Abstract
Several examples suggest a relationship between in vitro migratory capacity and bone marrow (BM) homing. Pertussis toxin (PTX) is a potent inhibitor of serpentine receptor–associated inhibitory trimeric guanidine nucleotide binding (Gi) protein signals. As such, it blocks hematopoietic progenitor cell migration in vitro, but contrary to expectation, no effects on BM homing were observed in previous studies. We therefore re-examined the effect of PTX on homing of murine BM and fetal liver (FL). We found that BM homing of PTX-incubated progenitor cells (colony-forming cells in culture [CFU-Cs]) from BM or FL in irradiated and nonirradiated recipients was reduced by more than 75%, with a concomitant increase in circulating CFU-Cs in peripheral blood. Additional studies confirmed the functional significance of this reduction in homing: PTX-treated cells did not provide radioprotection, and their short-term engraftment in BM and spleen was drastically reduced. Furthermore, several approaches show that cell-intrinsic rather than host-derived mechanisms are responsible for the PTX-induced homing defect. In summary, we show that Gi protein signals are required for BM homing and, as such, provide a new example of the association between BM homing and in vitro migration. Moreover, our data suggest that the behavior of hematopoietic progenitors in obeying Gi signaling does not diverge from that of mature leukocytes.
Introduction
In hematopoietic cell transplantation, intravenously administered progenitor cells travel to bone marrow (BM), where they lodge in the vicinity of cells providing appropriate signals for their retention, survival, and proliferation/differentiation. The early stages of BM seeding, which precede proliferation/differentiation, are collectively termed “homing.” Adequate homing is critical for successful engraftment. Despite the relevance of homing for clinical transplantation, the precise mechanisms governing it are poorly understood. It has been proposed that homing is a multistep process involving reversible adhesion and directed migration, similar in principle to leukocyte recruitment from circulation to sites of inflammation.1,2 Furthermore, it has been widely demonstrated that homing to BM can be modulated by altering the migratory and/or adhesive behavior of donor cells, similarly to approaches used for modulation of migration and/or adhesion of mature leukocytes. For example, disrupting the stromal cell–derived factor 1 (SDF-1)/CXC chemokine receptor 4 (CXCR4) or the very late antigen 4 (VLA-4)/vascular cell adhesion molecule 1 (VCAM-1) pathways has led to impairments in homing of progenitor cells to BM or leukocyte migration to tissues.3-7 Signaling through serpentine receptors such as CXCR4 involves inhibitory trimeric guanidine nucleotide binding (Gi) proteins and can be readily blocked by the widely used inhibitor pertussis toxin (PTX). PTX blocks signaling pathways involved in migration, such as calcium flux and activation of phosphatidylinositol 3 (PI3) kinase and small rho–guanosine triphosphatases (rho-GTPases).8-10 In vitro, the effects of PTX on hematopoietic progenitor cells are similar to its effects on mature leukocytes. Thus, PTX inhibits both chemokinetic and chemotactic migration of progenitor cells and mature leukocytes toward the most important chemoattractant for hematopoietic progenitor cells thus far identified, the chemokine SDF-1.11-13 These chemotactic responses were dependent on the expression of the SDF-1 receptor CXCR4, and they were abrogated by treating the cells with anti-CXCR4 antibodies.3,5 Similarly, treating hematopoietic donor cells with antibodies to CXCR4 resulted in significant inhibition of BM homing.3 Although, on the basis of in vitro observations, blocking downstream signals of SDF-1 would be expected to do likewise, previous studies have not found an effect of PTX on BM homing or short-term engraftment,12,14,15 despite the fact that several other examples have shown a correlation between in vitro migration and BM homing.3,12,16-19 Because of the apparent discrepancies between in vitro findings and in vivo behavior of PTX-treated cells, we re-examined Gi protein signaling in hematopoietic progenitor cell homing to BM. Using several in vitro and in vivo approaches and slightly different conditions from the ones used previously, we demonstrate in the present paper that Gi protein signaling is important for successful homing of hematopoietic stem cells and their short-term engraftment. Consequently, our findings suggest that for homing to BM spaces, intracellular signaling pathways need to be engaged. As far as the CXCR4/SDF-1 pathway signals are concerned, our data further suggest that primitive hematopoietic cells do not follow molecular pathways divergent from those used by mature cells to migrate from circulation to tissues.
Materials and methods
Animals
Inbred B6/129 mice (Jackson Laboratories, Bar Harbor, ME) served as donors and recipients for most experiments. Pooled donor BM was collected by flushing long bones from adult donors killed by cervical dislocation. Fifteen days after coitus (15 dpc), fetal livers (FLs) were pooled from one litter of a killed female. As sources of G418-resistant BM cells, B6/129 α4flox/flox donors were used; these mice were generated as described.20 Where indicated, adult B6/129 recipients were lethally irradiated with a single dose of 1150 cGy with the use of a cesium source (at a dose rate exceeding 100 cGy/min), and received transplants within 1 to 2 hours of irradiation. For some assays, C57BL/6 mice were used as recipients of C57BL/6 green fluorescent protein (GFP) BM cells. Donor BM or FL cells were injected into the lateral tail vein. Mice were housed and bred in the Specific Pathogen Free facility at the University of Washington (Seattle, WA) with water and chow ad libitum. All procedures were done in agreement with institutional animal care and use committee (IACUC) protocols approved by the local animal review board. Three to 5 mice were used per group for each experiment, and untreated controls were included in all experiments.
Enrichment of c-kit+ BM cells
Single-cell suspensions of BM or FL cells were successively incubated with Fc-blocking antibody (CD16/CD32) (BD Pharmingen, San Diego, CA), biotinylated anti–c-kit–AB (clone 2B8) (BD Pharmingen), and antibiotin antibody coupled to paramagnetic beads (Miltenyi Biotec, Auburn, CA). Cells were passed over a Mini-MACS column (Miltenyi Biotec) as directed. As assessed by flow cytometry (FACS Calibur) (BD Immunocytometry Systems, San Jose, CA) by means of a phycoerythrin-labeled anti–c-kit antibody (clone ack45) (BD Pharmingen), c-kit+ purity was regularly greater than 90%.
Migration assay
The c-kit–enriched cells were suspended in X-Vivo20 medium (BioWhittaker, Walkersville, MD) plus recombinant murine stem cell factor (rmuSCF) (100 ng/mL) (Peprotech, Rocky Hill, NJ) at a density of 2.5 × 106 cells per milliliter, and 100 μL cells per transwell insert were pipetted in quadruplicate into the upper chamber of 5-μm pore size transwells (Corning Costar, Cambridge, MA). The lower chamber contained X-Vivo20 plus SCF, with or without SDF-1 (100 ng/mL; Peprotech), to test SDF-1–induced chemotactic migration or chemokinetic motility. Transwell migration into the lower chamber was quantified after 4 hours as described previously.21
Actin polymerization assay
Chemokine-induced actin polymerization was measured as described previously.11 Briefly, cells were incubated with SDF-1 at 37° C for 0 to 300 seconds, fixed in 1.5% formalin, and permeabilized with saponin. F-actin was stained with Alexa568-conjugated phalloidin (Molecular Probes, Eugene, OR). Staining intensity was measured by fluorescence-activated cell sorter (FACS) or visualized by means of DeltaVision deconvolved white-field microscopy (Applied Precision, Issaquah, WA) with an Olympus fluorescence microscope (IX70; Olympus, Melville, NY), 60 × oil immersion objective, acquisition and deconvolution using a Cool Snap HQ CCD camera (Roper Scientific, Tucson, AZ), Applied Precision softWoRx, and Universal Imaging (Downington, PA) MetaMorph software.
Parallel plate flow chamber adhesion assay
The c-kit+ cells were incubated as described in the presence or absence of PTX. First, 35-mm Petri dishes were coated with RetroNectin (Takara Bio, Ostu, Japan) dissolved in water at a concentration of 20 μg/mL, to serve as the bottom plate in a 20-mm (length) × 2.5-mm (width) × 250-μm (height) parallel plate flow chamber (GlycoTech, Rockville, MD). To test adhesion, cells suspended in X-Vivo20 medium plus rmuSCF (100 ng/mL) at a concentration of 3 × 106 cells per milliliter were warmed to 37° C for 10 minutes; the cell suspension was pumped through the chamber at a wall shear rate of 0.5 dynes per square centimeter by means of a no. 975 pulse-free syringe pump (Harvard Apparatus, Holliston, MA). Cell accumulation was monitored with an inverted Nikon (Tokyo, Japan) TE 200 microscope with a 10 × phase contrast objective by means of a Roper Scientific high-resolution Cascade charge-coupled device (CCD) camera. To blur out free-flowing cells, the camera shutter speed was set to 180 milliseconds, 6 times slower than the shutter speed needed to observe cells moving at the hydrodynamic velocity at the chamber wall. For each accumulation experiment, images of a 750-μm by 555-μm area of the flow chamber were recorded twice per second for 200 seconds by means of MetaMorph video acquisition software (Universal Imaging). Linear cell accumulation over time was tested by determination of cell accumulation at 100 and 200 seconds, by counting adherent events using MetaMorph's manual cell-counting function.
Ribosylation assay
Jurkat cells (ATCC, Manassas, VA) were incubated in the presence of 100 ng/mL PTX (List Biological Laboratories, Campbell, CA) for 0, 2, or 20 hours. Membrane-rich lysates were prepared by disrupting the cells on the French Pressure Cell Press (American Instruments, Silver Spring, MD) and pelleting unbroken cells and nuclei by successive centrifugation at 2500g and 15 000g. Proteins were then precipitated by centrifugation at 250 000g. Gi proteins were ribosylated as previously described22 with PTX in the presence of 10 mM thymidine, 4 mM adenosine triphosphate (ATP), 4 mM guanosine triphosphate (GTP), 5 mM MgCl2, 4 mM EDTA (ethylenediaminetetraacetic acid), and 4 μCi (0.148 MBq) (P32)–nicotinamide adenine dinucleotide ((P32)NAD) (Amersham Biosciences, Piscataway, NJ). Under the influence of an excess of PTX, Gi proteins not previously ribosylated will incorporate P32. The reaction mix was boiled in Laemmli buffer plus βmercaptoethanol (Bio-Rad, Hercules, CA) and separated on 12% sodium dodecyl sulfate (SDS)–polyacrylamide gels. Gels were dried and analyzed by means of a phosphoimager. The presence of a band migrating at approximately 41 kDa indicated that free, that is, nonribosylated, Gi proteins had been present in the cells.
Colony-forming cells in culture (CFU-Cs) assay
CFU-C assays were performed as described, with the use of commercially available methylcellulose medium (StemCell Technologies, Vancouver, BC, Canada). After 7 days, CFU-Cs were counted under 2.5 × original magnification by means of a dissecting microscope. CFU-Cs were plated in at least duplicate. For the enumeration of G418-resistant clones, G418 (Calbiochem, La Jolla, CA) was added to the medium at a concentration of 1.5 mg/mL, a dose that in pilot studies had been found to be nontoxic to resistant cells, but sufficient to completely kill at least 2 × 106 wild-type BM cells per milliliter.
Homing assay
Total FL or adult BM cells were incubated in X-Vivo20 plus rmuSCF (100 ng/mL) (Dulbecco minimum essential medium [DMEM] plus 10% fetal calf serum [FCS] in some experiments) at a concentration of 2.5 × 106 cells per milliliter for 20 hours (2 hours in some experiments) in the presence or absence of PTX (100 ng/mL). Cells were washed twice in phosphate-buffered saline (PBS)/bovine serum albumin (BSA) 0.5%, and then injected intravenously into irradiated (at 1 to 2 hours after irradiation) or nonirradiated isogeneic hosts. Then, 2 × 104 bone marrow cells per milliliter methylcellulose medium were plated in hexaplicate, to quantify the number of injected CFU-Cs. Since incubation with PTX did not affect CFU-C proliferation, in keeping with previously documented data,11,14 similar numbers of normal and PTX-treated CFU-Cs were injected in all instances. At 24 hours after transplantation, fractions of peripheral blood, BM, and spleen were plated in methylcellulose medium in duplicate. After 7 days, CFU-Cs were enumerated; calculated as CFU-Cs per femur, CFU-Cs per milliliter blood, or CFU-Cs per spleen; and expressed as a fraction of input CFU-Cs. To test homing in nonirradiated recipients, 2 different approaches were used. Distribution of total BM cells from C57BL/6 GFP mice in C57BL/6 hosts was tested 24 hours after injection by means of flow cytometry. Alternatively, BM cells from B6/129 hosts harboring the neomycin-resistance gene were used as donor cells for nonirradiated B6/129 wild-type mice; peripheral blood and fractions of BM and spleen were plated in methylcellulose (MC) medium containing G418. To test for background colony growth, equal fractions of nontransplanted host blood, BM, and spleen were also plated in G418-containing MC medium.
Radioprotection assay
Total BM cells were incubated for 20 hours with or without PTX. Then, 2.5 × 105 total cells were injected intravenously into lethally irradiated hosts (n = 10 per group). The injected CFU-Cs were quantified as described. Survival of transplant recipients was monitored daily. Radioprotection was assumed if recipients survived until day 30, at which time surviving recipients were killed, and peripheral blood, BM, and spleen CFU-C content was assessed as described.
Short-term engraftment
Lethally irradiated B6/129 mice were injected with 1.5 × 106 total BM cells, incubated in SCF with or without PTX for 20 hours. Input CFU-Cs were quantified as described. Recipients were killed 8 days after transplantation, and CFU-C content was assessed in peripheral blood, BM, and spleens.
Competitive homing assay
To test for cell-extrinsic effects of PTX-treated donor cells, equal numbers of in vitro–incubated PTX-treated and nontreated BM cells were mixed immediately before injection into lethally irradiated recipients. In addition, lethally irradiated recipients that received only incubated control cells were concurrently tested, to detect a potential “trans” effect of PTX-treated cells on homing of untreated companion cells. Injected CFU-Cs were quantified as described. At 24 hours after injection, the recipients were killed, and peripheral blood, BM, and spleens were taken. PTX and control donor cells were differentiated by virtue of the neomycin-resistance (neo-resistance) gene present in one of the donor populations. Aliquots of blood, BM, and spleen were cultured in duplicate in both normal and in G418-containing MC media. Wild-type CFU-Cs were calculated as the difference between total CFU-Cs and G418-resistant CFU-Cs. A cross-over design, using G418-resistant marrow as control BM in some of the recipients, and as PTX-treated BM in the others, controlled for potential effects of G418 on colony development.
Results
In vitro effects of PTX on c-kit+ BM and FL cells
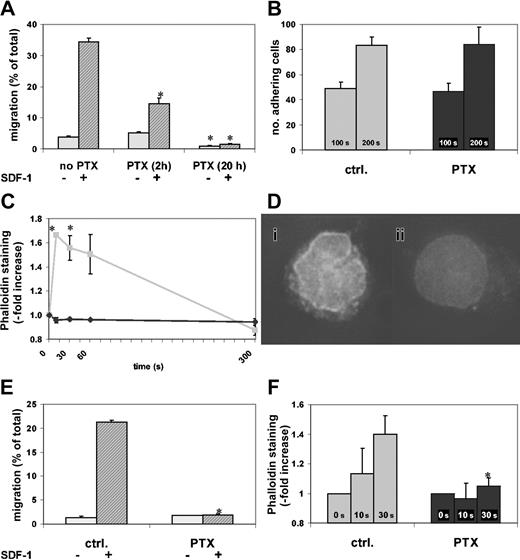
According to previous studies, incubation in SCF increases the in vitro motility of hematopoietic progenitor cells and thus sensitizes them to SDF-1.3 To confirm this effect of SCF incubation on cell motility, we incubated c-kit+ BM cells in SCF and tested their migratory capacity at different times. Control c-kit+ BM cells incubated for 2 hours in SCF had 4% migration toward SDF-1. After 20 hours of SCF incubation, chemotactic migration of control c-kit+ cells had increased more than 5-fold compared with migration at 2 hours (data not shown). Because of this migration-enhancing capacity of SCF and its ability to secure cell survival,23 subsequent studies were all performed in the presence of SCF. We next examined the effect on migration of different incubation times with PTX. More than one third of cells treated for 20 hours with SCF alone migrated toward SDF-1, and 5% migrated spontaneously. Cells that had been incubated in SCF and exposed to PTX for the last 2 hours of the incubation period had more than 50% reduced chemotactic migration, whereas both spontaneous and chemotactic migration of cells exposed to SCF plus PTX for 20 hours were almost completely abrogated (Figure 1A).
Effect of PTX on in vitro migration and actin polymerization. PTX blocks in vitro migration and actin polymerization of BM and FL c-kit+ cells. The c-kit+ cells from BM or 15-dpc FL cells were enriched as described in “Materials and methods” and incubated in SCF with or without PTX (2 hours or 20 hours for panel A, 20 hours for panels B-F). *P < .05 compared with control. (A) Chemokinetic and chemotactic (SDF-1) transwell migration of c-kit+ BM cells was assessed. SDF-1–induced chemotaxis was significantly attenuated after 2 hours of PTX incubation (middle), but migration was almost completely blocked after 20 hours of PTX incubation (right) (chemokinetic migration, light gray bars; chemotactic migration, dark gray bars). (B) Adhesion of c-kit+ BM cells to RetroNectin under laminar flow was identical in PTX-treated and control cells (control cells, ▦; PTX-treated cells, ▪). (C) SDF-1–induced actin polymerization was measured by staining F-actin with phalloidin. The rapid and transient SDF-1–induced actin polymerization in control cells was blocked in PTX-incubated c-kit+ BM cells (control cells, ▦; PTX-treated cells, ♦). (D) After a 10-second stimulation with SDF-1, control (i), but not PTX-treated (ii), cells showed increased phalloidin staining with a predominantly perinuclear pattern (DeltaVision; original magnification × 60). (E) Transwell migration of c-kit+ FL cells was tested as described in “Materials and methods.” Chemokinetic migration of c-kit+ FL cells was low. SDF-1–induced chemotaxis was completely inhibited by PTX (right) compared with control (left) (chemokinetic migration, light gray bars; chemotactic migration, dark gray bars). (F) SDF-1 induced actin polymerization in control, but not in PTX-treated, c-kit+ FL cells. The actin response of FL cells was slower than that of BM cells, where the maximum was reached after 10 seconds (control cells, ▦; PTX-treated cells, ▪). Error bars indicate SEM.
Effect of PTX on in vitro migration and actin polymerization. PTX blocks in vitro migration and actin polymerization of BM and FL c-kit+ cells. The c-kit+ cells from BM or 15-dpc FL cells were enriched as described in “Materials and methods” and incubated in SCF with or without PTX (2 hours or 20 hours for panel A, 20 hours for panels B-F). *P < .05 compared with control. (A) Chemokinetic and chemotactic (SDF-1) transwell migration of c-kit+ BM cells was assessed. SDF-1–induced chemotaxis was significantly attenuated after 2 hours of PTX incubation (middle), but migration was almost completely blocked after 20 hours of PTX incubation (right) (chemokinetic migration, light gray bars; chemotactic migration, dark gray bars). (B) Adhesion of c-kit+ BM cells to RetroNectin under laminar flow was identical in PTX-treated and control cells (control cells, ▦; PTX-treated cells, ▪). (C) SDF-1–induced actin polymerization was measured by staining F-actin with phalloidin. The rapid and transient SDF-1–induced actin polymerization in control cells was blocked in PTX-incubated c-kit+ BM cells (control cells, ▦; PTX-treated cells, ♦). (D) After a 10-second stimulation with SDF-1, control (i), but not PTX-treated (ii), cells showed increased phalloidin staining with a predominantly perinuclear pattern (DeltaVision; original magnification × 60). (E) Transwell migration of c-kit+ FL cells was tested as described in “Materials and methods.” Chemokinetic migration of c-kit+ FL cells was low. SDF-1–induced chemotaxis was completely inhibited by PTX (right) compared with control (left) (chemokinetic migration, light gray bars; chemotactic migration, dark gray bars). (F) SDF-1 induced actin polymerization in control, but not in PTX-treated, c-kit+ FL cells. The actin response of FL cells was slower than that of BM cells, where the maximum was reached after 10 seconds (control cells, ▦; PTX-treated cells, ▪). Error bars indicate SEM.
Actin polymerization, which is requisite for cell motility, was tested in the presence or absence of PTX. In untreated cells, SDF-1 induced a rapid, almost 2-fold increase of F-actin, peaking at 10 seconds, which was spontaneously reversible within 5 minutes. This increase of F-actin was not seen in PTX-incubated cells (Figure 1C). Fluorescence microscopy demonstrated that in untreated cells SDF-1 induced a predominantly perinuclear accumulation of F-actin that was not observed in resting or in PTX-incubated SDF-1–treated cells (Figure 1D). These studies confirmed the sensitivity of migration of c-kit+ BM cells to PTX. The data indicated that inhibition of migration by PTX is a not a rapid process, since it is not complete after 2 hours' incubation, and also showed that incubation in SCF markedly increased cell motility in control cells, but not in PTX-treated cells, thus amplifying the difference between the migrated control and PTX-treated cell pools.
We also studied adhesion of control or PTX-treated c-kit+ BM cells (20 hours' incubation in serum-free medium plus SCF with or without PTX) to RetroNectin-coated Petri dishes under laminar flow with a constant shear force. PTX had no effect on cell adhesion to RetroNectin (Figure 1B), indicating that in keeping with some earlier studies of adhesion of hematopoietic progenitor cells,15 adhesion of hematopoietic progenitor cells does not require Gi protein signaling. Additional studies of adhesion under static conditions confirmed these findings (data not shown).
Studies of 15-dpc FL c-kit+ cells treated for 20 hours with SCF and with or without PTX, testing chemotaxis to SDF-1 and actin polymerization after SDF-1 stimulation, demonstrated essentially identical effects of PTX on fetal and adult BM c-kit+ cells (Figure 1E-F). In summary, our in vitro studies of c-kit+ BM and FL cells support and extend previous studies on the negative effect of PTX on cell motility. They do not show effects of PTX on c-kit+ BM cell adhesion under either static or flow conditions.
Ribosylation levels of PTX-treated cells
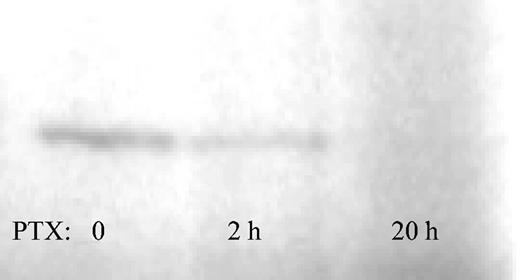
To test the availability of nonribosylated, that is, not PTX-affected, Gi protein, membranes of Jurkat cells treated with PTX for 0, 2, or 20 hours were subjected to in vitro ribosylation with the use of (P32)NAD in the presence of excess PTX. Gi proteins in membranes of untreated or 2-hour–treated Jurkat cells could be labeled with P32, whereas Gi proteins from Jurkat cells treated with PTX for 20 hours were completely ribosylated, as evidenced by the absence of a 41-kDa band (Figure 2). Thus, Gi protein ribosylation in Jurkat cells was not complete after 2 hours of PTX incubation. This confirms the data reported about the kinetics of the ribosylase activity of PTX in some of the original studies from the 1980s (61% reduction after 2 hours of PTX incubation in WBC264-9C cells).24 Labeling of Gi proteins in membranes of c-kit+ BM cells was attempted but was not successful, possibly owing to a requirement for intracellular processing of PTX, as described for several other cell lines.25
Kinetics of Gi protein ribosylation. Membrane-rich protein from Jurkat cells treated with PTX for 0, 2, or 20 hours was prepared as described. Nonribosylated Gi proteins in untreated cells incorporated P32 under the influence of an excess of PTX, as evidenced by a band migrating at 41 kDa (left). The presence of the 41-kDa band at 2 hours of PTX incubation evidences incomplete Gi protein ribosylation at this time point (middle). Gi protein ribosylation was complete at 20 hours (right).
Kinetics of Gi protein ribosylation. Membrane-rich protein from Jurkat cells treated with PTX for 0, 2, or 20 hours was prepared as described. Nonribosylated Gi proteins in untreated cells incorporated P32 under the influence of an excess of PTX, as evidenced by a band migrating at 41 kDa (left). The presence of the 41-kDa band at 2 hours of PTX incubation evidences incomplete Gi protein ribosylation at this time point (middle). Gi protein ribosylation was complete at 20 hours (right).
Effects of PTX on BM homing and short-term engraftment
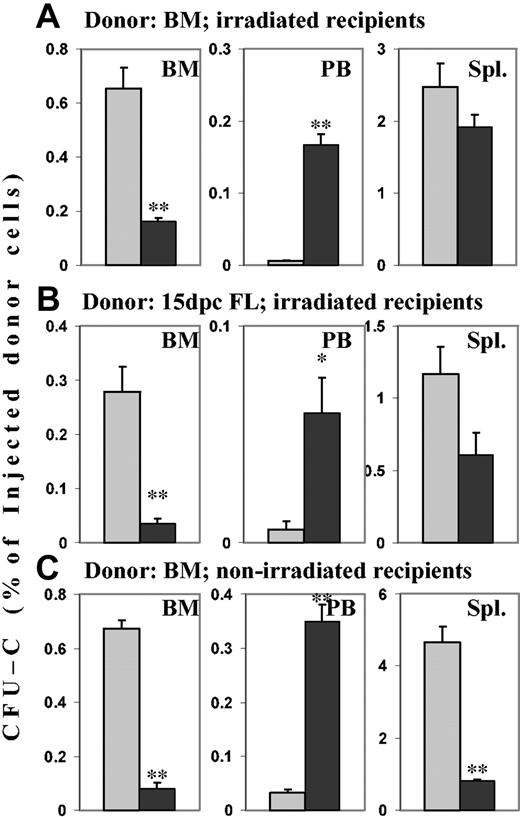
To test the consequences of the in vitro findings on BM homing, cells incubated for 2 time durations in SCF with or without PTX were used. When 20-hour–incubated control cells (SCF only) were transplanted into lethally irradiated hosts, 24 hours later 0.65% ± 0.077% of the injected CFU-Cs could be recovered per femur, and almost no CFU-Cs (fewer than 1 in 40 000 injected CFU-Cs per millililter blood) were circulating in blood. In contrast, in recipients of cells treated for 20 hours with SCF plus PTX, 0.16% ± 0.015% of injected CFU-Cs could be recovered per femur, a reduction of greater than 75% (P < .005 compared with controls). At the same time, significant numbers of CFU-Cs—the equivalent of the content of one femur per milliliter of blood—remained in circulation (P < .005 compared with controls). In irradiated hosts, splenic distribution of CFU-Cs was not affected by incubation with PTX (Figure 3A). Because previous studies had incubated BM with PTX for only 2 hours, we also tested homing after this shorter time. A smaller decrease of BM homing (25%) was observed than after 20-hour incubation with PTX, although the number of circulating CFU-Cs was markedly increased. Both changes reached statistical significance in comparison with controls (P < .005; data not shown). In keeping with earlier studies,26 whether BM cells were incubated in SCF for 2 hours or 20 hours did not affect CFU-C frequency, nor was the fraction of CFU-C homing to irradiated BM different between 2-hour and 20-hour incubated BM cells (data not shown). Since all our previous studies had treating donor cells in serum-free media, whereas a previous study had used serum, we also transplanted BM cells incubated in serum with or without PTX for 2 hours or 20 hours, to rule out an effect of serum on the PTX-induced inhibition of homing. An inhibition of BM homing and a concomitant increase in circulating donor cells were observed in the recipients of PTX-treated cells at both time points, similar to those in cells incubated in serum-free media with SCF. The data ruled out a protective effect of serum (data not shown). Additional homing studies were performed with nonirradiated recipients to assess the relevance of irradiation-induced changes in the host, such as up-regulated chemokines, adhesion molecules, and cytokines, and damage to endothelial layers. For this purpose, neomycin-resistant BM cells were injected into wild-type recipients. Essentially identical changes as in irradiated hosts were observed in BM (greater than 85% decreased homing of PTX-treated CFU-Cs; P < .005 compared with controls) and in blood (greater than 10-fold increase of PTX-treated CFU-Cs; P < .005 compared with controls). In spleens, however, an almost 6-fold reduction of homed PTX-treated CFU-Cs was found, suggesting that irradiation up-regulated a PTX-insensitive cell-retention mechanism in the spleen (P < .005 compared with controls) (Figure 3C). There was no quantitative difference in BM homing of control BM in irradiated and nonirradiated recipients. Homing studies using GFP+ donor cells in GFP– recipients confirmed these findings (data not shown).
Effects of PTX incubation of hematopoietic progenitor cells on homing. Lethally irradiated hosts received transplants of BM or FL cells, and nonirradiated hosts received transplants of BM cells from neo-resistant donors. Transplants incubated in SCF for 20 hours in the absence or presence of PTX were injected into the hosts. After 24 hours, recovery of CFU-Cs (neo-resistant CFU-Cs in the nonirradiated hosts) from BM, peripheral blood, and spleen was assessed in colony assays. Numbers are given as the percentage of injected CFU-Cs per femur (mean ± SEM), per milliliter blood, and per spleen. ▦ indicates control cells; and ▪, PTX-treated cells. **P < .005. *P < .01 compared with control. (A) Homing of PTX-treated adult BM CFU-Cs to BM was reduced more than 75%. Concomitantly, circulating CFU-Cs were several-fold increased in recipients of PTX-treated cells. (B) BM homing of PTX-treated day-15 FL CFU-Cs also was reduced by more than 75%, with a similar increase of circulating CFU-Cs. (C) In nonirradiated hosts, BM homing of PTX-treated BM CFU-Cs was similarly reduced as in irradiated hosts, and circulating CFU-Cs were similarly increased. In contrast to the irradiated spleens, nonirradiated spleens also showed greatly attenuated homing (> 75% decrease) of PTX-treated donor CFU-Cs from adult BM.
Effects of PTX incubation of hematopoietic progenitor cells on homing. Lethally irradiated hosts received transplants of BM or FL cells, and nonirradiated hosts received transplants of BM cells from neo-resistant donors. Transplants incubated in SCF for 20 hours in the absence or presence of PTX were injected into the hosts. After 24 hours, recovery of CFU-Cs (neo-resistant CFU-Cs in the nonirradiated hosts) from BM, peripheral blood, and spleen was assessed in colony assays. Numbers are given as the percentage of injected CFU-Cs per femur (mean ± SEM), per milliliter blood, and per spleen. ▦ indicates control cells; and ▪, PTX-treated cells. **P < .005. *P < .01 compared with control. (A) Homing of PTX-treated adult BM CFU-Cs to BM was reduced more than 75%. Concomitantly, circulating CFU-Cs were several-fold increased in recipients of PTX-treated cells. (B) BM homing of PTX-treated day-15 FL CFU-Cs also was reduced by more than 75%, with a similar increase of circulating CFU-Cs. (C) In nonirradiated hosts, BM homing of PTX-treated BM CFU-Cs was similarly reduced as in irradiated hosts, and circulating CFU-Cs were similarly increased. In contrast to the irradiated spleens, nonirradiated spleens also showed greatly attenuated homing (> 75% decrease) of PTX-treated donor CFU-Cs from adult BM.
FL cells may home quantitatively less well than adult CFU-Cs.27 To test, therefore, whether homing requirements differed in PTX-treated and untreated cells of adult versus fetal origin, we tested 24-hour homing of 15-dpc FL cells. First, 20 × 106 cells incubated in SCF with or without PTX for 20 hours were injected into lethally irradiated recipients, and homing was assessed after 24 hours. The picture was very similar to homing of adult cells, in that BM homing of PTX-treated CFU-Cs was reduced more than 80% (P < .005 compared with controls), and circulating CFU-Cs were almost 10-fold increased (P < .01 compared with controls). In contrast to adult BM cells, splenic homing of PTX-treated FL cells was reduced by almost 50% compared with controls (P < .05) (Figure 3B). Thus, FL cell homing to BM was Gi protein dependent, similar to BM homing of adult BM cells.
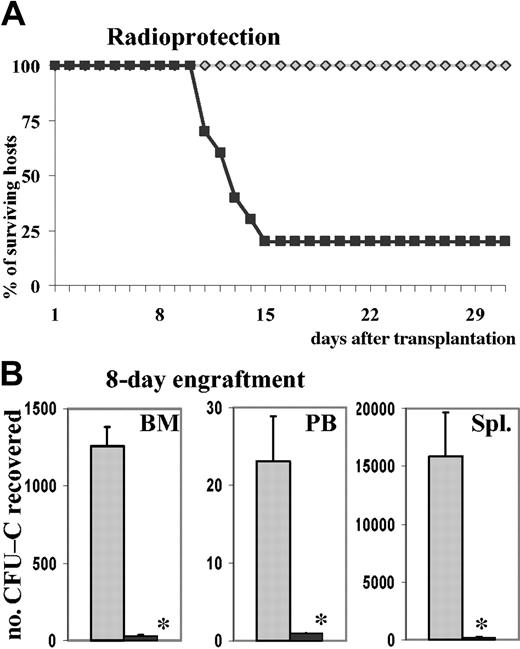
To test whether the decreased homing of PTX-treated cells was of functional consequence in posttransplantation reconstitution of hematopoiesis, we assessed radioprotection of mice receiving transplants of untreated versus PTX-treated cells. First, 2.5 × 105 total BM cells treated with or without PTX, representing 783 ± 58 or 740 ± 37 CFU-Cs, respectively, were injected into lethally irradiated recipients (2.5 × 105 BM cells were estimated from previous experiments to be approximately 5 times the normal median lethal dose [LD50] required for radioprotection). Ten of 10 recipients of normal cells survived until day 30, whereas only 2 of 10 recipients of PTX-treated cells were radioprotected (Figure 4A). The 8 deaths in the PTX group occurred between days 11 and 15, which suggested that they were likely to have been due to graft failure secondary to the impaired BM homing of PTX-treated cells. On day 30, 5 of the recipients of normal cells and the 2 surviving recipients of PTX-treated cells were killed, and the CFU-C content of peripheral blood, BM, and spleen were assessed: the CFU-C content of femurs and blood of the control recipients indicated complete engraftment, even though the spleens were still large and their CFU-C content was elevated. The surviving recipients of PTX-treated cells, however, had markedly reduced CFU-C content in peripheral blood, femurs, and spleens (less than 50%, less than 65%, and less than 65%, respectively; not shown) compared with recipients of untreated BM cells. To consolidate these findings, we made additional observations in a subsequent group of lethally irradiated recipients given 1.5 × 106 BM cells, treated for 20 hours with SCF and with or without PTX (representing 5940 ± 127 control CFU-Cs and 5880 ± 119 PTX-treated CFU-Cs), which were killed 8 days after transplantation. In recipients of PTX-treated cells, CFU-C numbers were dramatically reduced in BM and spleens (P < .005 compared with controls), and no circulating CFU-Cs were found in peripheral blood (P < .005 compared with controls) (Figure 4B). Similarly, nucleated cell counts were significantly reduced in all 3 tissues (data not shown). Since it has been noted that PTX does not affect proliferation and differentiation of hematopoietic progenitor cells, this markedly decreased short-term engraftment of mice receiving transplants of PTX-treated BM was probably the consequence of decreased homing. The marked decrease of splenic short-term engraftment compared with a virtually normal homing (Figure 3A) invited additional conclusions. It seemed that irradiated spleen efficiently retained cells by a PTX-insensitive mechanism but that recruitment of CFU-Cs to proliferative niches was inhibited.
Effect of PTX on engraftment. PTX attenuates short-term engraftment. (A) Lethally irradiated hosts were injected with 250 000 BM cells, incubated for 20 hours with SCF in the absence or presence of PTX. This cell dose, equivalent to 740 ± 37 control CFU-Cs and 783 ± 58 PTX-treated CFU-Cs, was sufficient to radioprotect 10 of 10 lethally irradiated recipients of control cells, whereas 8 of 10 of the recipients of PTX-treated cells died between days 11 and 15 (recipients of control cells, gray diamonds; recipients of PTX-treated cells, black squares). (B) Lethally irradiated hosts received 1.5 × 106 BM cells, equivalent to 5940 ± 127 control CFU-Cs and 5880 ± 119 PTX-treated CFU-Cs, incubated for 20 hours with SCF in the absence or presence of PTX. At 8 days after transplantation, the recipients were killed. CFU-Cs were enumerated in BM, peripheral blood, and spleen and depicted as CFU-Cs per tissue (mean ± SEM). CFU-C contents were dramatically reduced in all 3 tissues of recipients of PTX-treated cells (control cells, ▦; PTX-treated cells, ▪). *P < .005 compared with control.
Effect of PTX on engraftment. PTX attenuates short-term engraftment. (A) Lethally irradiated hosts were injected with 250 000 BM cells, incubated for 20 hours with SCF in the absence or presence of PTX. This cell dose, equivalent to 740 ± 37 control CFU-Cs and 783 ± 58 PTX-treated CFU-Cs, was sufficient to radioprotect 10 of 10 lethally irradiated recipients of control cells, whereas 8 of 10 of the recipients of PTX-treated cells died between days 11 and 15 (recipients of control cells, gray diamonds; recipients of PTX-treated cells, black squares). (B) Lethally irradiated hosts received 1.5 × 106 BM cells, equivalent to 5940 ± 127 control CFU-Cs and 5880 ± 119 PTX-treated CFU-Cs, incubated for 20 hours with SCF in the absence or presence of PTX. At 8 days after transplantation, the recipients were killed. CFU-Cs were enumerated in BM, peripheral blood, and spleen and depicted as CFU-Cs per tissue (mean ± SEM). CFU-C contents were dramatically reduced in all 3 tissues of recipients of PTX-treated cells (control cells, ▦; PTX-treated cells, ▪). *P < .005 compared with control.
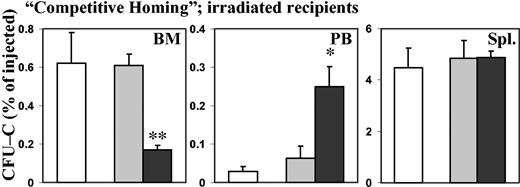
Previous experiments as well as our own experience have shown that with the injection of PTX-treated cells, functional amounts of PTX are introduced into the recipients.28 Thus, we observed that peripheral blood leukocyte counts were increased in nonirradiated recipients after injection of PTX-treated cells, to a degree that could not be explained by the number of donor cells alone. While recipients of normal control cells had 10 000 white blood cells (WBCs) per microliter (10 × 109/L), with 170 donor cells per microliter, recipients of PTX-treated cells had 35 000 WBCs per microliter (35 × 109/L) with 2600 donor cells per microliter (P < .005; data not shown). Therefore, to test whether the effect of PTX on homing was mediated through cell-intrinsic or host-derived mechanisms, different approaches were taken. PTX-treated cells were coinjected with untreated cells, or recipients were treated with PTX. In “competitive homing” assays, lethally irradiated hosts were injected with a 1:1 mix of control (SCF for 20 hours) and PTX-treated (SCF plus PTX for 20 hours) BM cells (see “Materials and methods”). If purely cell-intrinsic mechanisms were responsible for reduced BM homing of PTX-treated cells, untreated companion cells would home normally. Alternatively, any significant effects on cell homing of untreated companion cells should be considered to be indirect. These could be mediated either by effects of PTX on coinjected companion cells or on the host environment. A group of mice injected only with control cells (SCF only) served as additional controls, for quantitative comparison of the homing of untreated cells in the presence and absence of PTX-treated cells. In these studies, BM homing of PTX-treated CFU-Cs was decreased to a similar degree (less than one third of control; P < .01) as in the previous experiments, in which only PTX-treated cells had been used as donor cells. Likewise, the number of PTX-treated CFU-Cs was increased several-fold in peripheral blood in comparison with control cells. At the same time, homing of control cells coinjected with PTX-treated cells was similar to that of control cells injected alone, except for a mild increase of circulating CFU-Cs in the coinjected control cells, which did not reach statistical significance. These data suggested a hematopoietic cell-intrinsic activity of PTX on the BM homing of hematopoietic progenitor cells (Figure 5). In additional experiments, we treated lethally irradiated recipients with PTX either before (24 hours prior to irradiation) or after transplantation (4 hours after injection of normal BM cells), and assessed homing after 24 hours. Unlike PTX incubation of BM cells in vitro, PTX injection of recipients, whether before or after transplantation, did not affect homing of normal BM cells to spleen or BM (data not shown), lending additional credence to the cell-intrinsic nature of the PTX effect.
“Competitive homing” of PTX-treated and control BM CFU-Cs. Lethally irradiated recipients were injected with a 1:1 mix of control (SCF only) and PTX-treated (SCF + PTX) BM cells. As an additional control, some recipients were injected with SCF-treated cells alone. After 24 hours, homing of control CFU-Cs injected alone, of control CFU-Cs coinjected with companion PTX-treated cells, and of PTX-treated CFU-Cs coinjected with companion control cells to BM, peripheral blood, and spleen was assessed by colony assays. Error bars indicate SEM. As in the previous experiments, even when PTX-treated CFU-Cs were coinjected with companion control BM cells, their homing to BM was significantly reduced, with a concomitant increase in circulating CFU-Cs, in comparison with control CFU-Cs coinjected with PTX-treated cells or injected alone. Splenic homing was not affected. At the same time, the companion PTX-treated cells had no effect on the homing of coinjected control cells, since there was no difference in homing between control cells injected alone and control cells coinjected with companion PTX-treated cells, evidencing the hematopoietic cell-intrinsic nature of the PTX effect (control cells injected alone, □; control cells injected with companion PTX-treated cells, ▦; PTX-treated cells injected with companion control cells, ▪). *P < .05. **P < .01.
“Competitive homing” of PTX-treated and control BM CFU-Cs. Lethally irradiated recipients were injected with a 1:1 mix of control (SCF only) and PTX-treated (SCF + PTX) BM cells. As an additional control, some recipients were injected with SCF-treated cells alone. After 24 hours, homing of control CFU-Cs injected alone, of control CFU-Cs coinjected with companion PTX-treated cells, and of PTX-treated CFU-Cs coinjected with companion control cells to BM, peripheral blood, and spleen was assessed by colony assays. Error bars indicate SEM. As in the previous experiments, even when PTX-treated CFU-Cs were coinjected with companion control BM cells, their homing to BM was significantly reduced, with a concomitant increase in circulating CFU-Cs, in comparison with control CFU-Cs coinjected with PTX-treated cells or injected alone. Splenic homing was not affected. At the same time, the companion PTX-treated cells had no effect on the homing of coinjected control cells, since there was no difference in homing between control cells injected alone and control cells coinjected with companion PTX-treated cells, evidencing the hematopoietic cell-intrinsic nature of the PTX effect (control cells injected alone, □; control cells injected with companion PTX-treated cells, ▦; PTX-treated cells injected with companion control cells, ▪). *P < .05. **P < .01.
Discussion
Several prior publications have indicated that both short-term engraftment14 and BM homing12,15 of hematopoietic cells treated ex vivo with PTX were unimpaired. Despite these findings, in vitro testing of PTX-treated BM cells showed virtually complete inhibition of their migration. Thus, considering the available in vitro and in vivo data together, one could surmise that the in vitro migratory capacity of donor cells does not predict their BM homing behavior in vivo. This concept, however, is in contrast to the currently accepted model of extravasation of mature lymphocytes from the circulation to inflammatory tissues, since inhibition of Gi protein signaling inhibited their in vitro migration and severely crippled their ability to leave the circulation in vivo.29 In addition to some increased or reversible adhesion, which requires integrin clustering, chemotactic responses through serpentine receptors are deemed necessary for migration of mature cells to inflammatory tissues.2 Thus, the molecular underpinning of hematopoietic progenitor cell migration to BM appeared to be in contrast to that of more mature cells. It is these conflicting concepts that stimulated us to carefully re-examine the necessity of Gi protein signaling for the homing and short-term engraftment of hematopoietic cells to BM. In contrast to previous findings, we found that PTX-sensitive signals are needed for BM homing. Importantly, as the total recovery of cells was increased in circulation, this implied absence of a nonspecific toxicity of PTX on the treated cells as the cause of the reduced lodgment to BM or other tissues. But why are our data different from those previously published? Several considerations are offered to explain these differences: First, there are differences in the conditions used for ex vivo treatment with PTX between our experiments and those previously published. Previous studies used cells treated with PTX for only 1 or 2 hours,12,14,15 an incubation time established as sufficient to block migration of lymphocytes. However, detailed studies have shown that the kinetics of Gi protein blockade by PTX are largely cell-type dependent,24,25,30-33 and systematic analyses of bone marrow hematopoietic progenitor cells have not been published. Following 2-hour incubation with PTX, migration is incompletely blocked in c-kit+ BM cells, and only 25% reduction of homing was found under these conditions. In contrast, the effect on homing after 20 hours' incubation (inhibition by at least 75%) was much greater, suggesting that the PTX effect at 2 hours was incomplete. Additionally, as we have seen, 20-hour incubation with SCF significantly increased motility only in control cells, not in PTX-incubated cells, which may have exaggerated the difference between our results and those of previous studies. Incomplete PTX effect after 2 hours and less overall migratory capacity of 2-hour–incubated control cells compared with 20-hour–incubated control cells might be jointly responsible for the relatively small effect of PTX on homing at the early time point. It is also important to emphasize that concurrent enumeration of circulating progenitors in blood, or studies in other tissues, have not been carried out previously. As we have shown, the number of circulating CFU-Cs was several-fold increased in recipients of cells incubated with PTX for 2 hours. Therefore, it is possible that our results differ from those published previously because of different conditions and the requirement for complete ribosylation of Gi proteins.
Additional considerations for our findings pertain to whether the PTX effects are strictly cell intrinsic or are dictated by events outside the cells, that is, the BM environment of the recipient. In other words, the concern is raised that the observed effects might be the result of PTX influence on the host. This is not a trivial issue, since PTX has been shown, for example, to render brain endothelial cells impenetrable to lymphocyte migration.34 An influence of the PTX-treated cells on the host is a distinct possibility, because of a leakage of PTX from the treated cells, as was observed by others28 and by us. However, as we show by several different approaches, there is no evidence for strong host effects of PTX, but clear evidence in favor of a cell-intrinsic action. When PTX-treated and untreated control cells were coinjected in irradiated hosts, the PTX-treated cells showed the expected reduction in homing, whereas the control cells coinjected with PTX-treated cells homed as efficiently as concurrent control cells injected alone. Moreover, homing was not affected when recipients of untreated cells were injected with PTX before or shortly after transplantation of normal cells. Thus, we believe that although alteration, direct or indirect, of the host environment due to PTX treatment should be considered, the weight of the evidence suggests that the effects we have seen are largely cell intrinsic, rather than influenced by the environment. Nevertheless, to definitively assess hematopoietic cell-intrinsic and cell-extrinsic effects of PTX, it will be useful to express the catalytic subunit of PTX in hematopoietic cells, in order to study the cells' homing behavior, and these experiments are in progress.
In addition to differences in bone marrow homing of ex vivo PTX-treated BM cells, we also observed novel differences in splenic homing. In contrast to the markedly reduced BM homing of PTX-treated BM cells, splenic homing of PTX-treated BM cells in irradiated recipients was essentially the same as for control cells. This was not the case in nonirradiated spleens, where retention was only one sixth control BM cells. This would indicate that following irradiation there are some changes in the splenic microenvironment. These include especially the up-regulation of VCAM-1, which was previously documented by Mazo et al.35 We have also documented these differences in our experiments (data not shown). However, even though the number of cells retained in irradiated spleen does not differ in control and PTX-treated cells, the PTX-treated cells had significantly reduced short-term engraftment in both the marrow and the spleen and failed to radioprotect lethally irradiated recipients, an activity largely dependent on spleen. This may imply that radiation induced up-regulation of at least transient retention mechanisms in spleen, but these cells, if they remain in the spleen, were unable to proliferate or to migrate to proliferative niches, similar in principle to what Cyster and Goodnow36 have demonstrated for the splenic distribution of PTX-treated lymphocytes. Decreased radioprotection and splenic hematopoiesis is also consistent with data of Wiesmann and Spangrude.14
It has been suggested that the 2-dimensional (2-D) transwell migration assays do not accurately model the complex events of migration into BM in vivo.37 While this may be true, our data and data from several other groups nevertheless support the emerging theme that the migratory behavior of hematopoietic cells in vitro predicts their successful homing in vivo. Thus, impaired migration and impaired homing were found in experiments using anti-CXCR4 antibody treatment,3 anti–VLA-4 treatment,4 protein kinase C inhibition,12 or inhibition of rho-GTPase and blockade of calcium flux using thapsigargin.18 Alternatively, increased migration and improved homing were achieved by incubation of BM with C3a16 or with a sphingosine 1 phosphate receptor agonist.17 The relevance of this concept to “normal” transplantation is suggested by the fact that for human autologous transplant recipients a linear relationship between in vitro migration to SDF-1 and bone marrow homing was observed.19
The concept put forth in this manuscript is of significance for the general understanding of BM homing. A number of serpentine receptors, which are associated with Gi proteins for intracellular signal transduction, have been implicated in hematopoietic progenitor cell homing. They include, but may not be limited to, chemokines, complement, sphingolipids, and wingless-related (wnt) proteins.8,16,17,38-41 BM homing and short-term engraftment are inhibited by PTX to a greater extent than has ever been achieved by inhibition of any single pathway, suggesting that several of these mediators cooperatively provide homing signals that converge in their use of Gi protein–mediated signals. The data presented here thus suggest that Gi protein signals might serve as a converging pathway for modulation of BM homing of hematopoietic progenitor cells by several agents. Of note, the results detailed in this manuscript were obtained with the use of c-kit+ or progenitor (CFU-C) cells, not long-term repopulating stem cells. Studies using hematopoietic cells transduced with an S1 expression vector are underway and are expected to clarify the dependence on Gi protein signals for stem cell homing to BM.
Prepublished online as Blood First Edition Paper, June 24, 2004; DOI 10.1182/blood-2004-04-1605.
Supported by a scholarship of the Deutsche Krebshilfe eV (Bonn, Germany), an American Society of Hematology (ASH) Fellow Scholar Award, and National Institutes of Health (NIH) grant DK56465 to the Fred Hutchinson Cancer Research Center (FHCRC) (H.B.); and by NIH grants HL46557 and HL58734 (T.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to Jan Abendroth, PhD, for his help with the membrane preparation, and to Paul Hendrie, MD, PhD, for advice on the ribosylation protocol. We acknowledge Greg Martin, Keck Imaging Center at the University of Washington, for expertly running the DeltaVision microscopy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal