Abstract
Selective depletion of alloreactive T cells from allogeneic stem cell grafts can reduce graft-versus-host disease (GVHD) while preserving beneficial effects of T cells including facilitation of engraftment, protection against opportunistic infection, and reduced relapse risk. Memory T cells (CD62L–) represent a population of T cells that have previously encountered pathogens and may contain fewer T cells capable of recognizing neoantigens including recipient allogeneic antigen (aAg). We investigated whether human naive (CD62L+) or memory (CD62L–) T cells had different capacities to respond to aAg by assessing their ability to proliferate in response to and lyse HLA-mismatched Epstein-Barr virus–transformed B cells. Freshly sorted and in vitro expanded CD62L– memory T cells were less responsive to aAg stimulation than were CD62L+ naive T cells but contained higher levels of cytomegalovirus (CMV)–specific T cells. Analysis of T cell receptor (TCR) repertoire showed restricted TCR diversity in the memory T-cell population possibly due to selection associated with chronic exposure to common pathogens. Memory T cells may represent a donor cell subpopulation suitable for enhancing immune reconstitution without increasing the risk of GVHD.
Introduction
Graft-versus-host disease (GVHD) and opportunistic infection (OI) represent 2 major causes of morbidity and mortality following allogeneic stem cell transplantation (SCT). Both of these conditions are mediated or controlled, respectively, by donor-derived T cells and represent a paradoxical problem where the presence of T cells may cause GVHD but protect from infection, whereas reducing T cells may result in a rise in infection rates. Furthermore, T cells play a critical role in promoting stem cell engraftment, encouraging rapid recovery of cellular immunity, and decreasing the probability of disease relapse.1-3 The separation of the effects of GVHD from the beneficial effects mediated by T cells has been a long-standing goal of transplantation immunology.
GVHD is caused by the recognition of allogeneic antigens (aAgs) by donor T cells. GVHD can be prevented through T-cell depletion of SCT grafts, but this results in unfavorable engraftment rates and increase in relapse due to the loss of the potent graft-versus-tumor (GVT) effect.4,5 Several groups have depleted activated aAg-reactive T cells using magnetic-activated cell sorting (MACS) or immunotoxins recognizing CD25 (interleukin 2 [IL-2] receptor α) following stimulation with recipient peripheral blood mononuclear cells (PBMCs) or Epstein-Barr virus (EBV)–transformed lymphoblastoid cell lines (LCLs).6-9 However, PBMCs are inefficient antigen-presenting cells and result in only partial depletion of aAg-specific T cells.8 The use of LCLs on the other hand, may deplete EBV-specific T cells that protect against lymphoproliferative disease in immunosuppressed patients. Furthermore, these procedures may not be easily implemented for routine clinical use because of their technical complexity.
Two recent publications have suggested an alternative approach for separating GVHD-inducing T cells from beneficial T cells using the lymphocyte homing receptor CD62L (L-selectin) to distinguish between naive (CD62L+) and “antigen-experienced” memory T cells (CD62L–).10,11 In these murine studies, naive T cells were responsible for the induction of GVHD, whereas memory T cells were able to engraft and respond to specific antigen challenge but were ineffective inductors of GVHD. These studies point to several differences between naive and memory T cells, which may account for their respective ability to induce GVHD. First, naive T cells express the homing receptors CD62L and CCR7 that allow efficient homing to antigen-rich secondary lymphoid organs, which may lead to activation by aAgs.12-15 These molecules are down-regulated following T-cell receptor (TCR) activation (leading to egress from the lymph node T-cell zone into the periphery and sites of inflammation) and are largely absent from the surface of memory T cells.12,13,16,17 Second, memory T cells should have increased specificity toward antigens that are commonly encountered or are persistent in the host (eg, cytomegalovirus [CMV]) resulting in a skewed TCR repertoire in this population. This may result in a reduced ability to recognize new antigens such as aAgs. These functional properties may result in qualitative and quantitative differences between naive and memory T cells in recognition and response to aAg stimulation.
Anderson and colleagues examined a CD4-dependent, minor histocompatibility murine model and found that CD4+ memory T cells (CD62L–CD44+) did not induce GVHD.10 This was not due to the presence of regulatory T cells (CD4+CD25+). In similar experiments, Chen and coworkers showed in a murine model that CD62L+-depleted T cells failed to proliferate against aAgs and were unable to induce GVHD.11 In both studies, memory T cells retained the ability to recognize antigens to which mice had previously been exposed. However, this principle has not yet been investigated in human T cells. In this study, we demonstrate that naive (CD62L+) and memory (CD62L–) T cells can be expanded in vitro and differ in their capacity to recognize aAgs, which is likely to be due to a decrease in TCR repertoire diversity in the memory T-cell pool.
Materials and methods
Donor samples and cell lines
PBMCs were isolated from healthy donors who had previously been tissue typed for HLA-A, -B, and DRB1 alleles (Table 1). PBMCs were used to generate an LCL (viral strain B95.8).
Tissue typing and CMV serology of donors
. | Tissue typing . | . | . | CMV serology . | ||
|---|---|---|---|---|---|---|
. | A . | B . | DRB1 . | . | ||
| Donor no. | ||||||
| 1 | 2, 24 | 8, 65 | 1, 3 | Pos | ||
| 2 | 2, 26 | 35, 41 | 11, 15 | Pos | ||
| 3 | 1, 26 | 35 | 11, 13 | Pos | ||
| 4 | 2* | ND | ND | Pos | ||
| 5 | 2* | ND | ND | Neg | ||
| 6 | 2* | ND | ND | Pos | ||
| 7 | 2, 3 | 7, 62 | 4, 7 | Pos | ||
| 8 | 2* | ND | ND | Pos | ||
| 9 | ND | ND | ND | Pos | ||
| LCLs | ||||||
| A | 24, 32 | 35, 44 | 7, 14 | NA | ||
| B | 3, 24 | 7, 35 | 13, 15 | NA | ||
| C | 2, 28 | 44, 57 | 7, 11 | NA | ||
| D | 3, 24 | 7, 35 | 13, 15 | NA | ||
. | Tissue typing . | . | . | CMV serology . | ||
|---|---|---|---|---|---|---|
. | A . | B . | DRB1 . | . | ||
| Donor no. | ||||||
| 1 | 2, 24 | 8, 65 | 1, 3 | Pos | ||
| 2 | 2, 26 | 35, 41 | 11, 15 | Pos | ||
| 3 | 1, 26 | 35 | 11, 13 | Pos | ||
| 4 | 2* | ND | ND | Pos | ||
| 5 | 2* | ND | ND | Neg | ||
| 6 | 2* | ND | ND | Pos | ||
| 7 | 2, 3 | 7, 62 | 4, 7 | Pos | ||
| 8 | 2* | ND | ND | Pos | ||
| 9 | ND | ND | ND | Pos | ||
| LCLs | ||||||
| A | 24, 32 | 35, 44 | 7, 14 | NA | ||
| B | 3, 24 | 7, 35 | 13, 15 | NA | ||
| C | 2, 28 | 44, 57 | 7, 11 | NA | ||
| D | 3, 24 | 7, 35 | 13, 15 | NA | ||
ND indicates not determined; and NA, not applicable.
HLA-A2 determined by CR11.351 antibody.
Flow cytometric analysis
Freshly isolated PBMCs and cultured T cells were analyzed by incubating cells with fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–conjugated monoclonal antibodies to CD3, CD4, CD8, CD19, CD56 (Becton Dickinson, San Jose, CA), CD45RA, CD45RO (Immunotech, Marseilles, France), and CD25 and CD62L (BD PharMingen, San Diego, CA). Cells were analyzed with HLA-A2 major histocompatibility complex (MHC) class I tetramer specific for CMV pp65 (amino acids 495-503, NLVPMVATV). After incubation on ice for 30 minutes, cells were washed with phosphate-buffered saline (PBS) and analyzed by flow cytometry using a FACSCalibur.
Cell sorting
For initial experiments, PBMCs were sorted into naive (TN, CD62L+CD45RA+), central memory (TCM, CD62L+CD45RA–), effector memory (TEM, CD62L–CD45RA–), and terminal differentiated T cells (TT, CD62L–CD45RA+). Second, PBMCs were fractionated into more generalized subsets by sorting CD62L+ or CD62L– T-cell populations alone or in combination with CD4+ or CD8+ antibodies. All sorting procedures were performed on freshly isolated PBMCs. Cells were labeled with the appropriate antibodies on ice for 30 minutes, washed with PBS, and sorted by flow cytometry using a FACSVantage cell sorter.
Cell expansion
T cells (2 × 105) from each subset were placed in microtiter well plates and stimulated with 5 ng/mL OKT3 (Ortho Biotech, Bridgewater, NJ) in AIM V media (Gibco, Grand Island, NY) supplemented with 10% pooled AB serum and 100 U/mL IL-2 (Chemicon, Temecula, CA). After 5 days of stimulation, a half-volume media change was performed and fresh media containing IL-2 was added. Cells were fed every 2 to 3 days by a half-volume media exchange. To determine whether CMV-specific T cells found in CD62L+ or CD62L– T-cell populations would respond to cognate antigen stimulation, CEM.T2 cells were pulsed with 10 μg/mL NLVPMVATV (NLV) peptide for 2 hours and cocultured with sorted memory and naive T cells at a 1:1 ratio. After 7 days in culture, T-cell cultures were assayed for the presence of CMV-specific T cells using pp65 tetramer complex.
Mixed lymphocyte reaction assay
Following cell expansion, 2 × 105 T-cell responders were plated in triplicate in 96-well flat bottom microtiter plates with 1 × 104 irradiated (60 Gy) HLA-mismatched LCL in a final volume of 200 μL. After 5 days, cultures were pulsed with 1 μCi (0.037 MBq) [3H]thymidine per well. After 16 hours, cells were harvested and counted using a microscintillant counter (Packard Biosciences, Canberra, Australia).
Cytotoxicity assays
Following cell expansion, 2 × 106 T cells were stimulated at a 1:40 responder-to-stimulator ratio with irradiated (60 Gy) HLA-mismatched LCL in media supplemented with 20 U/mL IL-2. After 1 week, T-cell cultures were restimulated with irradiated LCL. On day 14, T cells were harvested and used in a standard 4-hour 51Cr-release assay against the stimulator LCL. Percent lysis was calculated using the following equation: Percent lysis = (experimental release–spontaneous release)/(maximum release–spontaneous release) × 100.
TCR spectratyping
TCR repertoire diversity in T-cell subsets was analyzed using TCR spectratyping by polymerase chain reaction (PCR). This technique measures complementary-determining region 3 (CDR3) of the variable β (Vβ) chain of the TCR that differs in length due to nucleotide excision and addition at the joining (Dβ) and diversity (Jβ) junctions.18 TCR diversity was examined for CD62L– and CD62L+ populations using TCR Vβ chain spectratyping. Fresh PBMCs were labeled with CD62L FITC for 30 minutes on ice. Cells were washed in PBS and sorted using the FACSVantage into CD62L+ and CD62L– T-cell populations. RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) from which cDNA was synthesized from 1 μg RNAusing an M-MLV reverse transcription kit (Gibco) in a 20-μL reaction. TCR repertoire diversity was analyzed by evaluating the number and frequency of Vβ families by PCR as previously described.19,20 cDNA from sorted cells was used in PCR reactions containing 23 Vβ forward primers and a second reverse β chain constant (Cβ) region-specific primer conjugated to the fluorescent dye FAM (Perkin Elmer, Cambridge, United Kingdom; Table 2). All experiments were performed on an ABI Sequence Detection System (Applied Biosystems, Foster City, CA). TCRVβ complexity was assessed by comparing the total number of peaks per Vβ family.
TCRVβ forward primers
TCRVβ . | Sequence . | Product, bp . |
|---|---|---|
| 1 | GCACAACAGTTCCCTGACTTGCAC | 195-207 |
| 2 | TCATCAACCATGCAAGCCTGACCT | 195-207 |
| 3 | GTCTCTAGAGAGAAGAAGGAGCGC | 190-208 |
| 4 | ACGATCCAGTGTCAAGTCGAT | 334-346 |
| 5 | CTGATCAAAACGAGAGGACAGCA | 354-375 |
| 6 | TCAGGTGTGATCCAATTTC | 329-347 |
| 7 | CCTGAATGCCCCAACAGCTCTC | 190-214 |
| 8 | GGTGACAGAGATGGGACAAGA | 355-373 |
| 9 | CACCTAAATCTCCAGACAAAGCT | 194-212 |
| 11 | TGTTCTCAAACCATGGGCCATGAC | 321-333 |
| 12 | GRCATGGGCTGAGGCTGAT | 267-290 |
| 13 | CTCTCCTGTGGGCArGTC | 408-425 |
| 14 | ACCCAAGTACCTCATCACAG | 328-383 |
| 15 | AGTGTCTCTCGACAGGCACAG | 193-208 |
| 16 | AAAGAGTCTAAACAGGATGAGCC | 241-256 |
| 17 | TTTCAGAAAGGAGATATAGCT | 226-241 |
| 18 | AGCCCAATGAAAGGACACAGTCAT | 325-337 |
| 20 | CTCTGAGGTGCCCCAGAA | 218-227 |
| 21 | GGCTCAAAGGAGTAGACTCC | 185-200 |
| 22 | ATGAAATCTCAGAGAAGTCT | 234-252 |
| 23 | GATCAAAGAAAAGAGGGAAAC | 358-370 |
| 24 | TACCCAGTTTGGAAAGC | 353-368 |
| 25 | CAGGTATGCCCAAGGAAAGA | 226-241 |
| Reverse primer | ||
| Cβ (FAM) | TTCTGATGGCTCAAACAC | NA |
TCRVβ . | Sequence . | Product, bp . |
|---|---|---|
| 1 | GCACAACAGTTCCCTGACTTGCAC | 195-207 |
| 2 | TCATCAACCATGCAAGCCTGACCT | 195-207 |
| 3 | GTCTCTAGAGAGAAGAAGGAGCGC | 190-208 |
| 4 | ACGATCCAGTGTCAAGTCGAT | 334-346 |
| 5 | CTGATCAAAACGAGAGGACAGCA | 354-375 |
| 6 | TCAGGTGTGATCCAATTTC | 329-347 |
| 7 | CCTGAATGCCCCAACAGCTCTC | 190-214 |
| 8 | GGTGACAGAGATGGGACAAGA | 355-373 |
| 9 | CACCTAAATCTCCAGACAAAGCT | 194-212 |
| 11 | TGTTCTCAAACCATGGGCCATGAC | 321-333 |
| 12 | GRCATGGGCTGAGGCTGAT | 267-290 |
| 13 | CTCTCCTGTGGGCArGTC | 408-425 |
| 14 | ACCCAAGTACCTCATCACAG | 328-383 |
| 15 | AGTGTCTCTCGACAGGCACAG | 193-208 |
| 16 | AAAGAGTCTAAACAGGATGAGCC | 241-256 |
| 17 | TTTCAGAAAGGAGATATAGCT | 226-241 |
| 18 | AGCCCAATGAAAGGACACAGTCAT | 325-337 |
| 20 | CTCTGAGGTGCCCCAGAA | 218-227 |
| 21 | GGCTCAAAGGAGTAGACTCC | 185-200 |
| 22 | ATGAAATCTCAGAGAAGTCT | 234-252 |
| 23 | GATCAAAGAAAAGAGGGAAAC | 358-370 |
| 24 | TACCCAGTTTGGAAAGC | 353-368 |
| 25 | CAGGTATGCCCAAGGAAAGA | 226-241 |
| Reverse primer | ||
| Cβ (FAM) | TTCTGATGGCTCAAACAC | NA |
NA indicates not applicable.
Statistics
Comparisons were analyzed using paired Student t tests. Pair-wise comparison between groups was adjusted for multiple comparisons using the Bonferroni method.
Results
Expansion and phenotype of isolated T-cell subsets
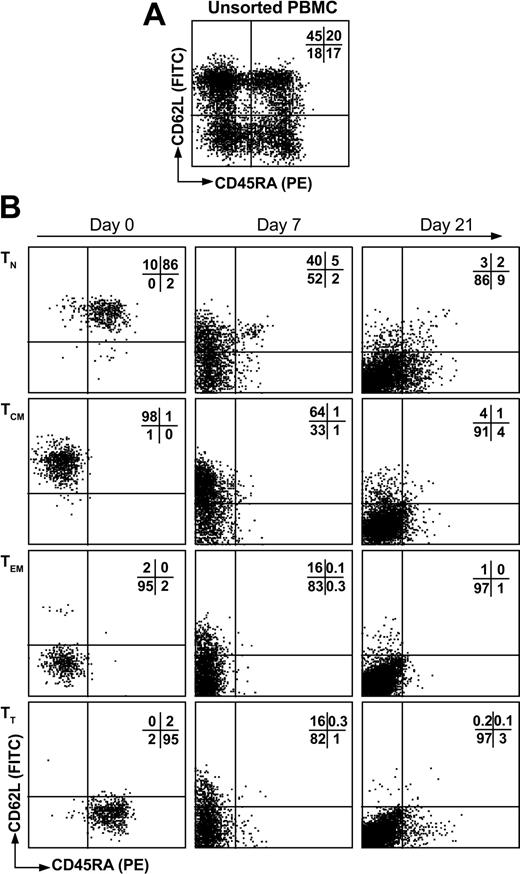
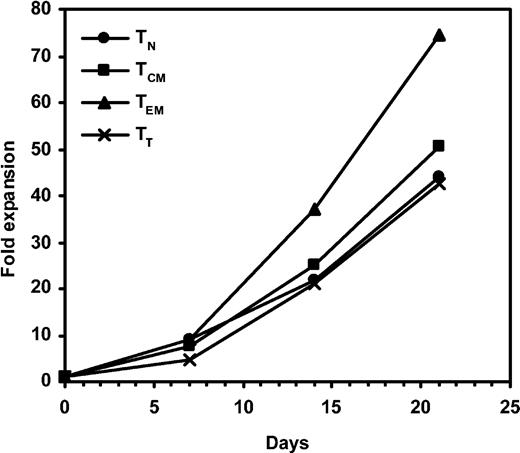
Healthy individuals were analyzed for EBV and CMV serology and tissue typed for HLA-A, -B, and -DRB1 when possible (Table 1). During initial experiments, T cells were sorted by flow cytometry into 4 different T-cell subsets based on expression of CD62L and CD45RA. These were naive (TN, CD62L+CD45RA+), central memory (TCM, CD62L+CD45RA–), effector memory (TEM, CD62L–CD45RA–), and terminal differentiated T cells (TT, CD62L–CD45RA+) using a nomenclature previously described.21 Initial labeling with CD62L and CD45RA produced 4 distinct populations of sufficient frequency for cell sorting where T-cell subsets were represented at 31% (range, 20%-49%), 34% (23%-45%), 18% (9%-27%), and 16% (9%-19%) for TN, TCM, TEM, and TT, respectively (Figure 1A). These 4 populations were easily sorted into high-purity T-cell subsets (approximately 90% pure) using flow cytometric cell sorting (Figure 1B first column). To assess the potential for in vitro culture for immunotherapy purposes as well as to obtain sufficient cell numbers for analysis, we stimulated 2 × 105 sorted cells with OKT3 in the presence of IL-2. All of the subsets responded to OKT3/IL-2 stimulation and we were able to obtain 40-fold expansion over a period of 21 days (Figure 2). In these experiments, TEM showed a slight growth advantage compared to other T-cell populations. Analysis of CD62L and CD45RA expression following TCR activation showed that both of these molecules were down-regulated during expansion (Figure 1B). This is consistent with the principle of effector differentiation following TCR activation.22-24
T-cell subset cell sorting and phenotype following expansion. Fresh PBMCs were labeled with CD62L FITC and CD45RA PE (A) and sorted by flow cytometry into CD62L+CD45RA+ (TN), CD62L+CD45RA– (TCM), CD62L–CD45RA– (TEM), or CD62L–CD45RA+ (TT) T-cell subsets (B, first column). Following isolation, T cells were stimulated with OKT3 in the presence of IL-2 and expanded for 21 days in which the phenotype of CD62L and CD45RA was monitored (B). The percentage of events in each quadrant is indicated.
T-cell subset cell sorting and phenotype following expansion. Fresh PBMCs were labeled with CD62L FITC and CD45RA PE (A) and sorted by flow cytometry into CD62L+CD45RA+ (TN), CD62L+CD45RA– (TCM), CD62L–CD45RA– (TEM), or CD62L–CD45RA+ (TT) T-cell subsets (B, first column). Following isolation, T cells were stimulated with OKT3 in the presence of IL-2 and expanded for 21 days in which the phenotype of CD62L and CD45RA was monitored (B). The percentage of events in each quadrant is indicated.
Expansion of T-cell subsets. PBMCs were labeled with CD62L FITC and CD45RA PE and sorted into TN (•), TCM (▪), TEM (▴), and TT (×) subsets and stimulated with OKT3 and IL-2. Cell culture were fed every 2 to 3 days and enumerated for viable cells for 21 days.
Expansion of T-cell subsets. PBMCs were labeled with CD62L FITC and CD45RA PE and sorted into TN (•), TCM (▪), TEM (▴), and TT (×) subsets and stimulated with OKT3 and IL-2. Cell culture were fed every 2 to 3 days and enumerated for viable cells for 21 days.
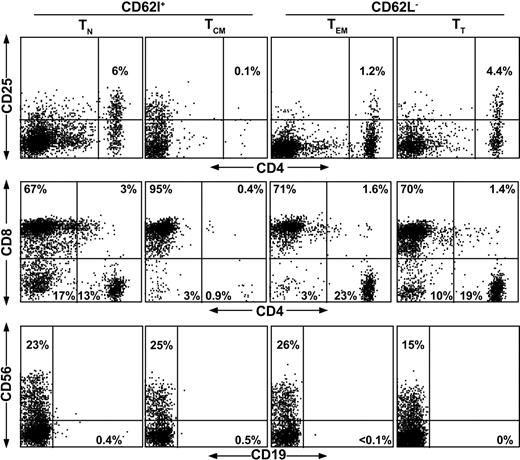
Through the selection and expansion process, it might be possible to inadvertently enrich for T regulatory (Treg) cells that may suppress the immune response to aAg.25-27 Therefore, we analyzed these subsets for the presence of CD4+CD25+ T cells (Figure 3). These data indicate that there were no significant differences in the frequency of Treg cells in the different T-cell populations after sorting and expansion. We also examined memory (CD62L–) cells versus unsorted PBMCs prior to expansion and found no differences between these populations indicating that Treg cells were not altered significantly in this process (data not shown). Further characterization at day 21 showed that the cultures, regardless of initial phenotype, were primarily CD8+, with a modest increase in the numbers of CD56+ lymphocytes (Figure 3).
Phenotype of naive and memory T cells after expansion. To examine the contribution of Treg cells and to assess the phenotype of T-cell subset cultures following expansion, T cells were analyzed for the presence of CD4+CD25+ regulatory T cells (top row), and for lymphocyte phenotype with CD4, CD8 (middle row), and CD19, CD56 (bottom row). CD62L+ and CD62L– subsets are TN and TCM or TEM and TT, respectively, as shown above the diagram.
Phenotype of naive and memory T cells after expansion. To examine the contribution of Treg cells and to assess the phenotype of T-cell subset cultures following expansion, T cells were analyzed for the presence of CD4+CD25+ regulatory T cells (top row), and for lymphocyte phenotype with CD4, CD8 (middle row), and CD19, CD56 (bottom row). CD62L+ and CD62L– subsets are TN and TCM or TEM and TT, respectively, as shown above the diagram.
Retention of responsive antigen-specific T cells
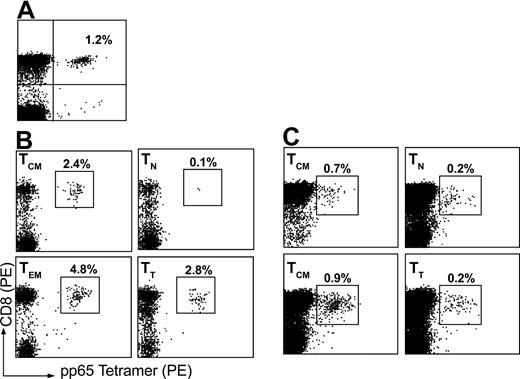
Memory T cells should contain a higher frequency of antigen-specific T cells recognizing previously encountered antigens. Therefore, we analyzed T-cell subsets for the presence of T cells recognizing CMVpp65 using MHC class I tetramers presenting HLA-A2–restricted peptide. Cell cultures were tested shortly after sorting (< 7 days) for the presence of CMV-specific T cells using a tetramer presenting the NLV epitope (Figure 4). We observed high frequencies of pp65-specific T-cell frequency in the memory T-cell subsets (eg, TCM, TEM, and TT) as compared with TN cells, where TEM showed the highest percentage (4.7%) compared with unsorted PBMCs that had a frequency of approximately 1% pp65-specificT cells (Figure 4A-B). This shows that the memory T-cell population is skewed toward the recognition of CMV and the majority of these cells are contained within the CD62L– population. After 21 days in culture, T-cell subsets were tested again for both pp65-specific T cells (Figure 4C). We found that T cells recognizing both antigens were present in the memory T-cell subsets, but were less frequent in naive T cells. This is consistent with other T-cell differentiation models where T cells specific for CMV are found principally in the memory and effector cell populations during chronic infection.28
Analysis of antigen-specific T cells in naive and memory T-cell subsets. Tetramer complex recognizing CMV pp65 was used to assess the relative frequency CMV-specific T cells in various T-cell subsets. (A) Unsorted PBMCs were first assayed for CMV-specific T-cell frequency. (B) T cells were then sorted on the basis of CD62L and CD45RA expression and assessed for the frequency of tetramer-positive CD8+ T cells shortly after cell sorting (< 7 days) or (C) after in vitro expansion for 21 days.
Analysis of antigen-specific T cells in naive and memory T-cell subsets. Tetramer complex recognizing CMV pp65 was used to assess the relative frequency CMV-specific T cells in various T-cell subsets. (A) Unsorted PBMCs were first assayed for CMV-specific T-cell frequency. (B) T cells were then sorted on the basis of CD62L and CD45RA expression and assessed for the frequency of tetramer-positive CD8+ T cells shortly after cell sorting (< 7 days) or (C) after in vitro expansion for 21 days.
To determine whether pp65-specific T cells were able to mount a proliferative response after cognate antigen stimulation, we sorted T cells from CMV-seropositive donors into CD62L+ and CD62L– fractions and cocultured with NLV-loaded T2 cells and measured the frequency of pp65-specific T cells using tetramer. Analysis of PBMCs from seropositive donors using tetramer and CD62L show that the majority of pp65-specific T cells are CD62L– (Figure 5A). Both populations responded to NLV peptide stimulation. The frequency of pp65-specific T cells increased from 0.9% and 4% (CD62L+ and CD62L–, respectively) to over 20% in both cell cultures (Figure 5B). Tetramer-positive CD62L+ T cells lost the expression of CD62L following antigen stimulation, whereas tetramer-positive CD62L– cells retained an effector phenotype. These data indicate that tetramer-positive T cells in both CD62L+ and CD62L– populations retain the ability to proliferate in response to cognate antigen stimulation.
Proliferation of pp65-specific T cells in response to peptide stimulation. (A) PBMCs from seropositive donors were sorted on CD62L into CD62L+ (R1) or CD62L– (R2). (B) Day 0 analysis with CD62L and tetramer and following stimulation with NLV peptide-pulsed T2 cells on day 7.
Proliferation of pp65-specific T cells in response to peptide stimulation. (A) PBMCs from seropositive donors were sorted on CD62L into CD62L+ (R1) or CD62L– (R2). (B) Day 0 analysis with CD62L and tetramer and following stimulation with NLV peptide-pulsed T2 cells on day 7.
Reduced recognition of CD62L– T cells against aAgs
Specificity of memory T cells is skewed toward recognition of previously encountered antigens as demonstrated by higher frequencies of pp65-tetramer–positive T cells in TCM, TEM, and TT populations. Therefore, memory T-cell subsets may have a lower frequency of T cells with the capacity to recognize aAgs. To test this hypothesis, we measured proliferative and cytotoxic responses of T-cell populations against HLA-mismatched LCLs, which are potent antigen-presenting cells. First, we looked at differences in proliferation between T-cell subsets. Figure 6 shows a representative experiment (donor 3) in which sorted T cells were cocultured with 3 different irradiated HLA-mismatched LCLs over a 6-day period. In these experiments, TEM and TT were significantly reduced in their ability to proliferate in response to aAg stimulation compared to TN (P = .01) and TCM (P = .04). These data indicate that T cells sorted on the basis of CD62L expression differ in alloreactivity, which is in agreement with published murine studies.10,11 We next examined whether aAg-specific responses could be generated by repetitive stimulation with HLA-mismatched LCLs. Sorted T-cell subsets were stimulated with LCLs twice on a weekly basis in the presence of low concentrations of IL-2. Subsequent to stimulation, T-cell subsets were assayed for cytotoxicity against stimulator cells in a standard 51Cr-release assay and each of the memory subsets was compared against TN (Figure 7). We found that whereas memory T cells (TCM, TEM, and TT) could generate a cytotoxic response following coculture with HLA-mismatched LCLs, the response was 50% less than that generated from the CD62L+CD45RA+ naive phenotype. These data agree with proliferation assays showing that antigen-experienced T cells respond to a lesser degree than do naive T cells when presented with aAgs.
Proliferation in response to aAg stimulation of naive and memory T-cell subsets. Donor cells (from donor 1, a representative sample) were isolated and expanded in culture for 7 days to obtain sufficient cell numbers. T-cell subsets were then cocultured against 3 separate irradiated HLA-mismatched LCLs for 6 days and proliferation measured by thymidine incorporation. Error bars represent the SEM for donor 3 against 3 separate LCLs. Statistical significance is shown by brackets comparing CD62L– against TN (P = .01) and TCM (P = .04).
Proliferation in response to aAg stimulation of naive and memory T-cell subsets. Donor cells (from donor 1, a representative sample) were isolated and expanded in culture for 7 days to obtain sufficient cell numbers. T-cell subsets were then cocultured against 3 separate irradiated HLA-mismatched LCLs for 6 days and proliferation measured by thymidine incorporation. Error bars represent the SEM for donor 3 against 3 separate LCLs. Statistical significance is shown by brackets comparing CD62L– against TN (P = .01) and TCM (P = .04).
Generation of cytotoxicity against aAg after repeated exposure to antigen. Donor cells were isolated and expanded in culture for 7 days and then stimulated with irradiated HLA-mismatched LCL for 2 stimulations (on a weekly basis) and then assayed against LCL stimulator target cells in a standard 51Cr-release assay. This figure shows representative data from donor 2 against 2 LCL (LCL1 and LCL2) where TN (⋄) TCM (□), TEM (▴) and TT (•) were compared.
Generation of cytotoxicity against aAg after repeated exposure to antigen. Donor cells were isolated and expanded in culture for 7 days and then stimulated with irradiated HLA-mismatched LCL for 2 stimulations (on a weekly basis) and then assayed against LCL stimulator target cells in a standard 51Cr-release assay. This figure shows representative data from donor 2 against 2 LCL (LCL1 and LCL2) where TN (⋄) TCM (□), TEM (▴) and TT (•) were compared.
Analysis of freshly isolated T-cell subsets for alloreactivity
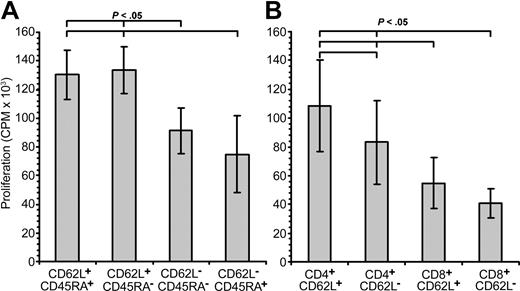
In vitro expansion of T-cell subsets by OKT3 and IL-2 stimulation may affect the ability of naive and memory T cells to proliferate in response to aAg stimulation. Therefore, we sorted fresh PBMCs from 5 healthy donors into TN, TCM, TEM, and TT populations and measured proliferation against 2 HLA-mismatched LCLs. We found that freshly isolated TEM and TT (both CD62L–) showed less proliferation (P < .05) than TN and TCM (both CD62L+; Figure 8A). When compared to proliferation from the TN population, there was a 24.5% (range, 21.5%-26.1%) and 49.8% (8.7%-98.7%) reduction in proliferation for TEM and TT, respectively.
Alloreactivity of freshly isolated naive and memory T cells. To measure the alloreactivity of uncultured naive and memory T cells, fresh PBMCs were sorted into CD62L+CD45RA+ (TN), CD62L+CD45RA– (TCM), CD62L–CD45RA– (TEM), or CD62L–CD45RA+ (TT) subsets (A) or CD4+CD62L+, CD4+CD62L–, CD8+CD62L+, or CD8+CD62L– (B) and measured for proliferation against 2 separate allogeneic LCLs. Data shown here are the mean values for each donor (n = 5) and stimulator (n = 2) pair done in triplicate. Error bars indicate statistical analyses performed between groups.
Alloreactivity of freshly isolated naive and memory T cells. To measure the alloreactivity of uncultured naive and memory T cells, fresh PBMCs were sorted into CD62L+CD45RA+ (TN), CD62L+CD45RA– (TCM), CD62L–CD45RA– (TEM), or CD62L–CD45RA+ (TT) subsets (A) or CD4+CD62L+, CD4+CD62L–, CD8+CD62L+, or CD8+CD62L– (B) and measured for proliferation against 2 separate allogeneic LCLs. Data shown here are the mean values for each donor (n = 5) and stimulator (n = 2) pair done in triplicate. Error bars indicate statistical analyses performed between groups.
To determine whether CD4+ or CD8+ memory or naive T cells mediated the aAg response, we sorted fresh PBMCs from 5 healthy donors into CD4+CD62L+ or CD62L– and CD8+CD62L+ and CD62L– populations and performed mixed lymphocyte reactions (MLRs) against 2 HLA-mismatched LCLs. Surprisingly, we were only able to observe a significant difference between CD4+CD62L+ and CD4+CD62L– T cells (P < .05) and not between CD8+CD62L+ and CD8+CD62L– T cells (Figure 8B). In the CD4+ population, CD62L– T cells showed a 21% (range, 0%-69%) reduction in aAg responsiveness, whereas in the CD8+ compartment, both CD62L+ and CD62L– cells responded equally.
TCRVβ analysis
Results from proliferation and cytotoxicity assays, as well as from tetramer analysis, indicated that TEM and TT (both CD62L–) had a reduced proliferation and cytotoxic potential when challenged with aAg as compared to TN, but also contained a higher frequency of pathogen-specific T cells (eg, CMV). One possible explanation for this phenomenon is that there is a difference in TCR diversity between CD62L+ and CD62L– T-cell subsets, resulting in lower recognition of antigens that have not previously been encountered.
To address this hypothesis, global diversity of the TCR repertoire was assessed using Vβ-specific primers (Table 2) to measure CDR3 length. The size spectrum of CDR3 regions provides a global description of TCR diversity, in that dominant peaks or absence of Vβ subfamilies represent the presence of an oligoclonal or clonal T-cell population or loss of T cells from a Vβ family, respectively.29 We sorted freshly isolated PBMCs into CD62L+ and CD62L– T-cell populations and compared the relative diversity of each Vβ family. We found a clear difference in the diversity between CD62L+ and CD62L– T-cell subsets. Figure 9A shows several TCRVβ families from CD62L+ or CD62L– T cells from donors 1 and 5, demonstrating the focusing of the TCR in memory cell populations compared to naive T cells. Several of the Vβ families in the CD62L– population show a single or double peak. To further characterize the overall diversity of these 2 subsets, the TCR complexity was assessed in CD62L+ and CD62L– T cells by counting the number of peaks per Vβ family (Figure 9B). This analysis shows that CD62L– cells have a decreased overall diversity, where an average of 3.6 peaks (70 total) per family compared to 6.6 peaks (126 total) in donor 1 and an average of 3.1 (62 total) versus 5.5 (109 total) in donor 5 were observed for CD62L– and CD62L+ T-cell subsets, respectively. The skewing of the TCR is presumably due to previous encounters with common antigens causing expansion of antigen-specific T-cell clones that persist in the memory T-cell compartment and may account for the inefficiency in recognizing aAgs.
Analysis of TCRVβ by spectratype. RNA was extracted from sorted CD62L+ (□) and CD62L– (▪) T cells from donors 1 and 5 immediately following isolation. (A) Spectratype analysis was performed on 23 Vβ families where 3 representative families are shown for donor 1 and 5 for CD62L+ and CD62L– T cells. (B) TCR complexity was assessed by counting the number of peaks per Vβ family for CD62L+-sorted and CD62L–-sorted T cells.
Analysis of TCRVβ by spectratype. RNA was extracted from sorted CD62L+ (□) and CD62L– (▪) T cells from donors 1 and 5 immediately following isolation. (A) Spectratype analysis was performed on 23 Vβ families where 3 representative families are shown for donor 1 and 5 for CD62L+ and CD62L– T cells. (B) TCR complexity was assessed by counting the number of peaks per Vβ family for CD62L+-sorted and CD62L–-sorted T cells.
Discussion
Allogeneic SCT is a common curative treatment for hematologic malignancies. However, transplant recipients often develop 2 life-threatening conditions, OI and GVHD. The first is due to a severe deficiency of immune cells, whereas alloreactive T cells transferred in the stem cell graft cause GVHD. Several treatment strategies have been examined to try to strike a balance between OI and GVHD, including T-cell depletion, donor lymphocyte infusion with thymidine-kinase suicide gene-transduced T cells, and adoptive transfer of pathogen-specific T cells.4,5,30-32 These methods attempt to preserve or enhance T-cell–mediated immunity toward OI while reducing the probability of GVHD.
Two recent studies have proposed another possible method for reducing GVHD following SCT using markers (eg, CD62L, CD44) for discriminating between “antigen-experienced” memory T cells and naive T cells, which may be functionally different.10,11 The first of these studies by Anderson and colleagues demonstrated in a CD4-dependent, minor histocompatibility antigen-incompatible murine model of chronic GVHD, that memory T cells (CD62L–CD44+) were ineffective at generating GVHD, whereas naive T cells (CD62L+CD44–) produced lethal GVHD but were still able to recognize antigen to which the mice had previously been primed.10 This was in contrast to naive T cells (CD62L+CD44–), which caused lethal GVHD shortly after transfer. Treg cells (CD4+CD25+), which promote self-tolerance and suppress GVHD, were not responsible for this effect.33,34 Subsequently, Chen and coworkers extended this work to include CD8+ memory T cells (here, CD62L–). This group provided further evidence in a murine system that memory T cells are less likely to cause GHVD than naive T cells.11 Remarkably, CD62L– T cells adoptively transferred from mice primed with BCL1 tumor were able to inhibit BCL1 tumor growth, showing that tumor specificity is preserved in CD62L– memory T cells while reducing GVHD.
Our study shows that human memory T cells (CD62L–) differ from naive T cells (CD62L+) in their ability to respond to aAg stimulation in both proliferation and cytotoxicity assays. As found by others, this was not caused by inadvertent enrichment of Treg cells. However, memory T cells contained higher levels of CMV-specific T cells (> 4% in TEM compartment of donor 1) than did naive T cells, indicating that the TCR repertoire may be skewed toward pathogens or neoplastic cells that the host may frequently encounter. Further analysis of TCR diversity (examining individual Vβ families) shows that the memory T-cell repertoire is markedly restricted compared to naive T cells. Similar to results published by Hamel et al, memory T cells had a distinct TCR repertoire with certain Vβ families dominated by single peaks.35 This restriction in diversity likely plays a role in the inability of memory T cells to generate a robust proliferative response following aAg exposure. Chen et al11 were also able to show that Vβ families were different in CD62L+ and CD62L– populations indicating that antigen-specific tolerance was in part caused by clonal deletion or anergy.
With these data, adoptive transfer of memory T cells is an attractive idea to restore pathogen-specific immunity in recipients of allogeneic stem cell transplants. Presumably, memory T cells would contain specificity toward many commonly occurring pathogens. Furthermore, these cells should recognize epitopes to multiple antigens and HLA-restricted peptides. The surprising differences in TCRVβ repertoire may also lend to selecting low-diversity Vβ families using monoclonal antibodies. This may further reduce the frequency of aAg-specific T cells and may represent a method for tailoring aAg-specific T-cell depletion in stem cell donors.
These data in combination with other reports suggest that selection of CD62L– (or depletion of CD62L+) T cells will reduce GVHD and facilitate immune reconstitution toward OI. Although CD62L+ depletion has the potential to remove T cells specific for unencountered minor histocompatibility antigens that could induce potent GVT responses, GVT-mediating T cells specific for overexpressed autoantigens such as PR1 and WT1 may still be present in the CD62L– T-cell populations. Rezvani et al demonstrated that PR1- and WT1-specific T cells in healthy donors were primarily of a memory phenotype.36 In one of our donors (donor 1), PR1-specific T cells were present in healthy lymphocytes and were found predominantly in the memory T-cell pool (TCM, TEM, and TT) when examined by tetramer (data not shown). Furthermore, Verdijk et al found that in healthy multiparous women, T cells specific for the minor antigens HY, HA-1, and HA-2, which are responsible for potent GVL effects in female-to-male SCT,37 were CD45RA–CD27+, indicating they were memory T cells. Further examination of healthy donors with a broader range of tumor antigens is needed to confirm whether T-cell memory includes specificity against self and differentiation tumor antigens.
Conversely, we recognize that severe or fatal GVHD could be caused by expansions of one or a small number of clones of T cells potentially of CD62L– phenotype.38 Nevertheless, preliminary data suggest that clinical allodepletion does reduce the incidence of severe GVHD8 and our data also suggest that depletion of alloreactive T cells is likely to reduce the frequency and severity of GVHD. Clearly, clinical trials will be required to address this issue.
This report has demonstrated that human memory T cells, as demonstrated in murine experiments, have a reduced capacity to recognize and respond to aAg stimulation and that this may potentially reduce GVHD while preserving immunity toward previously encountered antigens (eg, CMV). Ultimately, the ability to home to secondary lymphoid organs, effector potential, and the diversity of the TCR repertoire will determine the degree of alloreactivity of memory T cells in vivo. However, using a T-cell population with a skewed TCR repertoire may be advantageous in avoiding GVHD. Adoptive transfer of CD62L– memory T cells or depletion of CD62L+ naive T cells may find use in the SCT setting allowing for rapid reconstitution of T cell–mediated immunity while minimizing GVHD.
Prepublished online as Blood First Edition Paper, July 1, 2004; DOI 10.1182/blood-2003-12-4431.
Supported by the Cure Cancer Foundation Australia (A.E.F).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Lyanne Weston, Tissue Typing, Australian Red Cross Blood Transfusion Service for technical assistance and Drs C. Traversari and V. Russo, Tumor Immunology and Gene Therapy Program, Hospital San Raffaele, Milan, Italy for lymphoblastoid cell lines.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal