Abstract
In human mast cells, derived from CD34+ peripheral blood cells, we observed that Kit ligand (KL) failed to induce degranulation but acted in synergy with antigen to markedly enhance degranulation, levels of cytokine gene transcripts, and production of cytokines. Further examination revealed that antigen and KL activated common and unique signaling pathways to account for these varied responses. KL, unlike antigen, failed to activate protein kinase C but activated phospholipase Cγ and calcium mobilization and augmented these signals as well as degranulation when added together with antigen. Both KL and antigen induced signals that are associated with cytokine production, namely phosphorylation of the mitogen-activated protein kinases, phosphatidylinositol 3–kinase–dependent phosphorylation of protein kinase B (also known as Akt), and phosphorylation of nuclear factor κB (NFκB). However, only KL stimulated phosphorylation of signal transducer and activator of transcription 5 (STAT5) and STAT6, whereas antigen weakly stimulated the protein kinase C–dependent induction and phosphorylation of c-Jun and associated activating protein-1 (AP-1) components, an action that was markedly potentiated by costimulation with KL. Interestingly, most signals were down-regulated on continuous exposure to KL but were reactivated along with cytokine gene transcription on addition of antigen. The findings, in total, indicated that a combination of FcϵRI and Kit-mediated signals and transcriptional processes were required for optimal physiologic responses of human mast cells to antigen.
Introduction
Studies of the regulation of release of inflammatory mediators from mast cells have focused largely on FcϵRI-mediated signaling pathways. However, FcϵRI operates physiologically in the context of Kit (CD117) activated signaling pathways. FcϵRI and Kit serve distinct as well as overlapping functions in mast cells. Cross-linking of FcϵRI by antigen through immunoglobulin E (IgE) leads to degranulation, de novo synthesis of inflammatory lipids, and the transcription of genes for a wide variety of cytokines, whereas activation of Kit by Kit ligand (KL) ensures the maturation and survival of mast cells.1-3 Mature mast cells continue to express Kit and respond to KL to maintain mast cell viability, expression of various receptors, and optimal responses to antigen.2,4 In particular, KL markedly enhances FcϵRI-mediated release of inflammatory mediators.5-9 KL itself may stimulate release of inflammatory mediators under certain conditions but the literature is inconsistent on this matter. While some reports indicate that KL stimulates degranulation, synthesis of inflammatory lipids,7,10-14 and transcription of cytokine genes7,15 when added to KL-deprived mast cells, other reports indicate weak or no such responses to KL.5,8,16 On continuous exposure to KL, human intestinal mast cells are reported to express mRNA for certain cytokines and then express an additional set of cytokine RNAs upon FcϵRI ligation.6 Blood-derived human mast cells, in contrast, exhibit minimal transcription of mRNA for cytokines and chemokines but can be stimulated via FcϵRI to produce a large array of cytokines and chemokines.17,18 The reason for these differences are unclear.
The synergistic interactions between KL and antigen in the release of inflammatory mediators might be explained by overlapping signaling pathways. Although FcϵRI relies on recruitment of soluble tyrosine kinases,19 whereas Kit possesses intrinsic tyrosine kinase activity,20 both receptors appear to use common downstream signaling pathways. These include activation of the phospholipases (PLs) C, D, and A2; phosphatidylinositol 3′-kinase (PI 3-kinase); the mitogen-activated protein (MAP) kinases3,20-23 ; and calcium mobilization.10,24,25 In addition, it is reported that FcϵRI and Kit both engage the Janus family kinase (JAK)/signal transducer and activator of transcription (STAT) pathways with recruitment of JAK3/STAT6 in the case of FcϵRI26 and STAT1 and STAT5 in the case of Kit.3,20 Synergistic interactions between Kit and FcϵRI have been noted for some of these pathways. Augmentation of c-Jun NH2-terminal kinase (JNK) activation, for example, is thought to account for the enhanced production of tumor necrosis factor α (TNFα) when cells are costimulated with antigen and KL.8 However, there are no reports in the literature of signaling pathways that are unique to FcϵRI or Kit that explain why FcϵRI primarily promotes release of inflammatory mediators and Kit promotes maturation and survival of mast cells.
We have compared the responses to antigen and KL to determine the similarities and differences in downstream signaling mechanisms activated through FcϵRI and Kit and how these mechanisms relate to release of inflammatory mediators. Studies were conducted with mast cells derived from human peripheral blood CD34+ cells. These cells can be cultured to a high degree of purity to yield an apparently mature27 and nonreplicating28 population of mast cells. Moreover, they have been used successfully to study signaling events, degranulation, and generation of cytokines in response to stimulation via FcϵRI and FcγRI.17,29
In the present studies, cells were either deprived of KL overnight (deprived cells) or exposed continuously to KL (nondeprived cells) to allow Kit and FcϵRI signaling systems to be studied separately or together. We show that while antigen and KL interact synergistically in the activation of common signaling events, there are critical differences. One is that KL fails to activate protein kinase C (PKC) and downstream events including induction of the activating protein-1 (AP-1) transcription factor, c-Jun, and degranulation. Another is that, in contrast to KL, antigen elicits no or minimal stimulation of STAT5 and STAT6 phosphorylation. However, the combination of KL and antigen elicits optimal activation of transcription factors and signals for production of cytokines and degranulation.
Materials and methods
Materials
Reagents were obtained from the following sources: Stem Pro-34 media and culture reagents from Invitrogen/GIBCO (Carlsbad, CA); recombinant human (rh) KL, interleukin 6 (IL-6), and IL-3 from Pepro Tech (Rocky Hill, NJ); recombinant mouse IL-3 from R&D Systems (Minneapolis, MN); chimeric Fc-specific anti–4-hydroxy-3-nitrophenylacetyl–IgE and 4-hydroxy-3-nitrophenylacetyl–bovine serum albumin from Serotec (Raleigh, NC) and Biosearch Technologies (Novoto, CA), respectively; BAY-11-7082 from Calbiochem (San Diego, Ca); monoclonal antibodies against PKCα, PKCβ, PKCα/β, PKCδ, PKCϵ, extracellular signal-related kinase 2 (ERK2), and c-Fos from BD Biosciences (San Diego, CA); monoclonal antibody against spleen tyrosine kinase (Syk) (4D10) from Upstate Inc (Lake Placid, NY); polyclonal antibodies against phosphorylated PLCγ1 (Tyr783) and PKC from Biosource (Camarillo, CA) and Cell Signaling Technology (Beverly, MA); polyclonal antibodies that detect activated phosphorylated ERK2 (Thr202/Tyr204), JNK (Thr183/Tyr185),30 p38 MAP kinase (Thr180/Tyr182),31 p90RSK (Thr360/Ser364), c-Jun (Ser73),32,33 activating transcription factor 2 (ATF-2; Thr71),34 STAT5 (Tyr694), STAT6 (Tyr641),35-37 and nuclear factor κB (NFκB) p65 (Ser536)38 from Cell Signaling Technology or New England Biolabs (Beverly, MA). All other chemicals were molecular biology grade from several sources.
Culture of human mast cells and experimental conditions
Human mast cells were obtained by culture of peripheral blood CD34+ mononuclear cells as described.27 Cultures of mature mast cells (> 95% mast cells at 8 weeks) were maintained in growth medium that consisted of Stem Pro-34 supplemented with L-glutamine (2 mM), penicillin (100 U/mL), streptomycin (100 μg/mL), rhIL-6 (100 ng/mL), and rhKL (100 ng/mL).
Experiments were performed with cultures of mature mast cells that had been incubated overnight (18 h) either in the above growth medium (nondeprived cultures) or medium in which the 2 growth factors, rh KL, and rhIL-6 were omitted (deprived cultures). A modified protocol was adopted for measurement of cytokine production as explained in “Assay of cytokine mRNA and protein.” In all cases, 1 μg/mL human IgE directed against 4-hydroxy-3-nitrophenylacetyl was included in the medium. Dye exclusion measurements indicated no loss of viability of deprived cells (> 95% viable) under these conditions. Cells were then washed 3 times, resuspended in fresh supplemented Stem Pro-34 growth medium, and aliquotted into tissue culture plates or tubes as needed. Cells were stimulated for 15 minutes for measurement of degranulation, for 2 hours for measurement of cytokine mRNA, and for the times designated for other measurements. Stimulants included antigen (4-hydroxy-3-nitrophenylacetylated bovine serum albumin, 100 ng/mL) and rhKL (100 ng/mL). These concentrations were previously found to be optimal for degranulation or support of culture growth.
Cell cultures were maintained in KL and IL-6 to achieve optimal maturation of mast cells.27 However, we found that in deprived cells, IL-6 had no effect on the activation of PKC, calcium signal, activation of the MAP kinases, or degranulation in cells stimulated by antigen or KL. Collectively, the studies indicated that in contrast to KL, IL-6 had no influence on the antigen-induced responses. For clarity, these studies are not described in further detail in this paper.
Electrophoretic separation of proteins and determination of protein and phosphorylation by densitometric analysis
Proteins in whole-cell lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 10% acrylamide gels in Tris (tris(hydroxymethyl)aminomethane)/glycine buffer for 3 hours at 135 milli-amperes. Proteins were transferred to nitrocellulose membranes and probed with the primary antibodies indicated in the figure legends. The antibodies included those known to detect the activated phosphorylated forms of PLCγ, the MAP kinases, and transcription activating factors as listed in “Materials.” Secondary antibodies included horseradish peroxidase–conjugated antibodies against rabbit IgG or mouse IgG. The immunoreactive proteins were visualized by the enhanced chemiluminescence system (Amersham, Piscataway, NJ), and the density of bands was determined on a Molecular Dynamics densitometer (Piscataway, NJ).
Measurement of degranulation and assay of inositol 1,4,5-trisphosphate
Degranulation was determined by measurement of release of the granule marker, β-hexosaminidase, by use of a colorimetric assay in which release of P-nitrophenol from P-nitrophenyl-N-acetyl-β-D-glucosaminide is measured. Values were expressed as the percent of intracellular β-hexosaminidase that was released into the medium.39
Inositol 1,4,5-trisphosphate was assayed by use of a commercially available receptor-binding assay kit (Perkin Elmer Life Sciences, Boston, MA). For this assay, trichloroacetic acid was used to prepare protein-free extracts of cells. Trichloroacetic acid was removed by extraction into a 1,1,2-trichloro-1,2,2-trifluoroethane/trioctylamine mixture before assay of inositol 1,4,5-trisphosphate according to the manufacturer's instructions.
Assay of cytokine mRNA and protein
Messenger RNAs for the individual cytokines and chemokines were assayed simultaneously by use of a multi-probe ribonuclease (RNase) protection assay kit and custom-made templates that recognized mRNA of the indicated cytokines and proteins (BD Biosciences). Radiolabeled RNA cytokine probes were prepared and used according to the manufacturer's instructions. Protected probes were separated by gel electrophoresis and detected by autoradiography.
The cytokines, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-8 were determined by enzyme-linked immunosorbent assay (ELISA) by use of kits supplied by R&D Systems. For these determinations, cells were deprived of growth factors for 3 hours, instead of overnight as was the case for other experiments, before addition of vehicle, KL, or antigen. Cultures were then incubated for an additional 16 hours for measurement of release of cytokines into the medium. The period of 3 hours was chosen to ensure minimal loss of cell viability during the course of the experiment.
Measurement of cytosolic Ca2+
Cells were incubated with 2 μM Fura-2 am ester (Molecular Probes, Eugene, OR) for 30 minutes in 96-well black culture plates (CulturPlate-96 F; Perkin Elmer Life Sciences; 5 × 104 cells/0.1 mL/well). Fluorescence was monitored in a Wallac VICTOR2 plate reader (Perkin Elmer Life Sciences) with alternating excitation at 340 nm and 380 nm and an emission wavelength set at 510 nm. Data were corrected for background fluorescence of cells that did not contain Fura-2, and the ratio of fluorescence at 340 nm and at 380 nm was determined.40
Results
Antigen and KL act in synergy to promote degranulation and transcription of cytokine genes
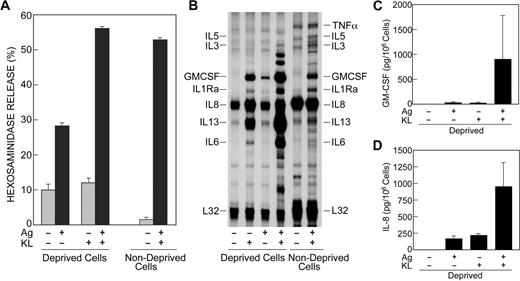
Cells deprived of KL overnight exhibited approximately 30% degranulation in response to antigen as assessed by measurement of release of the granule marker, β-hexosaminidase (Figure 1A). KL, by itself, failed to stimulate degranulation but in combination with antigen markedly potentiated degranulation. In nondeprived cells, antigen induced degranulation to the same extent as the combination of KL and antigen in deprived cells (Figure 1A).
KL and antigen act in synergy to promote degranulation and transcription of cytokine genes. (A,B) Human mast cells were incubated overnight in the presence of antigen-specific IgE in the absence (deprived cells) or presence (nondeprived cells) of KL and IL-6. Cells were left unstimulated or stimulated with 100 ng/mL KL or antigen (Ag), individually or in combination. Release of the granule marker, β-hexosaminidase, was measured 15 minutes after addition of stimulants (A). Values are expressed as percent of intracellular β-hexosaminidase that was released into the medium and are the mean ± SEM from 6 separate experiments. For measurement of the cytokine mRNAs by an RNase protection assay, stimulation was stopped 2 hours after addition of KL or antigen (B). The blots shown are from 1 of 3 experiments. The expected positions of the cytokine mRNA probes are as indicated. (C,D) Cells were incubated overnight in the presence of IgE, KL, and IL-6 and then for 3 hours in the absence of growth factors. Cells were subsequently stimulated or not with antigen and KL for 16 hours for measurement of GM-CSF and IL-8 in the culture medium by ELISA. Values are mean ± SEM from 3 experiments and have been corrected for values in the absence of stimulants (ie, GM-CSF, 45 pg/106 cells; and IL-8, 380 pg/106 cells).
KL and antigen act in synergy to promote degranulation and transcription of cytokine genes. (A,B) Human mast cells were incubated overnight in the presence of antigen-specific IgE in the absence (deprived cells) or presence (nondeprived cells) of KL and IL-6. Cells were left unstimulated or stimulated with 100 ng/mL KL or antigen (Ag), individually or in combination. Release of the granule marker, β-hexosaminidase, was measured 15 minutes after addition of stimulants (A). Values are expressed as percent of intracellular β-hexosaminidase that was released into the medium and are the mean ± SEM from 6 separate experiments. For measurement of the cytokine mRNAs by an RNase protection assay, stimulation was stopped 2 hours after addition of KL or antigen (B). The blots shown are from 1 of 3 experiments. The expected positions of the cytokine mRNA probes are as indicated. (C,D) Cells were incubated overnight in the presence of IgE, KL, and IL-6 and then for 3 hours in the absence of growth factors. Cells were subsequently stimulated or not with antigen and KL for 16 hours for measurement of GM-CSF and IL-8 in the culture medium by ELISA. Values are mean ± SEM from 3 experiments and have been corrected for values in the absence of stimulants (ie, GM-CSF, 45 pg/106 cells; and IL-8, 380 pg/106 cells).
We next determined the effects of antigen and KL on levels of cytokine gene transcripts by use of a RNase protection assay kit. The cytokine mRNAs examined were those known to be most prominently up-regulated when human mast cells were stimulated via FcϵRI and FcγRI. These included the IL-1 receptor antagonist (IL-1Ra), IL-3, IL-5, IL-6, IL-8, IL-13, TNFα, and GM-CSF.17 Deprived cells expressed abundant message for IL-8 and some message for IL-13 without stimulation. KL stimulated production of message for GM-CSF, IL-1Ra, and IL-6 and substantially enhanced expression of message for IL-8 and IL-13 (Figure 1B). Antigen stimulated only modest increases in levels of mRNAs for GM-CSF, IL-1Ra, and IL-13. However, KL and antigen together caused robust expression of broad array of cytokine mRNAs with significant augmentation in the production of mRNA for GM-CSF, IL-1Ra, and IL-13. In nondeprived cells, antigen stimulated the same array of cytokine mRNAs but to a lesser extent than the combination of antigen and KL in deprived cells (Figure 1B) and, in addition, induced small increases in message for TNFα and possibly IL-3 and IL-5. Of particular note, the basal expression of cytokine mRNAs in deprived and nondeprived cells was similar, suggesting that transcription of cytokine genes is down-regulated by continuous exposure to KL. Even so, transcription could be reactivated by antigen.
To assess whether or not comparable changes were apparent in production of cytokine proteins, GM-CSF and IL-8 were assayed by ELISA. In nonstimulated cultures, the levels of GM-CSF (∼45 pg/106 cells) and IL-8 (∼380 pg/106 cells) in the culture medium appeared to reflect the relatively low levels of GM-CSF mRNA and high levels of IL-8 mRNA in cells. Antigen and KL individually induced small or modest increases in the levels of these 2 cytokines and in combination they induced substantial increases in cytokine levels (Figure 1C-D), as was the case for the cytokine transcripts (ie, Figure 1B).
Stimulation of PLCγ, calcium mobilization, and PKC by KL and antigen
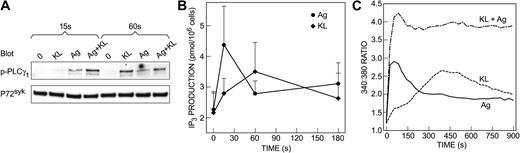
An increase in cytosolic Ca2+ and the activation of PKC are primary signals for degranulation of mast cells.39,41 For this reason, we investigated the effects of KL and antigen on these and related signals. In KL-deprived cells, both KL and antigen stimulated phosphorylation of PLCγ1 (Figure 2A), production of inositol 1,4,5-trisphosphate (Figure 2B), and an increase in cytosolic Ca2+ (Figure 2C). Both stimulants evoked responses of similar magnitude, but the responses to KL were delayed when compared with the responses to antigen. This delay was particularly evident with the increase in levels of inositol 1,4,5-trisphosphate and cytosolic Ca2+ (Figure 2B-C). KL and antigen together interacted synergistically to augment the early phosphorylation of PLCγ1 and the generation of a calcium signal (Figure 2A-B). The activation of PKC was monitored by translocation of PKC and its phosphorylated isoforms to the membrane fraction. PKC isoforms are phosphorylated before recruitment to the plasma membrane42,43 through the production of diglycerides by PLC and possibly via PLD.44-46 In contrast to antigen, which stimulated translocation of all isoforms tested, KL induced minimal or no detectable translocation of PKCα, PKCβ, PKCδ, PKCϵ, and the phosphorylated forms of PKC (Figure 3). Therefore, the ability of KL to augment antigen-induced degranulation appeared to correlate with its ability to augment the calcium signal, and its inability to induce degranulation appeared to correlate with its inability to activate PKC.
Both KL and antigen stimulate PLCγ1 phosphorylation; production of inositol 1,4,5-trisphosphate; and calcium mobilization. Human mast cells were incubated overnight in the presence of anti–4-hydroxy-3-nitrophenylacetyl–IgE in the absence of KL and IL-6. Cells were not stimulated or were stimulated with 100 ng/mL KL, antigen (Ag), or both in combination for the periods indicated to assess the extent of tyrosine phosphorylation of PLCγ1 (p-PLCγ1) by immunoblotting (A); intracellular concentrations of inositol 1,4,5-trisphosphate (IP3; B); and concentration of intracellular-free Ca2+ (C) as described in “Materials and methods.” Data are from 1 of 3 representative experiments (A,C) or are the mean ± SEM of values from 3 separate experiments (B). The data in panel C indicate the ratio of fluorescence at 510 nm when Fura-2–loaded cultures in multi-well plates were excited at 340 nm and 380 nm and are the average value from 3 cultures.
Both KL and antigen stimulate PLCγ1 phosphorylation; production of inositol 1,4,5-trisphosphate; and calcium mobilization. Human mast cells were incubated overnight in the presence of anti–4-hydroxy-3-nitrophenylacetyl–IgE in the absence of KL and IL-6. Cells were not stimulated or were stimulated with 100 ng/mL KL, antigen (Ag), or both in combination for the periods indicated to assess the extent of tyrosine phosphorylation of PLCγ1 (p-PLCγ1) by immunoblotting (A); intracellular concentrations of inositol 1,4,5-trisphosphate (IP3; B); and concentration of intracellular-free Ca2+ (C) as described in “Materials and methods.” Data are from 1 of 3 representative experiments (A,C) or are the mean ± SEM of values from 3 separate experiments (B). The data in panel C indicate the ratio of fluorescence at 510 nm when Fura-2–loaded cultures in multi-well plates were excited at 340 nm and 380 nm and are the average value from 3 cultures.
Antigen but not KL induces translocation of PKC. Human mast cells were incubated overnight in the presence of IgE and in the absence of KL and IL-6, as described in the legend for Figure 1, and then either not stimulated or stimulated with 100 ng/mL antigen (Ag), KL, or both stimulants for 5 minutes. Immunoblots were prepared from the membrane fraction of whole-cell lysates using antibodies against the indicated isoforms of PKC, phosphorylated PKCα/β [p-PKC (α+β)], or phosphorylated pan-PKC [p-PKC (pan)]. The photographs were overexposed to maximize detection of membrane bound PKC in KL-stimulated cells. Identical results were obtained in 2 other experiments.
Antigen but not KL induces translocation of PKC. Human mast cells were incubated overnight in the presence of IgE and in the absence of KL and IL-6, as described in the legend for Figure 1, and then either not stimulated or stimulated with 100 ng/mL antigen (Ag), KL, or both stimulants for 5 minutes. Immunoblots were prepared from the membrane fraction of whole-cell lysates using antibodies against the indicated isoforms of PKC, phosphorylated PKCα/β [p-PKC (α+β)], or phosphorylated pan-PKC [p-PKC (pan)]. The photographs were overexposed to maximize detection of membrane bound PKC in KL-stimulated cells. Identical results were obtained in 2 other experiments.
Phosphorylation of MAP kinases and Akt
MAP kinases and class 1A PI 3-kinase are known to regulate production of at least some cytokines in mast cells.16,47-50 Site-specific antiphospho antibodies (see “Materials and methods” under “Materials”) were used to detect activating phosphorylations of the MAP kinases and, as a proxy for PI 3-kinase activation, the phosphorylation of protein kinase B (also known as Akt), which is dependent on activation of PI 3-kinase.51
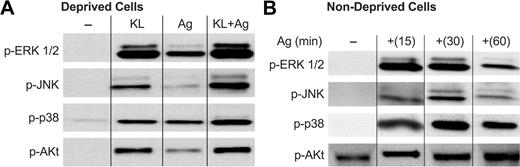
In deprived cells, none of the kinases were extensively phosphorylated before addition of stimulant (Figure 4A). KL, as well as antigen, induced phosphorylation of all 3 MAP kinases and Akt. The phosphorylation of these molecules were augmented when cells were stimulated with KL and antigen in combination. The interaction appeared to be synergistic in the case of JNK and additive or less than additive in the case of ERK1/2, p38 MAP kinase, and Akt. A similar pattern of augmentation in the phosphorylation of the MAP kinases has been noted in MC/9 cells costimulated with KL and antigen.8
Both antigen and KL stimulate activating phosphorylations of the MAP kinases and Akt. Human mast cells were incubated overnight in the presence of antigen-specific IgE either in the absence (Deprived Cells, A) or presence (Nondeprived Cells, B) of KL and IL-6. Cells were left unstimulated or stimulated with 100 ng/mL KL, antigen (Ag), or both for 15 minutes in panel A or with antigen for the times indicated in panel B. Proteins were immunoblotted with antibodies that detect the activated phosphorylated forms of the MAP kinases and Akt (designated with the prefix “p-”). Similar results were obtained in 2 other experiments.
Both antigen and KL stimulate activating phosphorylations of the MAP kinases and Akt. Human mast cells were incubated overnight in the presence of antigen-specific IgE either in the absence (Deprived Cells, A) or presence (Nondeprived Cells, B) of KL and IL-6. Cells were left unstimulated or stimulated with 100 ng/mL KL, antigen (Ag), or both for 15 minutes in panel A or with antigen for the times indicated in panel B. Proteins were immunoblotted with antibodies that detect the activated phosphorylated forms of the MAP kinases and Akt (designated with the prefix “p-”). Similar results were obtained in 2 other experiments.
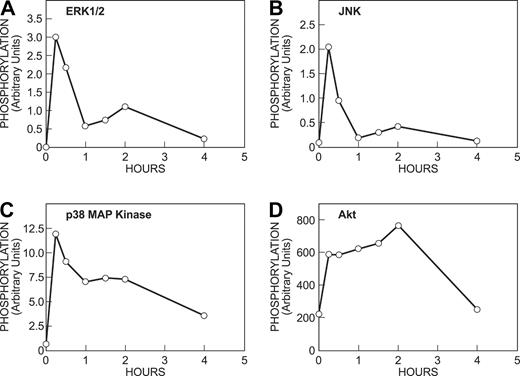
In nondeprived cells, Akt, but none of the MAP kinases, was phosphorylated before addition of antigen. This suggests that continuous exposure to KL results in constitutive stimulation of PI 3-kinase and down-regulation of the MAP kinases (Figure 4B, —). The addition of antigen caused robust phosphorylation of all 3 MAP kinases as well as additional phosphorylation of Akt (Figure 4B at 15, 30, and 60 minutes). The phosphorylation of the MAP kinases was transient and that of Akt more sustained (Figure 5). The time course of these responses was similar to that induced by antigen in KL-deprived cells (data not shown). Thus, antigen remained capable of stimulating phosphorylation of the MAP kinases in cells that had been continuously stimulated by KL and in which the MAP kinases had been down-regulated by KL.
Time course of phosphorylation of MAP kinases and Akt in response to antigen. Cells were incubated overnight with antigen-specific IgE, IL-6, and KL (nondeprived cells). Cells were then stimulated with 100 ng/mL antigen for the indicated periods of time as described for Figure 4. Immunoblots were quantitated bydensitometric scanning. Similar results were obtained in a second experiment.
Time course of phosphorylation of MAP kinases and Akt in response to antigen. Cells were incubated overnight with antigen-specific IgE, IL-6, and KL (nondeprived cells). Cells were then stimulated with 100 ng/mL antigen for the indicated periods of time as described for Figure 4. Immunoblots were quantitated bydensitometric scanning. Similar results were obtained in a second experiment.
Induction and phosphorylation of downstream transcription factors
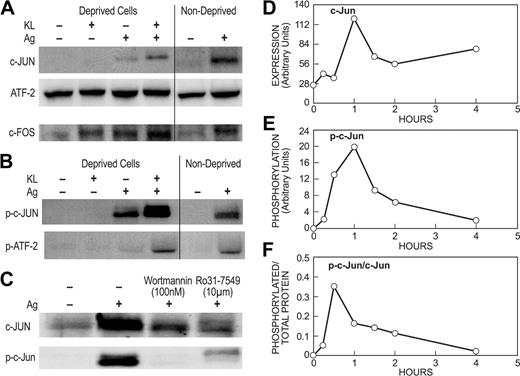
We next investigated the effects of KL and antigen on the induction and phosphorylation of downstream transcription factors. The dimeric AP-1 is one of several transcription factors thought to promote cytokine gene transcription in mast cells.52 The core component of AP-1, c-Jun, is an inducible protein whose synthesis is dependent on PI 3-kinase53 and PKC,54 whereas enhancement of its activation potential requires phosphorylation of Ser63 and Ser73 by JNK.55 c-Jun can form dimers with itself or associate with other factors such as ATF-2 and c-Fos.56 Examination of these factors revealed that KL failed to induce production of c-Jun but it markedly potentiated the induction of c-Jun by antigen in deprived and nondeprived cells (Figure 6A). ATF-2 was expressed constitutively in human mast cells, although antigen, but not KL, induced a modest 1.5- to 2-fold increase (range of values in 3 experiments) in expression of ATF-2 in deprived and nondeprived cells. The levels of c-Fos increased in response to either KL or antigen and this induction was enhanced by the combination of the 2 stimulants.
KL and antigen interact synergistically in the expression and phosphorylation of AP-1–related transcription factors. Deprived and nondeprived mast cell cultures were incubated overnight with antigen-specific IgE as described for previous figures. For panels A and B, deprived and nondeprived cultures were stimulated for 1 hour with 100 ng/mL KL, antigen (Ag), or both stimulants as indicated. For panel C, 100 nM wortmannin or 10 μM Ro31-7549 was added to nondeprived cells 10 minutes before addition of antigen and the reaction was terminated 1 hour later. Immunoblots were probed with antibodies against c-Jun, ATF-2, or c-Fos proteins or the activated phosphorylated forms of these proteins (designated with the prefix “p-”). For panels D-F, nondeprived cultures were stimulated with 100 ng/mL antigen for the time periods indicated. The increase in amount of c-Jun (D) and levels phosphorylated c-Jun (E) as determined by densitometric scans are shown for a typical experiment. For panel F, the data were calculated in terms of specific activity (ie, phosphorylated c-Jun/total c-Jun). All data are representative of results from at least 3 sets of experiments.
KL and antigen interact synergistically in the expression and phosphorylation of AP-1–related transcription factors. Deprived and nondeprived mast cell cultures were incubated overnight with antigen-specific IgE as described for previous figures. For panels A and B, deprived and nondeprived cultures were stimulated for 1 hour with 100 ng/mL KL, antigen (Ag), or both stimulants as indicated. For panel C, 100 nM wortmannin or 10 μM Ro31-7549 was added to nondeprived cells 10 minutes before addition of antigen and the reaction was terminated 1 hour later. Immunoblots were probed with antibodies against c-Jun, ATF-2, or c-Fos proteins or the activated phosphorylated forms of these proteins (designated with the prefix “p-”). For panels D-F, nondeprived cultures were stimulated with 100 ng/mL antigen for the time periods indicated. The increase in amount of c-Jun (D) and levels phosphorylated c-Jun (E) as determined by densitometric scans are shown for a typical experiment. For panel F, the data were calculated in terms of specific activity (ie, phosphorylated c-Jun/total c-Jun). All data are representative of results from at least 3 sets of experiments.
Synergistic interactions between KL and antigen were most evident from the pattern of phosphorylation of c-Jun and ATF-2 for which phospho-specific antibodies were available. No phosphorylated c-Jun or ATF-2 were apparent in deprived cells stimulated with KL (Figure 6B). Antigen, in contrast, stimulated phosphorylation of c-Jun and some phosphorylation of ATF-2. These phosphorylations were substantially enhanced when antigen was added together with KL in deprived cells or when antigen was added to nondeprived cells where KL was already present. As noted above, the induction of c-Jun is dependent on PI 3-kinase and PKC. Accordingly, the induction and phosphorylation of c-Jun was suppressed by the PI 3-kinase inhibitor, wortmannin, and the PKC inhibitor, Ro31-7549 (Figure 6C).
The induction and phosphorylation of c-Jun by antigen in nondeprived cells was examined in further detail. As shown in Figure 6D-E, maximal levels of c-Jun protein and phosphorylated c-Jun were reached 1 hour after antigen stimulation. In terms of specific activity (ie, phosphorylated c-Jun/total c-Jun), peak activity was reached by 30 minutes (Figure 6F). Thereafter, the levels of c-Jun protein remained elevated for at least 4 hours while the levels of phosphorylated c-Jun declined to near basal levels by this time, consistent with the rapid decline in levels of activated phosphorylated JNK (Figure 5B). Collectively, these studies showed that while KL was inactive by itself, it was required for optimal induction and phosphorylation of c-Jun by antigen by a process that was dependent on PKC and PI 3-kinase.
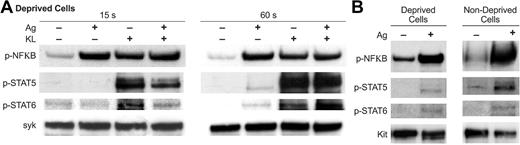
Investigation of other transcription factors believed to regulate cytokine production in mast cells26,49,57-59 showed that antigen stimulated phosphorylation of NFκB and, minimally so, STAT5 and STAT6 in deprived cells, whereas KL caused robust phosphorylation of all 3 transcription factors (Figure 7A). However, in contrast to the phosphorylation of c-Jun and ATF-2, the phosphorylation of NFκB and the STATs was not augmented by the combination of KL and antigen. A comparison of deprived and nondeprived cells indicated that the stimulatory effects of KL on NFκB and STAT5/6 phosphorylation were no longer apparent in nondeprived cells (Figure 7B). Addition of antigen elicited substantial phosphorylation of NFκB and, to a much lesser extent, STAT5 and STAT6. A role for NFκB in cytokine gene transcription was confirmed by use of IκB/NFκB pathway inhibitor, Bay-11-7082.60 Bay-11-7082 blocked induction of all cytokine mRNAs except for IL-13 whether cells were stimulated by KL or antigen, individually or in combination (data not shown). These data suggested that NFκB was required for optimal transcription of most cytokine genes in mast cells.
Phosphorylation of NFκB, STAT5, and STAT6 in response to KL and antigen. Cultures were incubated overnight in the absence (Deprived Cells) or presence (Nondeprived Cells) of IL-6 and KL and presence of antigen-specific IgE. Deprived cells were stimulated for 15 or 60 seconds with 100 ng/mL KL, antigen (Ag), or both stimulants as indicated (A). Nondeprived cells were stimulated with antigen for 60 seconds (B). Immunoblots were probed with antibodies against the activated phosphorylated forms of NFκB, STAT5, and STAT6 (designated with the prefix “p-”). Blots were also probed for the tyrosine kinase Syk or Kit to verify uniformity of loading. The blots are representative of 3 experiments.
Phosphorylation of NFκB, STAT5, and STAT6 in response to KL and antigen. Cultures were incubated overnight in the absence (Deprived Cells) or presence (Nondeprived Cells) of IL-6 and KL and presence of antigen-specific IgE. Deprived cells were stimulated for 15 or 60 seconds with 100 ng/mL KL, antigen (Ag), or both stimulants as indicated (A). Nondeprived cells were stimulated with antigen for 60 seconds (B). Immunoblots were probed with antibodies against the activated phosphorylated forms of NFκB, STAT5, and STAT6 (designated with the prefix “p-”). Blots were also probed for the tyrosine kinase Syk or Kit to verify uniformity of loading. The blots are representative of 3 experiments.
Discussion
FcϵRI-mediated signals have been widely studied in RBL-2H3 cells and other neoplastic mast cell lines. However, these cells express a constitutively active Kit.61,62 FcϵRI-mediated signals are thus influenced to an unknown extent by Kit-related signaling events in these cell lines. For this reason, we used human blood–derived mast cells as a means to examine FcϵRI-mediated signals in the absence and presence of Kit activation. As noted earlier, these cells exhibit physiologic responses to stimulation via FcϵRI and FcγRI when cells are conditioned with interferon γ (IFN-γ).17,29 As shown here, the human mast cells also respond robustly to KL after temporary withdrawal of KL. Under these conditions, KL failed to stimulate degranulation (Figure 1A) but it did induce some increase in levels of cytokine gene transcripts (Figure 1B). Antigen, in contrast, induced degranulation but induced only a minimal increase in levels of cytokine gene transcripts. These responses were substantially augmented when KL and antigen were used in combination. Degranulation was thus enhanced as was the expression of cytokine mRNA and protein. We did not determine whether the increase in expression was due to increased transcription of cytokine genes or increased stability of the transcripts through an arbitrary unit (AU)–rich element (ARE)–dependent mechanism,63-65 but it was apparent that antigen and KL act in synergy to markedly enhance the extent and array of cytokine transcripts and proteins expressed.
Examination of the signaling events revealed a pattern that accounted for the inability of KL to stimulate degranulation and yet had the ability of potentiating antigen-induced degranulation. While KL and antigen both caused phosphorylation of PLCγ1 as well as the associated production of inositol 1,4, 5-trisphosphate and rise in cytosolic Ca2+ (Figure 2), KL failed to activate PKC (Figure 3) which, along with an increase in cytosolic Ca2+, is an essential signal for degranulation in mast cells.39,41 However, the combination of KL and antigen resulted in a significantly enhanced and sustained increase in cytosolic Ca2+. This enhanced calcium signal in combination with antigen stimulation of PKC would likely augment degranulation. The reason why KL failed to activate PKC is unclear but ongoing studies with a mouse bone marrow–derived mast cell line indicate that compared with antigen, KL is a weak stimulant of PLD, which appears to be critical for the activation of certain PKC isoforms and degranulation in mast cells (Ze Peng and M.A.B., unpublished data, 2003).
The pathways that regulate expression of cytokine gene transcripts in mast cells are less well defined than those for degranulation. As noted earlier, the transcription of cytokine genes is dependent, at least in part, on PI 3-kinase and the MAP kinases. Both KL and antigen appeared to activate PI 3-kinase as indicated by Akt phosphorylation as well as the phosphorylation of all 3 MAP kinases (Figure 4). These responses were augmented by costimulation with KL and antigen and may thus contribute to the enhanced expression of message for KL-inducible cytokines such as GM-CSF, IL-1Ra, IL-6, and IL-13. However, the enhanced phosphorylation of these kinases would not account for the expression of additional cytokine transcripts by the combination of KL and antigen. As will be discussed, additional communication between FcϵRI and Kit pathways may occur at the level of activation of transcription factors that regulate cytokine gene transcription.
It is probable that multiple transcription factors are involved in the transcription of cytokine genes. The TNFα and IL-4 genes, for example, contain promoters for AP-1, AP-2, nuclear factor of activated T cells (NF-AT), E twenty-six (Ets) domain transcription factor, AP-1/ATF, NF-AT/AP-1, c-Maf, and NFκB (see citations in Ishizuka et al16 ). NFκB is believed to be the primary transcription factor for regulating production of TNFα,57,59 IL-6,58 and possibly other cytokines,52 although IL-4 and IL-5 appear not to be regulated by NFκB in antigen-stimulated cells.58 Studies with inhibitors point to additional contributions by AP-1 and NF-AT, both of which are activated by antigen.16,52,66 STAT6 has also been implicated in antigen-mediated production of TNFα and IL-6, as neither cytokine is produced in STAT6-deficient mast cells.26 The role of STAT proteins in KL-mediated cytokine production in mast cells has not been investigated.
In the present studies, KL and antigen differed in that only KL induced substantial phosphorylation of STAT5 and STAT6 (Figure 7), and only antigen induced synthesis and phosphorylation of the early response transcription factor, c-Jun. Nevertheless, optimal induction/phosphorylation of c-Jun and other interacting AP-1 components such as ATF-2 and c-Fos was achieved only in the presence of KL (Figure 6). Both stimulants, however, induced phosphorylation of NFκB (Figure 7). The failure of KL to promote synthesis of c-Jun was probably due to lack of PKC activation in KL-stimulated cells. When these results are compared with the pattern of cytokine mRNA's expression, it would appear that phosphorylation of NFκB and partial activation of AP-1 components are insufficient for inducing extensive expression of cytokine transcripts, as was the case in antigen-stimulated cells. Optimal expression of cytokine transcripts and proteins apparently required the phosphorylation of NFκB in combination with phosphorylation of STAT proteins and full activation of AP-1 components as in cells stimulated with the combination of KL and antigen. NF-AT, a family of Ca2+/calcineurin-regulated transcription factors, was not investigated in this study but, as noted in the previous paragraph, it is a likely participant in the regulation of cytokine production.
It is notable that, in contrast to other signaling events, the phosphorylation of the STATs or of NFκB was not augmented by costimulation of cells with KL and antigen (Figure 7). Therefore, there appeared to be no synergy between FcϵRI- and Kit-mediated pathways in the activation of STATs, as has been described for cytokine receptors with other receptors.67 A plausible interpretation of these results is that the augmentation of cytokine gene transcription when KL and antigen are used in combination lies in the combination of transcription factors brought into play by these 2 stimulants in addition to synergistic interactions on signaling pathways.
The above considerations apply to cells temporally deprived of KL, a situation unlikely to occur in mast cells in situ, which depend on continuous exposure to KL to remain viable. Studies with nondeprived cells, so as to mimic physiologic conditions, indicated that the phosphorylation of the MAP kinases, NFκB, and STAT proteins, as well as the transcription of cytokine genes, is no longer apparent after continuous exposure to KL (Figures 4B, 6-7). However, Kit remains active under these conditions as indicated by the phosphorylation of Akt (Figure 4B), which we presume to be indicative of sustained activation of PI 3-kinase. Nevertheless, antigen is still capable of activating the pathways that are down-regulated in KL-exposed cells, although reactivation of STAT phosphorylation is relatively modest. In this situation, the activation of AP-1 components by antigen may reinforce or supersede the action of STATs in regulating the expression of cytokine transcripts. If NFκB is a critical transcription factor, as the studies with BAY-11-7082 suggest, then augmentation of expression could be achieved by activation of either STAT proteins, as in deprived cells costimulated with KL and antigen, or AP-1, as in nondeprived cells stimulated with antigen.
In conclusion, Kit and FcϵRI use common signaling cascades with the important exceptions of the PKC and STAT signaling pathways. The downstream consequences of lack of PKC activation in KL-stimulated cells are failure to initiate degranulation and the induction/phosphorylation of c-Jun. Antigen, in contrast, fails to stimulate STAT phosphorylation. KL and antigen interact synergistically via the calcium signal to enhance degranulation and, by expanding the repertoire of transcription factors activated, to markedly enhance expression of cytokine transcripts and proteins. Extended exposure to KL leads to down-regulation of critical cytokine-related signaling and transcriptional processes that can be reactivated by antigen. This down-regulation could serve to limit constitutive production of inflammatory cytokines under normal physiologic conditions. Therefore, the pattern of cytokines produced in response to antigen may well vary depending on the prior exposure of mast cells to KL and possibility other growth factors.
Prepublished online as Blood First Edition Paper, June 24, 2004; DOI 10.1182/blood-2004-02-0631.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 3. Antigen but not KL induces translocation of PKC. Human mast cells were incubated overnight in the presence of IgE and in the absence of KL and IL-6, as described in the legend for Figure 1, and then either not stimulated or stimulated with 100 ng/mL antigen (Ag), KL, or both stimulants for 5 minutes. Immunoblots were prepared from the membrane fraction of whole-cell lysates using antibodies against the indicated isoforms of PKC, phosphorylated PKCα/β [p-PKC (α+β)], or phosphorylated pan-PKC [p-PKC (pan)]. The photographs were overexposed to maximize detection of membrane bound PKC in KL-stimulated cells. Identical results were obtained in 2 other experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/8/10.1182_blood-2004-02-0631/6/m_zh80200467680003.jpeg?Expires=1769130179&Signature=2Kidbf9oN9jniOYcX3CJ0sekKKdQmbCV-w6gubE~awyG4YWil9WL8UL3c3bCdW3PQt9pP7fMz6R2D-Oj~7DeCIFBJB9lGXFu3WlQq7lOC5D5Wx~XNehLdpxdRgadPGWHcJM~ewoZMIpNRdGsPcXG-Vt9POZN3LdUZCtqRlRjDwbNyfU1i67t9lQqBJlgxHFAFhs4mvoPEHExzMSeQusBw6eg43dJDBC1foKmtvqPMh9WJ7G~lE~9ItAaCqnIoZucKcizkz1L261V5uVIHuwS-cXiZg0kDVH8EUsgR7hxcVTzXn3CHHgiPsigx52TIdRKUw-xcvDDfoFXmyCTEQ2~Rg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal