Abstract
To identify candidate antigens in aplastic anemia (AA), we screened proteins derived from a leukemia cell line with serum of an AA patient and identified diazepam-binding inhibitor-related protein 1 (DRS-1). Enzyme-linked immunosorbent assay (ELISA) revealed high titers of anti–DRS-1 antibodies (DRS-1 Abs) in 27 (38.0%) of 71 AA patients displaying increased paroxysmal nocturnal hemoglobinuria (PNH)–type cells (PNH+), 2 (6.3%) of 32 PNH– AA patients, 5 (38.5%) of 13 PNH+ myelodysplastic syndrome (MDS) patients, and none of 42 PNH– MDS patients. DRS-1 gene was abundantly expressed in myeloid leukemia cell lines and in CD34+ cells derived from healthy individuals. Stimulation of T cells from an AA patient displaying high DRS-1 Abs with a putative CD4+ T-cell epitope (amino acid residues [aa's] 191-204) presented by HLA-DR15, which overlapped with a hot spot (aa's 173-198) of DRS-1 Ab epitopes, gave rise to T cells cytotoxic for L cells (murine fibroblasts) that were transfected with DRB1*1501 and DRS-1. Enzyme-linked immunospot assay demonstrated increased frequency of T-cell precursors specific to the DRS-1 peptide in other HLA-DR15+ AA patients displaying high DRS-1 Ab titers. These findings indicate that DRS-1 may serve as an autoantigen eliciting immune attack against hematopoietic stem cells in a subset of AA patients characterized by increased PNH-type cells.
Introduction
Acquired aplastic anemia (AA), a bone marrow failure syndrome characterized by pancytopenia and bone marrow hypoplasia, has been the subject of study by hematologists for many years, as more than 70% of AA patients improve under immunosuppressive therapies such as antithymocyte globulin (ATG) and cyclosporine (CsA).1-3 The dramatic effects of such T-cell suppressants on in vivo hematopoiesis suggest that immune system attack against hematopoietic stem cells plays an essential role in the development of AA.4-6 However, despite extensive efforts to clarify the immune mechanisms of AA, the key antigens provoking immune response against hematopoietic stem cells remain unknown. This is largely due to a lack of animal models and the heterogeneity of pathogenesis in AA. Lack of good progenitor cell assays in humans has also hindered the elucidation of immune mechanisms in AA.
In organ-specific autoimmune diseases, such as insulin-dependent diabetes mellitus (IDDM) and multiple sclerosis where autoreactive T cells play a primary role in pathogenesis, autoantibodies against target proteins of the pathogenic T cells are often detected.7-10 Although such antibodies do not usually contribute to the pathogenesis of T-cell–mediated diseases, detection of the antibodies may prove useful in both identifying autoantigens and diagnosing immune mechanisms underlying the diseases.11 We recently demonstrated that HLA-DRB1*1501 and increased paroxysmal nocturnal hemoglobinuria (PNH)–type cells represent prognostic markers for the immune mechanisms of AA.12,13 Extensive investigation of antibodies in the sera of patients possessing HLA-DRB1*1501 and a minor population of PNH-type cells may be useful in identifying novel autoantigens in AA. Using immunofluorescent analysis, we previously found that antibodies to UT-7, a megakaryoblastic cell line, are frequently detectable in sera of AA patients who display increased PNH-type cells (PNH+ patients; unpublished observation, T.C. and S.N., May 2001). These antibodies may recognize antigens that elicit T-cell responses against hematopoietic stem cells, allowing expansion of PNH-type stem cells.14,15
To examine these hypotheses, we screened proteins derived from UT-7 cDNA library using serum from a PNH+ patient with HLA-DRB1*1501. Serologic identification of antigens by recombinant expression cloning (SEREX) analysis identified diazepam-binding inhibitor-related protein 1 (DRS-1) as an autoantigen that raises both antibody production and T-cell responses to antigen-presenting cells transfected with DRS-1 gene.
Patients, materials, and methods
Study subjects
Sera or plasma were obtained from 103 patients with AA (45 with severe AA and 58 with moderate AA); 55 patients with myelodysplastic syndrome (MDS), consisting of 46 with refractory anemia (RA) and 9 with refractory anemia with excess of blasts (RAEB); 5 patients with florid PNH; and 52 healthy individuals. Samples were cryopreserved at –80° C until use. All patients and controls provided informed consent according to the Declaration of Helsinki before supplying samples. This study was approved by the human research committee of Kanazawa University Graduate School of Medical Science.
AA and MDS were diagnosed in patients at Kanazawa University Hospital and other hospitals taking part in the bone marrow failure study group led by the Ministry of Health, Labor, and Welfare of Japan. MDS was diagnosed on the basis of cytopenia in peripheral blood, hypercellularity or normocellularity in the sternal or iliac bone marrow, and presence of dysplasia in at least 2 lineages of bone marrow cells. Cytogenetic abnormalities such as trisomy 8 and del(20)(q11) were noted in 14 of 46 RA patients and in 1 of 9 RAEB patients.
Detection of PNH-type cells
Percentages of CD55–CD59– cells in CD11b+ granulocytes and in glycophorin A+ red blood cells (RBCs) were determined using 2-color flow cytometry as described previously.12,16,17 Based on analytical results from 68 healthy individuals, presence of more than 0.003% CD11b+ granulocytes and 0.005% glycophorin A+ RBCs was considered abnormal.12,17 Both thresholds exceeded the mean + 4 standard deviation (SD) for PNH-type granulocytes (0.0025%) and RBCs (0.0032%) determined on healthy individuals. Of the 103 AA patients, 71 (68.9%) displayed PNH-type cells ranged from 0.005% to 6.09%. The percentage of PNH-type cells was 0.005% to 0.01% in 7 (9.9%) patients, 0.01% to 0.1% in 22 (31.0%) patients, 0.1% to 1.0% in 32 (45.1%) patients, and 1.0% to 6.09% in 10 (14.1%) patients. Thirteen of the 46 (28.3%) RA patients displayed increased PNH-type cells, whereas none of the 9 RAEB patients did.
Preparation of cDNA library and SEREX
Poly(A) RNA was purified from UT-7 cells (kindly provided by Dr N. Komatsu of Jichi Medical School, Japan), and a cDNA expression library was constructed with a λZAPII expression vector using a cDNA library kit (Stratagene, La Jolla, CA). Screening for antigens recognized by autoantibodies in the patient's serum was performed as described previously.18 Briefly, XL1-Blue Escherichia coli (Stratagene) was transformed with recombinant phages, plated on agar at 5 × 104 plaques per 150-mm Petri dish, and cultured at 37° C for 6 to 8 hours. Expression of recombinant proteins was induced by incubating bacterial lawns with an overlay of iso-propyl β-D-thiogalactoside (IPTG; Promega, Madison, WI) saturated nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Transfer of released proteins was allowed to proceed for an additional 4 hours at 37° C. Membranes were then washed with 25 mM tris(hydroxymethyl)aminomethane (Tris)–buffered solution (TBS) containing 150 mM NaCl, 2.5 mM KCl, and 0.05% Tween 20 (TBST; pH 7.5) to remove excess agar and blocked overnight with 5% nonfat dry milk in TBS at 4° C.
Serum was obtained from an untransfused 71-year-old AA patient (patient 1) who demonstrated CsA-dependent recovery of hematopoiesis and displayed an increase in PNH-type cells.19 HLA-DRB1 alleles in this patient included 1501 and 0405. Serum was preabsorbed with bacterial lysates to minimize nonspecific antibody binding. Membranes were then incubated with the serum diluted at 1:200 in TBS containing 1% bovine serum albumin (BSA/TBS). Specific binding of immunoglobulin G (IgG) antibodies to recombinant proteins expressed on the lytic plaques was detected by incubating the membranes with alkaline phosphatase–conjugated antihuman IgG antibody (1:2000; Jackson ImmunoResearch, West Grove, PA). Antigen-antibody complexes were visualized by adding 5-bromo-4-chloro-3-indolyl phosphate (BCIP; KPL, Guildford, United Kingdom) and nitroblue tetrezolium (NBT; KPL). cDNA inserts from reactive clones were subcloned to monoclonality, excised in vivo to the pBluescript SK(–) phagemid (Stratagene), and sequenced using an ABI PRISM3100 sequencer (PE Applied Biosystems, Foster, CA).
Purification of bacterially expressed fusion proteins and Western blotting
Full-length DRS-1 cDNA obtained from SEREX analysis was subcloned into the pET-44a (+) vector (Novagen, Madison, WI) for expression of a His-tag fusion protein. Synthesized proteins were purified using a His bind kit (Novagen) according to the manufacturer's instructions. A His-tag encoded by pET-44a (+) without the insert was also purified for use as a negative control. Native DRS-1 protein was released from His-tag DRS-1 protein using a thrombin cleavage kit (Novagen). Size of the recombinant proteins was confirmed by Western blotting using mouse anti-His monoclonal antibody (mAb; Amersham Pharmacia Biotech, Piscataway, NJ) as described previously.20 To detect specific antibodies in serum, 1:200 diluted serum was incubated with blotted membranes.
Enzyme-linked immunosorbent assay (ELISA)
Each well of a 96-well Nunc-Immuno plate (Nalge-Nunc International, Roskilde, Denmark) was covered with 100 μL of coating buffer (50 mM carbonate/bicarbonate buffer, pH 9.6) containing 1 μg/mL of purified recombinant DRS-1 protein and kept overnight at 4° C. Plates were washed and covered with phosphate-buffered saline (PBS) containing 10% fetal calf serum overnight at 4° C to block nonspecific binding of serum protein to DRS-1. Sera from patients were added to a final dilution of 1:1000 and incubated at room temperature for 1 hour. After washing, plates were incubated with peroxidase-conjugated goat antihuman IgG antibody (1:10 000; Jackson ImmunoResearch) at room temperature for 1 hour. Finally, plates were washed and incubated with 3,3′,5,5′-tetramethylbenzidine substrate (Pierce, Rockford, IL) at room temperature for 30 minutes, and the optic density (OD) absorbance at 450 nm was read using an SLT EAR 340AT ELISA reader (SLT-Labinstruments, Grödig, Austria). A positive reaction was defined as an absorbance value exceeding the mean + 2 SDs for the OD absorbance value of sera from the 52 controls.
Cell lines
A chronic myeloid leukemia cell line KH88 was kindly provided by Dr M. Yasukawa of Ehime University. K562, KU812, Daudi, U937, HEL, and Molt-4 were purchased from RIKEN BRC (Ibaraki, Japan). A murine fibroblast cell line, L cell, transfected with HLA-DRB1*1501 (1501-L cell) or with HLA-DRB1*0101 (0101-L cell) was provided by Dr Y. Nishimura of Kumamoto University. Lymphoblastoid cell lines (LCLs) were established from peripheral blood mononuclear cells (PBMCs) of 7 healthy individuals using B95-8 (ATCC, Manassas, VA).
Ribonuclease protection assay (RPA)
RNA probes were prepared for detecting gene expression of DRS-1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by amplifying a cDNA fragment of DRS-1 and that of GAPDH using specific primer sets (sense primer 5′-CTATTCGATGCCGTGTATGC-3′, and antisense primer 5′-GCCTGGTCCAGACTTCTTTC-3′ for DRS-1; sense primer 5′-TGAACGGGAAGCTCACTGGC-3′, and antisense primer 5′-AGGTCCACCACCCTGTTGCT-3′ for GAPDH) followed by subcloning into a pGEM-T Easy vector (Promega). Linearized plasmid DNA containing DRS-1 or GAPDH by cutting with SalI were used as templates to synthesize biotin-labeled RNA probes using MAXIscript T7 kit (Ambion, Austin, TX) with minor modifications. A total of 10 ng of the RNA probe was used for hybridization with 20 μg total RNA of each kind of cell line. RPA was performed using the Ribo-Quant RPA kit (PharMingen, San Diego, CA), and chemiluminescent signals were detected using the Non-Rad Detection kit (PharMingen) according to the manufacturer's instructions.
Isolation of CD34+ cells and CD4+ T cells
CD34+ cells were isolated from the bone marrow of 3 healthy volunteers using a CD34 progenitor cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. CD4+ T cells were separated from cultured T cells using Dynabeads M-450 CD4 (Dynal Biotech ASA, Oslo, Norway).
Quantification of DRS-1–specific mRNA with real-time polymerase chain reaction (PCR)
Total RNA was extracted from PBMCs, CD34+ cells of healthy volunteers, and leukemia cell lines using Isogen (Nippon Gene, Tokyo, Japan) according to the manufacturer's instructions. Reverse transcription of 1 μg of RNA into cDNA was performed using superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) and stored at –20° C until use. Quantification of DRS-1 gene expression was performed using a LightCycler (Roche Diagnostics, Tokyo, Japan) with specific primers described under “Ribonuclease protection assay (RPA).” The LightCycler with a GAPDH primer kit (Search LC, Heidelberg, Germany) was used for quantification of mRNA for GAPDH, a housekeeping gene, in the same samples. The relative amount of DRS-1 mRNA to GAPDH mRNA (DRS-1/GAPDH) was used to represent expression level of the DRS-1 gene.
Epitope mapping
In order to detect hot spots of epitopes recognized by DRS-1 Abs of different patients, epitope mapping was performed using the NovaTope library construction and screening system (Novagen). Briefly, DRS-1 cDNA was randomly digested using DNase I into small fragments of 50 to 150 bp. After both ends were blunted and a single 3′-deoxyadenosine residue added, fragments were ligated into the pSCREEN T-Vector. NovaBlue (DE3)–competent cells were transformed using the constructed pSCREEN T-Vector for peptide expression. Colonies were transferred onto nitrocellulose filters and lysed in a chloroform vapor chamber. After denaturation and blocking, filters were incubated with sera from AA patients possessing DRS-1 Abs overnight at 4° C, then immunodetection was performed by incubating membranes with alkaline phosphatase–conjugated antihuman IgG antibodies (1:2000; Jackson ImmunoResearch). Antigen-antibody complexes were visualized by adding BCIP and NBT. Positive colonies were selected, and plasmid DNA was extracted from the colonies using a miniprep DNA purification system (Promega). Proteins derived from positive clones were purified using a His bind kit (Novagen) as described under “Purification of bacterially expressed fusion proteins and Western blotting.”
Preparation of peptides as an epitope candidate within DRS-1
Peptide sequences within DRS-1 that can be presented by HLA-DR15 were deduced based on the TEPITOPE algorithm21 with a prediction threshold (ie, percentage of best-scoring natural peptides) of 5%. Two positive peptides, amino acid residues (aa's) 191-204 (AVLLREFVGCFIDF, peptide 1) and aa's 351-364 (TNAVVNFLSRKSKL, peptide 2), as well as a negative peptide, aa's 95-108 (SSQVEPGTDRKSTG, peptide 3), which was predicted to display no binding to the HLA-DR15 molecule, were synthesized using a Rainin Symphony multiple-peptide synthesizer (Rainin, Woburn, MA). Synthetic peptides were lyophilized, reconstituted in dimethyl sulfoxide at 5 mg/mL, and diluted in RPMI 1640 (Gibco, Grand Island, NY) as needed.
Transduction of L cells with DRS-1 gene
The DRS-1 minigene was amplified by PCR using pBluescript SK(–) harboring full-length DRS-1 cDNA as a template. The primer set used for PCR included 5′-GGGCTCGAGCCCGCCGCCACCATGCTGACTAACTTCACTGATATT-3′ and 5′-GCGGCCGCTCACAGTTTTGATTTTCTGGATAA-3′ appended XhoI and NotI sites (underlined sequences) at the 5′ and 3′ ends of the DRS-1 minigene, respectively. PCR products were inserted into a pGEM-T Easy vector (Promega). After propagation, DRS-1 minigene was released by digestion with XhoI and NotI, then subcloned into pCAGIPuro vector (kindly provided by Dr H. Niwa, RIKEN). The pCAGIPuro vector harboring the DRS-1 minigene was used to transfect 1501- or 0101-L cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Stable transfectants were obtained by selection using RPMI 1640 medium containing puromycin (5 μg/mL; BD Biosciences Clontech, Palo Alto, CA).
Establishment of DRS-1–specific T cells and 3H-thymidine incorporation assay
PBMCs obtained from an AA patient (patient 2) with HLA-DR15 displaying high titers of DRS-1 Abs were cultured for 14 days with irradiated 1501-L cells that were pulsed with DRS-1 peptides, and CD4+ T cells were separated. Proliferative responses of cultured CD4+ T cells to 1501-L cells or 0101-L cells transfected with the DRS-1 gene were measured using 3H-thymidine incorporation assay. A total of 5 × 104 CD4+ T cells were cultured in 96-well U-bottomed plates (IWAKI, Chiba, Japan) with the same number of 45 Gy-irradiated L-cell transfectants. After 3 days of incubation, 1 μCi (0.037 MBq) of 3H-thymidine (6.7 Ci/mmol [2.48 × 1011 Bq/mmol]; Dupont NEN Products, Boston, MA) was added to each well. Cultured cells were harvested after 6 hours, and 3H-thymidine incorporation was measured. Data were represented as relative proliferative index calculated as 3H-thymidine incorporation by T cells cultured with L-cell transfectants relative to 3H-thymidine incorporation by T cells cultured without L-cell transfectants.
51Cr-release assay
L-cell transfectants were incubated with 100 μCi (3.7 MBq) of 51Cr at 37° C for 1 hour after washing with PBS. Labeled cells were washed 3 times with PBS and suspended in complete medium containing 10% pooled human AB serum. Labeled cells (5 × 103) were incubated with various numbers of DRS-1–specific CD4+ T cells for 4 hours. The release of 51Cr into medium was measured using a γ-counter. Percentage of specific lysis (mean ± SD) obtained in the 51Cr-release assay was determined from triplicate cultures as follows: 100 × (experimental release cpm – spontaneous release cpm)/(maximum release cpm – spontaneous release cpm), where cpm indicates counts per minute.
Determination of T-cell precursor frequencies specific to DRS-1 peptides
Approximately 2 × 106 PBMCs were cultured for 7 days in RPMI 1640 supplemented with heat-inactivated human serum (10%) and l-glutamine (2 mM) containing 20 μg/mL of a DRS-1–derived peptide. On day 7, 2 × 104 1501-L cells were pulsed with 20 μg/mL of the same peptide. After a 4-hour incubation, L cells were washed twice with RPMI1640, irradiated, and added to the cultured PBMCs. Interleukin 2 (IL-2) was added on day 8 at 100 U/mL. On day 14 of culture, CD4+ T cells were separated and subjected to enzyme-linked immunospot assay (ELISPOT) using an interferon γ (IFN-γ) ELISPOT assay kit (BioSource International, Camarillo, CA). Briefly, 105 induced CD4+ T cells were transferred into each well of the ELISPOT plate then cocultured with 2 × 104 peptide-pulsed L cells overnight. IFN-γ spots were then detected according to the manufacturer's instructions.
Statistics
Differences in prevalence of DRS-1 Ab titers in serum among different patient groups and in responses of DRS-1–specific T cells to different target cells were examined using Fisher exact test and Student t test, respectively. The logistic procedure was used to identify factors significantly associated with a good response to immunosuppressive therapy (IST).
Results
Identification of cDNA clones recognized by serum of an AA patient
SEREX analysis using the UT-7 cDNA library and diluted serum from an AA patient (patient 1) identified 6 independent clones including DRS-1, Homo sapiens KIAA0907 protein, α1HB subunit of voltage-dependent T-type calcium channel, U2 small nuclear ribonucleoprotein auxiliary factor 35-kDa subunit related-protein 2, hemoglobin γ-1 chain, and lens epithelium-derived growth factor. When sera from another 10 AA patients were screened for antibodies to these clones using a phage plate assay, only the DRS-1 clone was recognized in 2 patients. Focus was thus placed on DRS-1 for further studies.
Detection of specific antibodies to DRS-1 in AA patients
To confirm the presence of antibodies specific to DRS-1 in the sera of AA patients, a recombinant His-tag DRS-1 protein was prepared in addition to His-tag and DRS-1 proteins. Figure 1A shows the results of Western blotting using these recombinant proteins. Serum of a PNH+ AA patient displayed both His-tag DRS-1 and DRS-1, but not His-tag, whereas serum of a healthy individual did not display any of these recombinant proteins (Figure 1A).
Specific antibody to recombinant DRS-1 in the serum of an AA patient. (A) Purified His-tag DRS-1, His-tag, and native DRS-1 were loaded in lanes 1, 2, and 3, respectively. Proteins were visualized using Coomassie blue staining. Blotted membranes were incubated with anti-His mAb, serum of a PNH+ AA patient, and serum of a healthy individual for detection of DRS-1 Abs. (B) An equal amount of purified His-tag DRS-1 protein was used to detect antibodies specific to DRS-1 in sera from 3 PNH+ AA patients (lanes 1-3), 3 PNH– AA patients (lanes 4-6), 3 PNH– MDS patients (lanes 7-9), and a healthy individual (Healthy).
Specific antibody to recombinant DRS-1 in the serum of an AA patient. (A) Purified His-tag DRS-1, His-tag, and native DRS-1 were loaded in lanes 1, 2, and 3, respectively. Proteins were visualized using Coomassie blue staining. Blotted membranes were incubated with anti-His mAb, serum of a PNH+ AA patient, and serum of a healthy individual for detection of DRS-1 Abs. (B) An equal amount of purified His-tag DRS-1 protein was used to detect antibodies specific to DRS-1 in sera from 3 PNH+ AA patients (lanes 1-3), 3 PNH– AA patients (lanes 4-6), 3 PNH– MDS patients (lanes 7-9), and a healthy individual (Healthy).
Figure 1B shows results of the same Western blotting for selected patients with AA or MDS and healthy individuals. Clear bands indicating the presence of DRS-1 Abs were produced by the sera of several PNH+ AA patients but not by the sera of AA patients without PNH-type cells (PNH– AA patients) or those of PNH– MDS patients.
Measurement of DRS-1 Ab titers with ELISA in patients with AA and MDS
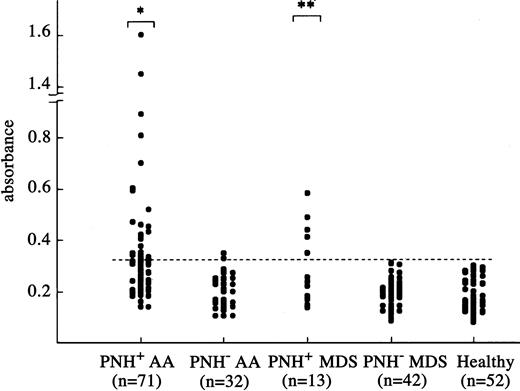
To measure titers of DRS-1 Abs in serum, we established an ELISA using recombinant DRS-1 protein. Figure 2 shows antibody titers in the sera of different groups of patients. AA and MDS patients were divided into 2 groups based on the presence of increased PNH-type cells, which represent a marker for immune pathophysiology in AA.12,13 Twenty-seven (38.0%) of the 71 PNH+ AA patients and 5 (38.5%) of 13 PNH+ MDS patients showed antibody titers greater than the cutoff value, which were significantly higher than that of PNH– AA (6.3%, 2 of 32) and PNH– MDS (0 of 42) patients, but there was no significant difference between PNH+ AA and PNH+ MDS patients (P = .976). All 5 MDS patients with DRS-1 Abs had RA without karyotypic abnormalities. None of 5 patients with florid PNH were positive for DRS-1 Abs (data not shown).
Titration of DRS-1 Abs in sera of patients using ELISA. Antibody titers against purified His-tag DRS-1 in the sera of 71 PNH+ AA patients, 32 PNH– AA patients, 13 PNH+ MDS patients, 42 PNH– MDS patients, and 52 healthy individuals were determined using sera diluted at a 1:1000 dilution. The dotted line denotes a cutoff value defined as mean + 2 SD for absorbance in 52 healthy individuals. Asterisks indicate a prevalence of DRS-1 Ab titers significantly higher than that of PNH– AA patients, PNH– MDS patients, and healthy individuals (*P < .001, **P < .05).
Titration of DRS-1 Abs in sera of patients using ELISA. Antibody titers against purified His-tag DRS-1 in the sera of 71 PNH+ AA patients, 32 PNH– AA patients, 13 PNH+ MDS patients, 42 PNH– MDS patients, and 52 healthy individuals were determined using sera diluted at a 1:1000 dilution. The dotted line denotes a cutoff value defined as mean + 2 SD for absorbance in 52 healthy individuals. Asterisks indicate a prevalence of DRS-1 Ab titers significantly higher than that of PNH– AA patients, PNH– MDS patients, and healthy individuals (*P < .001, **P < .05).
Response to IST in AA patients with DRS-1 Abs
To determine if the presence of DRS-1Abs reflects immune pathophysiology in AA, we selected 22 patients whose sera were tested for DRS-1 Abs before IST and compared response rates to IST between DRS-1 Ab+ patients and DRS-1 Ab– patients. Response to IST was evaluated at 6 months after therapy according to the response criteria of Camitta.22 All 11 (100%) DRS-1 Ab+ patients responded to ATG + CsA (4) or CsA (7), whereas 6 (55%) of the 11 DRS-1 Ab– patients improved with ATG + CsA (4) or CsA (2). Several factors that may influence response to IST were analyzed using multivariate analysis. The presence of DRS-1 Abs (P = .0026) was significantly associated with a good response to IST, whereas age (P = .2439), sex (P = .3852), severity of AA (P = .4159), and increased PNH-type cells (P = .7389) did not affect response to IST in the 22 patients.
Expression of DRS-1 gene by hematopoietic cells
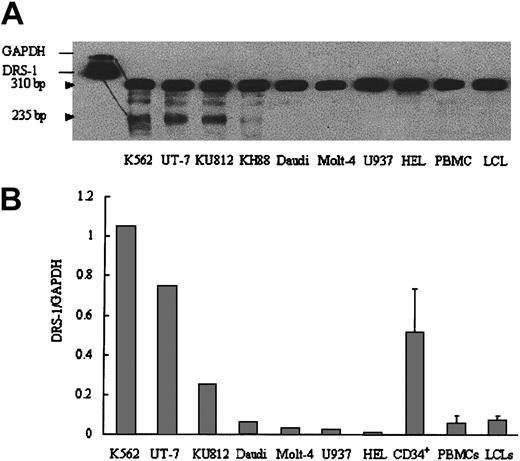
Although DRS-1 gene expression is reportedly ubiquitous, expression of the gene by hematopoietic cells has not been studied in detail. We studied DRS-1 gene expression in various leukemia cell lines using RPA. Myeloid leukemia cell lines such as K562, UT-7, KU812, and KH88 displayed high expression of the DRS-1 gene (Figure 3A). Conversely, lymphoid or monocytoid leukemia cell lines did not display detectable levels of DRS-1 mRNA. When expression of the DRS-1 gene was quantified using real-time PCR, DRS-1/GAPDH ratios of K562, UT-7, and bone marrow CD34+ cells from healthy individuals were 1.62, 0.75, and 0.51, respectively, which were 35-, 16-, and 11-fold higher than DRS-1/GAPDH ratios for PBMCs from healthy individuals (Figure 3B). Other leukemia cell lines such as Daudi, Molt-4, U937, and HEL, as well as LCLs derived from healthy individuals, displayed expression levels similar to those of normal PBMCs.
DRS-1 gene expression in hematopoietic cells. (A) Total RNA (20 μg) from each cell line was subjected to ribonuclease protection assay using biotin-labeled DRS-1 RNA probe and GAPDH RNA probe. The protected GAPDH probe and DRS-1 probe were visualized at 310 bp and 235 bp, respectively. (B) The same amounts of cDNA derived from each cell line or CD34+ cells were used to amplify DRS-1 or GAPDH, respectively. Relative expression levels of DRS-1 to GAPDH were determined as DRS-1/GAPDH. The levels for CD34+, PBMCs, and LCLs represent mean + SD of 3, 6, and 7 healthy individuals, respectively.
DRS-1 gene expression in hematopoietic cells. (A) Total RNA (20 μg) from each cell line was subjected to ribonuclease protection assay using biotin-labeled DRS-1 RNA probe and GAPDH RNA probe. The protected GAPDH probe and DRS-1 probe were visualized at 310 bp and 235 bp, respectively. (B) The same amounts of cDNA derived from each cell line or CD34+ cells were used to amplify DRS-1 or GAPDH, respectively. Relative expression levels of DRS-1 to GAPDH were determined as DRS-1/GAPDH. The levels for CD34+, PBMCs, and LCLs represent mean + SD of 3, 6, and 7 healthy individuals, respectively.
Antibody epitopes of DRS-1 protein
To determine whether there is a common epitope recognized by antibodies derived from different AA patients, randomly cut fragments of DRS-1 were ligated into pSCREEN T-Vector, and DRS-1 fragments derived from ligated plasmids were examined for reactivity to sera that were positive for DRS-1 Abs. Immunoblotting using the original serum revealed 3 antibody epitopes corresponding to aa's 61 to 74, aa's 144 to 166, and aa's 173 to 198 of DRS-1 (Figure 4A). Among these 3 epitopes, only aa's 173 to 198 were recognized by sera from other patients including patients 2 and 3 carrying HLA-DR15 (Figure 4B). Antibodies to this epitope were present in 7 (53.8%) of the 13 DRS-1 Ab+ patients.
Mapping of antibody epitopes in DRS-1 protein. (A) Lysates of transformed E coli expressing 3 different DRS-1 fragments (aa's 61-74, aa's 144-166, aa's 173-198) were tested for reactivity to the original serum from patient 1 (pt 1). (B) Recombinant proteins derived from one epitope clone aa's 173 to 198 were purified and subjected to Western blotting using the sera of AA patients who exhibited antibodies specific to the native DRS-1 protein. Patient 2 (pt 2) and patient 3 (pt 3) represent other PNH+ AA patients who showed high titers of DRS-1 Abs.
Mapping of antibody epitopes in DRS-1 protein. (A) Lysates of transformed E coli expressing 3 different DRS-1 fragments (aa's 61-74, aa's 144-166, aa's 173-198) were tested for reactivity to the original serum from patient 1 (pt 1). (B) Recombinant proteins derived from one epitope clone aa's 173 to 198 were purified and subjected to Western blotting using the sera of AA patients who exhibited antibodies specific to the native DRS-1 protein. Patient 2 (pt 2) and patient 3 (pt 3) represent other PNH+ AA patients who showed high titers of DRS-1 Abs.
T-cell responses to endogenous DRS-1 protein
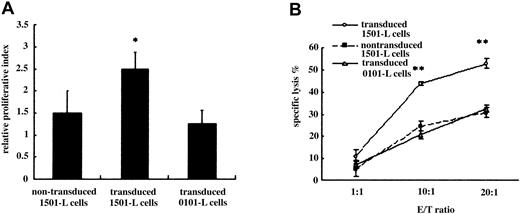
To determine if DRS-1 can elicit T-cell responses to antigen-presenting cells (APCs) expressing DRS-1, we looked for peptides that can be presented by HLA-DR15 in the aa sequence of DRS-1 and identified 2 putative CD4+ T-cell epitopes, peptide 1 and peptide 2. Interestingly, peptide 1 (aa's 191-204) was found to overlap with the common antibody epitope aa's 173-198. PBMCs from patient 2 stimulated by peptide 1 were examined for reactivity to 1501-L cells transduced with the DRS-1 gene. Primed CD4+ T cells exhibited significantly higher proliferative response to 1501-L cells transduced with DRS-1 gene than to nontransduced 1501-L cells or to 0101-L cells transduced with DRS-1 gene (P < .05; Figure 5A). DRS-1–specific CD4+ T cells also killed DRS-1–transduced L cells in a dose-dependent fashion. Cytotoxicity against DRS-1–transduced 1501-L cells reached 52.8% at an effector-target ratio of 20, significantly higher than that against DRS-1–transduced 0101-L cells or nontransduced 1501-L cells (P < .001; Figure 5B). These findings suggest that DRS-1 can be processed in APCs, and the DRS-1 peptide presented by HLA-DR15 may provoke T cells to attack APCs expressing the DRS-1 gene.
Response of DRS-1–specific T cells to APCs with DRB1*1501 expressing the DRS-1 gene and immature myeloid cells. (A) 3H-thymidine incorporation of DRS-1–specific T cells to DRS-1–transduced 1501-L cells and 0101-L cells, as well as nontransduced 1501-L cells. Values represent mean + SD of triplicate cultures from 4 different experiments. (B) Cytotoxicity of DRS-1–specific T cells against DRS-1–transduced 1501-L cells and 0101-L cells, as well as nontransduced 1501-L cells. L-cell transfectants were incubated with differing numbers of DRS-1–specific T cells in a 4-hour cytotoxicity assay. Values represent mean ± SD of duplicate cultures from 3 different experiments. Asterisks in Figure 5A-B indicate values significantly different from nontransduced 1501-L cells or transduced 0101-L cells (*P < .05, **P < .001). E/T indicates effector-target ratio.
Response of DRS-1–specific T cells to APCs with DRB1*1501 expressing the DRS-1 gene and immature myeloid cells. (A) 3H-thymidine incorporation of DRS-1–specific T cells to DRS-1–transduced 1501-L cells and 0101-L cells, as well as nontransduced 1501-L cells. Values represent mean + SD of triplicate cultures from 4 different experiments. (B) Cytotoxicity of DRS-1–specific T cells against DRS-1–transduced 1501-L cells and 0101-L cells, as well as nontransduced 1501-L cells. L-cell transfectants were incubated with differing numbers of DRS-1–specific T cells in a 4-hour cytotoxicity assay. Values represent mean ± SD of duplicate cultures from 3 different experiments. Asterisks in Figure 5A-B indicate values significantly different from nontransduced 1501-L cells or transduced 0101-L cells (*P < .05, **P < .001). E/T indicates effector-target ratio.
T-cell precursors specific to DRS-1 peptides in peripheral blood of DRS-1 Ab+ patients
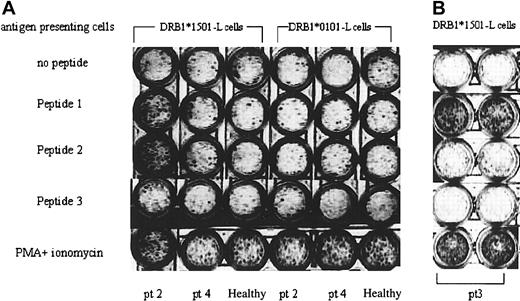
In order to determine the frequency of T-cell precursors specific to DRS-1 peptides in peripheral blood T cells of DRS-1 Ab+ AA patients, another peptide (peptide 3) with low affinity to HLA-DR15 was prepared, and PBMCs in patient 2 and patient 3 were examined using ELISPOT assay. Stimulation of PBMCs from patient 2 with peptide 1 and peptide 2 induced a higher number of INF-γ spots than stimulation with peptide 3 (Figure 6A). Such high induction of INF-γ spots was not induced in PBMCs of a DRS-1 Ab– AA patient (patient 4) and a healthy individual. Stimulation with 0101-L cells pulsed with peptide 1 and peptide 2 also failed to induce INF-γ spots from the same PBMCs. In patient 3, only peptide 1 induced as many as INF-γ spots as phorbol myristate acetate (PMA) plus ionomycin did (Figure 6B).
Frequency of T-cell precursors specific to the DRS-1 epitope. (A) PBMCs of a DRS-1 Ab+ patient (pt 2), a DRS-1 Ab– patient (patient 4; pt 4), and a healthy individual were subjected to ELISPOT assay using different combinations of APCs and DRS-1–derived peptides. (B) Another DRS-1 Ab+ patient (pt 3) was subjected to ELISPOT assay. The figure shows results of duplicate culture.
Frequency of T-cell precursors specific to the DRS-1 epitope. (A) PBMCs of a DRS-1 Ab+ patient (pt 2), a DRS-1 Ab– patient (patient 4; pt 4), and a healthy individual were subjected to ELISPOT assay using different combinations of APCs and DRS-1–derived peptides. (B) Another DRS-1 Ab+ patient (pt 3) was subjected to ELISPOT assay. The figure shows results of duplicate culture.
Discussion
DRS-1 was identified as a novel candidate for autoantigen in AA, using the SEREX method with a cDNA library derived from UT-7 and serum from an AA patient possessing increased PNH-type cells and HLA-DRB1*1501. Several findings in the present study suggest that DRS-1 is involved in the immune pathophysiology of AA. These include a high prevalence of DRS-1 Abs in PNH+ AA and PNH+ RA patients, high rate of response to immunosuppressive therapy in patients with this antibody, high expression of DRS-1 gene by myeloid leukemia cell lines and CD34+ cells from healthy individuals, inducibility of specific T cells recognizing APCs that express the DRS-1 gene, and the presence of T-cell precursors specific to DRS-1 in peripheral blood from AA patients displaying DRS-1 Abs.
DRS-1 is identical to monofunctional peroxisomal Δ,3 Δ2-enoyl-CoAisomerase (PECI).23 ECI is unique in that its activity is essential for the β-oxidation of all unsaturated fatty acids,24,25 and presence of this enzyme has been demonstrated in mammalian peroxisomes and mitochondria.23 This gene is abundantly expressed in various tissues, including the heart, lung, brain, and liver, but not in peripheral blood leukocytes.23 Expression of PECI may be increased in immature hematopoietic cells because they require active utilization of fatty acids as an energy source.23 Indeed, the results of our RPA and real-time PCR indicate that DRS-1 is highly expressed by immature myeloid cells, including CD34+ cells, supporting our hypothesis that DRS-1 could represent a target antigen of immune system attack directed against hematopoietic progenitor cells in AA. Although DRS-1 is ubiquitously expressed, only hematopoietic progenitor cells may be vulnerable to cellular immune responses to DRS-1 due to the expression of functional HLA-DR molecules.26
Antibodies to DRS-1 have been detected in patients with autoimmune diabetes,27 breast cancer,28 renal cancer,29 and hepatocellular carcinoma30 using the SEREX method. However, the significance of antibodies in the pathophysiology of these diseases has remained unclear due to the low prevalence (≤ 8%) of antibodies in these diseases. ELISA in the present study revealed significantly higher titers of DRS-1 Abs compared with healthy controls in 38.0% of PNH+ AA patients and 38.5% of PNH+ MDS patients, both of which are considered to have immune-mediated bone marrow failure.12,13,31 As for MDS patients, DRS-1 Abs were detected only in PNH+ RA patients, supporting the significance of a small number of PNH-type cells as a marker for immune pathophysiology. Antibody production against DRS-1 is not a secondary phenomenon associated with destruction of PNH-type cells because none of 5 patients with florid PNH displayed DRS-1 Abs. In PNH+ AA patients who do not show increased titers of DRS-1 Abs, antigens other than DRS-1 may be involved in immune pathogenesis of AA. Multivariate analysis identified presence of DRS-1 Abs as a factor predicting a good response to IST. PNH+ bone marrow failure is thus the first disease where antibody response to DRS-1 has been implicated in an autoimmune pathophysiology.
Overlap of immunodominant T- and B-cell epitopes has been observed in pyruvate dehydrogenase complex for primary biliary cirrhosis,32-35 myelin basic protein36,37 and proteolipid protein for multiple sclerosis,38-41 and glutamic acid decarboxylase 65 for IDDM,42-44 suggesting that this is a common theme for autoimmune diseases. The hot spot of the antibody epitope we identified in epitope mapping was shared by 53.8% of DRS-1 Ab+ AA patients, and the C-terminal half of the epitope sequence overlapped with a deduced CD4+ T-cell epitope (aa's 191-204) presented by HLA-DR15. Colocalization of the immunodominant T- and B-cell epitopes of DRS-1 may be important in the development of autoimmune responses against DRS-1 in AA. Antigen-specific B cells play important roles as APCs by way of uptake of antigens via surface immunoglobulin.45,46 Antibody binding to specific antigen modulates antigen processing by human B lymphoblastoid cells.46 In antigen-presentation of tetanus toxoid, a single bound antibody or associated F(ab) fragment can enhance the presentation of one T-cell determinant while strongly suppressing the presentation of a different T-cell determinant. Both suppressed and boosted determinants fall within an extended domain of antigen stabilized by the antibody during proteolysis.47
Some intracellular proteins can be processed in the cytoplasm and chaperoned to HLA class II molecules by the invariant chain peptide.48 Whether proteosome proteins like DRS-1 can take this pathway to be presented by HLA-DR is unknown. The specific response of DRS-1–specific T cells to HLA-DRB1*1501-L cells transfected with DRS-1 gene strongly suggests that the cytoplasmic protein can be processed by APCs and the cells can be targeted through recognition of the DRS-1 peptide–HLA-DR15 complex by specific T cells. Several studies have shown that HLA-DR molecules in hematopoietic progenitor cells bind some intracellular proteins such as tubulin β-chain, prolidase, thrombospondin 1, and granzyme.49 ELISPOT assay in the present study demonstrated that T-cell precursors specific to peptide 1 were increased in 2 AA patients carrying HLA-DR15 and DRS-1 Abs. The DRS-1 epitope may thus stimulate T cells to raise both antibodies and CD4+ T cells specific to DRS-1. Although we could not examine the effect of DRS-1–specific T cells on the growth of hematopoietic progenitor cells due to the unavailability of autologous CD34+ cells, these findings suggest that DRS-1–specific CD4+ T cells may contribute to the development of AA by directly killing hematopoietic progenitor cells. The high prevalence of immune response to DRS-1 in PNH+ AA patients appears to support this hypothesis, as CD4+ T cells specific to certain antigens on hematopoietic cells allow expansion of PNH clones.14
Hirano et al50 recently identified kinectin as a possible antigen in AA, using the SEREX method with a cDNA library derived from fetal liver cells. They induced kinectin-specific CD8+ T cells from PBMCs of HLA-A2–positive healthy donors and demonstrated that T cells inhibited in vitro growth of hematopoietic progenitor cells in an HLA-A2–restricted fashion. Although the number of AA patients they studied was very low, CD8+ T cells specific to endogenous proteins like kinectin may conceivably play a role in bone marrow failure for some AA patients. However, the high incidence of HLA-DR15 and increased PNH-type cells in immune-mediated AA suggest the importance of CD4+ T cells rather than CD8+ T cells in the development of AA.13 Our study demonstrated for the first time that immune responses to a protein abundantly expressed in hematopoietic progenitor cells by both T and B cells are operative in immune-mediated AA. Identification of DRS-1–specific T cells with HLA class II tetramers and subsequent functional analysis would further clarify the roles of DRS-1 in the pathogenesis of AA.
Prepublished online as Blood First Edition Paper, June 24, 2004; DOI 10.1182/blood-2004-05-1839.
Supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Technology, Sports, and Culture of Japan (KAKENHI 15390298) and grants from the Research Committee for Idiopathic Hematopoietic Disorders, the Ministry of Health, Labor, and Welfare, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr Haruo Tsuchiya for his helpful discussion and ideas.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal