furthermore, little polyploidy of the liver cells was seen at the time the liver sections were analyzed.
It is known that the most intensive polyploidization of the liver is characteristic of mammals with a body mass less than 250 g and that the mean ploidy level in adult sheep is 2.13%, which is similar to the level of ploidy of human liver but significantly lower than that in mouse or rat.30-34 Furthermore, in normal tissues, ploidy increases as cells terminally differentiate, and the onset of cellular polyploidy seems to be associated with late fetal development and postnatal maturation. In the context of normal animal physiology, polyploidy is associated with weaning and with the assumption of normal feeding.32-34 Thus, given that in most of our experiments we analyzed sheep before birth, it is expected that the level of polyploidy found in our liver tissue sections would be minimal. Of interest also is the finding that biliary and endothelial cells within the same acini are of human origin, suggesting the possibility that a functional acini of donor origin can be generated. These results can also provide a feasible explanation for the generation of donor hepatocytes in our model because several authors have shown the role of endothelial cells in early liver development and the association of blood production with blood vessel morphogenesis.35-37 It is thus possible that the generation of human endothelial cells in the fetal liver at this time of gestation, in which the liver is essentially a hematopoietic organ, in combination with the presence of human hematopoietic cells, can provide the right conditions for the development of human hepatocytes from HSCs if these cells harbor that potential. In addition, several groups have reported the role of stromal-derived factor-1α (SDF-1α) as an important mediator for recruiting HSCs to the injured liver.27,38
Although the sheep model is a noninjury model and thus SDF-1 and other potential stress signals would not be up-regulated, Ara et al39 have shown the role of SDF-1 in the homing of HSCs during fetal development. Thus, it is possible that in the sheep fetal model, SDF-1 also plays a role in attracting human HSCs to the liver environment, allowing these cells to contribute to the stem cell pool of the liver. Similar to what has been reported by Wang et al,8 the hepatocytes generated in our model appear to be functional because they express markers of normal hepatocytes and they produce and secrete albumin into the sera of the sheep that underwent transplantation. We also tested different sources of human HSCs, namely, CB, mobilized PB, and adult BM. In these studies, we chose to transplant a small number of cells because we knew this number would still produce hematopoietic engraftment in our model while it facilitated the analysis and enumeration of human hepatocytes and enabled accurate comparison between the experimental groups. By using this approach, we were able to show that on a cell-per-cell basis, HSCs derived from adult BM appear to have the highest hepatocytic potential of the 3 HSC sources tested at early time points after transplantation. In sheep analyzed at late time points (11 months after transplantation), CB HSCs seemed to have high hepatocytic potential. However, at earlier time points, this effect is not so evident; it is possible that CB HSCs need more time to give rise to hepatocytes than adult HSCs need. Mobilized peripheral blood HSCs were also able to generate hepatocytes, raising the possibility that HSC mobilization could be used to mediate liver repair in clinical patients in whom liver function has been compromised.
These data are in agreement with those of Kucia et al,28 who show that G-CSF mobilization in humans and mice leads to an increase of potential liver-specific cells in the circulation. Of note also was the increase in human hepatocytes in sheep liver sections with time after transplantation. It is known that during development, liver cells continuously proliferate through an autocrine mechanism; thus, human cells would be able to proliferate at their own rate, independently of the proliferation of sheep hepatic cells.38,40,41 Although we do not believe that there is a selective advantage in the growth of human hepatocytes in the sheep model, it is possible that until the postnatal period, at which time hepatocytes start to require exogenously provided growth factors, human hepatocytes grow at a different rate than do sheep hepatocytes, potentially accounting for the relative increase of human hepatocytic cells in sheep livers. Overall, our findings demonstrate that purified HSC populations from different sources, including BM, CB, and mobilized PB, all have the ability to generate hepatocytes in vivo, but to different degrees. Furthermore, our fetal model system allowed us to demonstrate for the first time that HSCs can give rise to significant numbers of functional hepatocytes in the absence of experimentally induced injury by a mechanism other than that of cell fusion. This finding is important from a basic science perspective because it demonstrates that HSCs still have a broad differentiation potential in a physiologically healthy population, without genetically or chemically induced depletion of a specific cell type. Whether this is truly the result of HSC differentiation into liver cells or of the presence of hepatic precursor cells within the BM compartment, as described by Kucia et al28 and Ratajczak et al29 in recent publications, is unclear. Studies are under way in which retroviral marking of transplanted cells and insertion site analysis will be used to clarify this issue. Moreover, the fact that the CD34+CD133+Lin– population of cells generated so few hepatocytes compared with the CD34+Lin–CD38– population is also surprising. Given that not all CD34+CD38– cells are CD133+, it is possible that a population enriched for hepatocytic potential existed, though at a lower frequency, when we selected for CD133. Other plausible explanations for this observation are that CD34+CD133+Lin– cells preferentially seed the BM or that they take longer to generate hepatocytes than do CD34+Lin–CD38– populations.
Whether our results are attributed to true differentiation or to the presence of liver-specific progenitor cells within our sorted HSC populations, they are still of clinical significance because they provide evidence that HSC transplantation could be a therapeutic option for patients who have a deficit in hepatocyte number or function. We and others42,43 have previously described the ability of in utero stem cell transplantation to cure human severe combined immunodeficiency (SCID). Thus, it is possible that in utero transplantation could cure genetic diseases in which a deficiency of liver cells or of their products threatens the life of the fetus or newborn.
Prepublished online as Blood First Edition Paper, July 1, 2004; DOI 10.1182/blood-2004-01-0259.
Supported by grants HL 70566, HL73737, HL52955, HL52955, and NAG9-1340 from the National Institutes of Health and the National Aeronautics and Space Administration.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal