Abstract
To assess the variation of thrombopoietin (TPO) responsiveness associated with megakaryocyte (MK) progenitor amplification, TPO dose-response curves were obtained for normal human, single-cell plated CD34+CD41+ cells. The number of MKs per well was determined in situ and expressed as number of doublings (NbD). Dose-response curves of the mean frequency of clones of each size versus log TPO concentration showed highly significant differences in the TPO concentration needed for half-maximum generation of clones of different sizes (TPO50): 1.89 ± 0.51 pg/mL for 1 MK clones; 7.75 ± 0.81 pg/mL for 2 to 3 MK clones; 38.5 ± 5.04 pg/mL for 4 to 7 MK clones, and 91.8 ± 16.0 pg/mL for 8 to 15 MK clones. These results were consistent with a prediction of the generation-age model, because the number of previous doublings in vivo was inversely correlated with the number of residual doublings in vitro. TPO responsiveness decreased in vitro by a factor of 3.5 per doubling, reflecting the recruitment of progressively more ancestral progenitors. In support of this hypothesis, the more mature CD34+CD41+CD42+ cell fraction had a lower TPO50 (P < .001), underwent fewer NbD (P < .001), and expressed a 2.8-fold greater median Mpl receptor density (P < .001) than the CD34+CD41+CD42– fraction. Progenitors that have completed their proliferative program have maximum factor responsiveness and are preferentially induced to terminal differentiation.
Introduction
Studies of megakaryocyte colonies have provided a simple, robust model of the genesis of clonal size heterogeneity in hemopoietic colonies. Dividing megakaryocyte colony-forming units (CFU-MKs) cultured in vitro leave the proliferative pool at a constant rate per doubling, so that the number of doublings (NbD) undergone by these progenitors are exponential distributions.1,2 The rate of exit from the proliferative compartment is decreased by stimulators of megakaryocyte colony formation and is lower in mixed erythroid-megakaryocyte than in pure megakaryocyte colonies.2
It was first thought that the exponential functions were generated as a result of a single random event arresting proliferation and occurring somewhat synchronously within individual clones. Among several, nonmutually exclusive possibilities, the critical event may be geared to the assembling or disassembling of a protein-protein complex binding to a regulatory DNA element,3 the thrombopoietin (TPO)–induced change in a critical component of the cell cycle, resulting in the abortive mitoses that induce polyploidy4-6 and/or the all-or-none, switchlike responses of the mitogen-activated protein kinase (MAPK) cascade7,8 that has been associated with induction of polyploidization in megakaryocyte cell lines9-11 and normal megakaryocytes.10,11 However, the random occurrence of the critical event and the validity of a stochastic model based on this premise are refuted by several data. In fact, data demonstrate that CFU-MKs determine in part the amplification of their progeny and, therefore, impart a degree of homogeneity to this progeny.12 In the granulocyte-macrophage series, the squared correlation coefficient of approximately 0.50 in NbD achieved by each of paired granulocyte-macrophage colony-forming unit (CFU-GM) daughter cells, which can be calculated from the data of Metcalf,13 indicates that one half of the total variance in NbD undergone by CFU-GM is related to preexisting, heritable characteristics of the progenitor cells. Thus, evidence of synchronization in whole clones implies that the commitment event is not random among the progeny of the colony-forming cell.2 A second major difficulty with a model based on stochastic commitment to polyploidization is the existence within the proliferative continuum of intrinsic differences which are correlated with proliferative potential.2,14 These intrinsic characteristics contradict the view that interclonal heterogeneity is generated by a homogeneous population of progenitors undergoing random cessation of proliferation. Therefore, it appears that clonogenic cells are themselves heterogeneous in parameters that predict, or even determine, their proliferative behavior. How this heterogeneity is created is not precisely understood. Among individual progenitors of the granulocytic,15 erythrocytic,16 and megakaryocytic14,17,18 series, intrinsic cytokine sensitivity varies inversely to proliferative potential. In megakaryocyte cultures, the progenitors of single megakaryocytes, of doublets, and of CFU-MKs that generate colonies with 3 or more megakaryocytes display progressively decreasing responsiveness to thrombopoietin, respectively.18 Generation-age may, therefore, be a determinant of progenitor heterogeneity. However, intrinsic heterogeneity of colony progenitors does not necessarily reflect an age structure or differences in mitotic history because large variations in proliferative potential have been demonstrated between paired daughter progenitor cells born from hemopoietic stem cells.19-22 In addition, there is evidence that some interlineage plasticity may persist during differentiation,23,24 and induction of a program of megakaryocytic differentiation associated with transcription of the TPO receptor and acquisition of TPO responsiveness has been described.25 Factors affecting progenitor sensitivity to growth regulators independently from generation-age may therefore be important. However, the precise relationship between age-dependent and age-independent variations in factor responsiveness remains unknown. A basic question is whether the number of doublings undergone by megakaryocyte progenitors in vitro depends on the NbD that previously occurred in vivo or whether they are set by some intrinsic properties, such as cytokine responsiveness, which would be totally or partially independent of progenitor past mitotic history.
In this study, we determined the TPO dose-response curves of human CD34+CD41+ megakaryocyte progenitors that generated clones of various sizes. We found that the range of responsiveness of individual progenitors covered 4 orders of magnitude and that TPO responsiveness was inversely correlated to clone size, expressed as number of doublings. To interpret these data, we tested a prediction of the generation-age model, namely that under constant culture conditions, the percentage of progenitors undergoing a given number of residual doublings (NbDr) in vitro is determined by the number of previous doublings (NbDp) they had undergone in vivo. The data established that, in the serum-deprived culture system used, 78% of the variation in TPO responsiveness of megakaryocyte progenitors could be interpreted as the effect of a 3.5-fold increase in responsiveness per previous progenitor doubling in vivo.
Materials and methods
Cell cultures
Bone marrow was obtained from healthy adult donors, using femoral bone fragments discarded during hip surgery. CD34+CD41+ cells (average, 2% of the CD34+ cells) were sorted as single cells into 96-well Terasaki plates containing 20 μL Iscove modified Dulbecco medium (Invitrogen, Cergy-Pontoise, France) that contained penicillin (250 U/mL), streptomycin (250 μg/mL), glutamine (2 mM; Invitrogen), 76 μM alphamonothioglycerol (Sigma, St Louis, MO), 1.5% deionized bovine serum albumin (BSA; Cohn fraction V; Sigma), 10 μL insulin-transferrin-selenium (Invitrogen), and a mixture of sonicated lipids (20 μL/mL) prepared as previously reported.26 The serum-deprived medium was then supplemented with various concentrations of a truncated form of recombinant human TPO, kindly provided by Kirin Brewery, Tokyo, Japan. Four plates were used for each of 8 to 11 TPO concentrations, ranging from 0 to 103 pg/mL. Cells were cultured for 5 days at 37° C in a fully humidified atmosphere containing 5% CO2 in air, fixed, and then counted in situ at × 100 magnification by a single microscopist. Previous studies have shown that only CD41+ cells develop under these conditions.27,28
For each experiment, at least 8 TPO concentrations were used and 4 96-well Terasaki plates were used for each concentration of TPO for a total of at least 32 plates (total = 3072 wells per experiment).
Fractionation of CD34+CD41+ progenitors
In 3 additional experiments, CD34+CD41+CD42a+ and CD34+CD41+CD42a– progenitors were separated by cell sorting, using anti-CD34 conjugated with phycoerythrin-cyanin 5 (PC5; Beckman Coulter, Villepinte, France), anti-CD41–allophycocyanin (APC), and anti-CD42a–fluorescein isothiocyanate (FITC; Becton Dickinson, Le Pont de Claix, France). Cell sorting was done as described previously.27 In one further experiment, Mpl expression on CD42a+ and CD42a– progenitors was compared. Mononucleated cells were treated with anti-Mpl monoclonal antibody (Becton Dickinson). Following treatment with anti–mouse immunoglobulin G (IgG)–phycoerythrin (PE), cells were incubated with excess mouse serum and further labeled with conjugated anti-CD34, anti-CD41, and anti-CD42a as above.
Regression and statistical analyses
Dose-response curves are hyperbola when response is plotted versus dose on a linear scale and S-shaped (sigmoid) curves with an inflection point (“transition”) when plotted on a log scale. Concentrations of TPO ranged from 0.1 to 1000 pg/mL; in addition, data obtained from culture wells to which no TPO had been added were plotted at the concentration of 0.01 pg/mL. Use of the log scale in all graphs and of log TPO concentrations in all calculations was justified by the 104-fold range in TPO concentrations required and because the distributions of log TPO thresholds for megakaryocyte progenitor stimulation obeyed the gaussian law or the very similar sigmoid model (see “Characterization of clone size classes”). Quadruplicate counts of megakaryocyte number per clone were determined for each TPO dose. The relative weight of each replicate was calculated as the inverse of its variance, so that the mean counts with the highest precision were given the greatest weight. The weighted means versus log TPO concentration were then fitted to the 29 built-in transition regression equations offered by Tablecurve 2Dv5 software (SPSS, Chicago, IL). Both the cumulative log dose-response curves and their analytical derivatives, the bell-shaped frequency distributions of TPO thresholds, were obtained. These analyses yielded the TPO50, defined as the TPO concentration corresponding to the log TPO concentration at half-plateau number of clones. They also provided a measure of progenitor responsiveness, defined as the inverse of TPO50 (1/TPO50).
Characterization of clone size classes
As described previously,2 the term “megakaryocyte clone” was used as a generic name for all megakaryocyte entities growing from single sorted cells. Such entities included colonies, generally defined as clones with 3 or more megakaryocytes, as well as single megakaryocyte clones or 2 megakaryocyte clones. Clone size was expressed as number of doublings (NbD), which were determined as log2 of megakaryocyte count per well (ie, log10 count × 3.3219). Log2 was chosen because it provides a suitable basis for expressing the number of doublings, although a log of any base could have been used and would have generated the same results. The overall curve was resolved into class-size dependent curves by fitting dose-response regression equations to the frequencies of clones with 1 (0 doubling; db), 2 to 3 (1-1.58 db), 4 to 7 (2-2.8 db), or 8 to 15 (3-3.9 db) megakaryocytes.
In some experiments, the aim was to determine the previous number of doublings, NbDp undergone in vivo by progenitors that undergo in vitro the residual number of doublings NbDr. For determination of NbDr, megakaryocyte counts per well were used. For determination of NbDp, a generationage structure was assumed, in which the progeny of the earliest TPO-responsive MK progenitor undergo a maturation process involving a nearly constant total NbD before mitotic arrest. For the size class NbDr, the corresponding NbDp was obtained from the plateau number of clones N yielded by the dose-response curve. N is proportional to 2NbDp (for example, if NbDp is 3, then N is proportional to 23 = 8) so that N = 2NbDp k, where k, a proportional factor accounting for sample size may be omitted if the NbDp of the earliest TPO-responsive MK progenitor is set at zero doubling. Then, log2N = NbDp and provides an estimate of the number of previous doublings elapsed since the earliest progenitor.
Results
Overall TPO dose-response curves
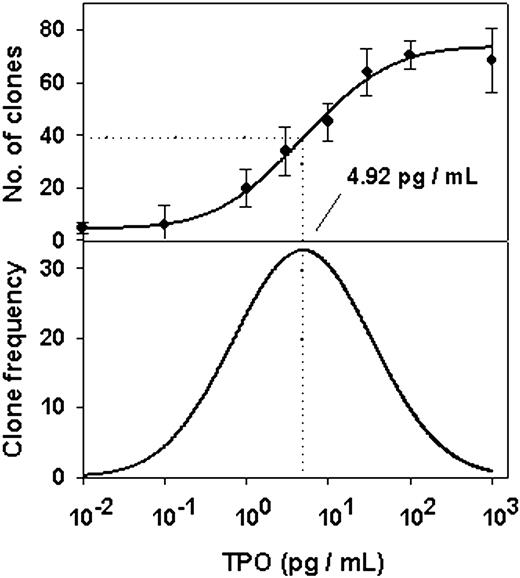
A log dose-response curve for all clones (≥ 1 megakaryocyte) is shown in Figure 1, top panel. In 3 independent experiments, the mean plateau cloning efficiency (percentage of wells containing at least 1 megakaryocyte) was 53.5% ± 19.9%. In each experiment, the gaussian cumulative model provided the best fit to data points when compared to 29 transition regression equations (r2 > 0.99; P < .001 in all cases). The inflection point, that is, the TPO concentration (TPO50) corresponding to the log TPO at half-plateau number of total clones, was 5.91 ± 0.72 pg/mL (mean ± 1 SEM; n = 3). The 95% confidence intervals of the estimates are given in Table 1. Also shown in Figure 1 (bottom panel) is the derivative curve, that is, the bell-shaped frequency distribution of TPO thresholds, the concentrations required to recruit individual progenitors. Note that the peak of this curve corresponds to the median TPO threshold for all clones, which is also the TPO50. The fact that this curve fit a gaussian model indicated that the distribution of TPO thresholds was log normal. Because counting showed that single megakaryocytes were present in some wells to which no TPO had been added, curves with an intercept parameter were preferred for both the gaussian and sigmoid models. The intercept on the ordinate of the dose-response curves for 1 MK and 1 to 15 MKs indicated that 7.62% ± 0.57% (mean ± 1 SEM; n = 6) of CD34+CD41+ cells plated were not dependent on TPO for survival in the conditions used.
Cumulative and derivative dose-response curves for the overall sample of clones derived from CD34+CD41+ progenitors. The cumulative dose-response curve is shown in the top panel, and derivative dose-response curve is shown in the bottom panel. Sorted progenitors were individually cultured for 5 days in serum-deprived medium with the indicated concentrations of TPO. Clones contained 1 to 16 megakaryocytes, corresponding to 0 to 4 progenitor doublings. Data points are the mean ± 1 SEM of quadruplicate determinations of the number of clones at day 5 per 96-well Terasaki plate. A gaussian cumulative equation gave the best fit to data among 29 functions based on transitional models (r2 = 0.99; P < .001). The bell-shaped bottom curve, the gaussian analytical derivative of the top curve, is the frequency distribution of TPO thresholds of individual progenitors. Counts obtained for wells with no TPO were assigned a concentration of 10–2 pg/mL to preclude entering the log of zero values in regressions. In this experiment, the mean absolute number of clones per 96-well plate which survived in absence of TPO was 4.44 ± 0.82 (ie, 6.05% of the total clones). The dotted vertical drop lines indicate TPO50 (ie, the TPO dose at which 50% of the plateau number of clones were counted). Results are from experiment number 1 and are representative of 3 experiments.
Cumulative and derivative dose-response curves for the overall sample of clones derived from CD34+CD41+ progenitors. The cumulative dose-response curve is shown in the top panel, and derivative dose-response curve is shown in the bottom panel. Sorted progenitors were individually cultured for 5 days in serum-deprived medium with the indicated concentrations of TPO. Clones contained 1 to 16 megakaryocytes, corresponding to 0 to 4 progenitor doublings. Data points are the mean ± 1 SEM of quadruplicate determinations of the number of clones at day 5 per 96-well Terasaki plate. A gaussian cumulative equation gave the best fit to data among 29 functions based on transitional models (r2 = 0.99; P < .001). The bell-shaped bottom curve, the gaussian analytical derivative of the top curve, is the frequency distribution of TPO thresholds of individual progenitors. Counts obtained for wells with no TPO were assigned a concentration of 10–2 pg/mL to preclude entering the log of zero values in regressions. In this experiment, the mean absolute number of clones per 96-well plate which survived in absence of TPO was 4.44 ± 0.82 (ie, 6.05% of the total clones). The dotted vertical drop lines indicate TPO50 (ie, the TPO dose at which 50% of the plateau number of clones were counted). Results are from experiment number 1 and are representative of 3 experiments.
Characteristics of TPO dose-response curves of CD34+CD41+ progenitors
Experiment no. . | Clone size, no. MK . | Clone size, mean NbD (range) . | Plateau no. of clones,*% of total (95% CI) . | TPO50, pg/mL (95% CI) . | CV† . |
|---|---|---|---|---|---|
| 1 | 1 | 0 (0) | 55.1 (51.9-58.3) | 1.26 (0.62-2.55) | 0.62 |
| 1 | 2-3 | 1.16 (1-1.58) | 20.2 (17.3-23.0) | 8.09 (4.87-13.4) | 0.51 |
| 1 | 4-7 | 2.28 (2-2.80) | 18.4 (17.9-19.0) | 41.1 (37.3-45.3) | 0.33 |
| 1 | 8-15 | 3.20 (3-3.90) | 6.23 (6.23-6.23) | 64.7 (64.4-64.9) | 0.19 |
| Total | 1-15 | 0.50 (0-3.90) | 102 (84.0-117)‡ | 4.92 (2.68-9.04) | 1.03 |
| 2 | 1 | 0 (0) | 50.6 (41.6-59.5) | 1.52 (0.64-3.62) | 0.51 |
| 2 | 2-3 | 1.24 (1-1.58) | 19.8 (15.9-23.6) | 6.22 (2.51-15.5) | 0.29 |
| 2 | 4-7 | 2.29 (2-2.80) | 21.8 (19.7-24.0) | 45.7 (34.7-60.2) | 0.31 |
| 2 | 8-15 | 3.21 (3-3.90) | 7.80 (7.80-7.80) | 90.7 (50.0-165) | 0.21 |
| Total | 1-15 | 0.73 (0-3.90) | 96.8 (88.5-105)‡ | 5.50 (3.64-8.00) | 1.00 |
| 3 | 1 | 0 (0) | 43.2 (33.0-53.1) | 2.89 (1.48-5.65) | 0.24 |
| 3 | 2-3 | 1.22 (1-1.58) | 25.6 (21.6-29.6) | 8.95 (7.31-11.0) | 0.26 |
| 3 | 4-7 | 2.30 (2-2.80) | 21.8 (21.1-22.5) | 28.8 (27.7-30.0) | 0.22 |
| 3 | 8-15 | 3.30 (3-3.90) | 9.42 (7.83-11.0) | 120 (88.0-165) | 0.27 |
| Total | 1-15 | 0.83 (0-3.90) | 100 (90.5-110) | 7.31 (5.53-9.66) | 0.47 |
Experiment no. . | Clone size, no. MK . | Clone size, mean NbD (range) . | Plateau no. of clones,*% of total (95% CI) . | TPO50, pg/mL (95% CI) . | CV† . |
|---|---|---|---|---|---|
| 1 | 1 | 0 (0) | 55.1 (51.9-58.3) | 1.26 (0.62-2.55) | 0.62 |
| 1 | 2-3 | 1.16 (1-1.58) | 20.2 (17.3-23.0) | 8.09 (4.87-13.4) | 0.51 |
| 1 | 4-7 | 2.28 (2-2.80) | 18.4 (17.9-19.0) | 41.1 (37.3-45.3) | 0.33 |
| 1 | 8-15 | 3.20 (3-3.90) | 6.23 (6.23-6.23) | 64.7 (64.4-64.9) | 0.19 |
| Total | 1-15 | 0.50 (0-3.90) | 102 (84.0-117)‡ | 4.92 (2.68-9.04) | 1.03 |
| 2 | 1 | 0 (0) | 50.6 (41.6-59.5) | 1.52 (0.64-3.62) | 0.51 |
| 2 | 2-3 | 1.24 (1-1.58) | 19.8 (15.9-23.6) | 6.22 (2.51-15.5) | 0.29 |
| 2 | 4-7 | 2.29 (2-2.80) | 21.8 (19.7-24.0) | 45.7 (34.7-60.2) | 0.31 |
| 2 | 8-15 | 3.21 (3-3.90) | 7.80 (7.80-7.80) | 90.7 (50.0-165) | 0.21 |
| Total | 1-15 | 0.73 (0-3.90) | 96.8 (88.5-105)‡ | 5.50 (3.64-8.00) | 1.00 |
| 3 | 1 | 0 (0) | 43.2 (33.0-53.1) | 2.89 (1.48-5.65) | 0.24 |
| 3 | 2-3 | 1.22 (1-1.58) | 25.6 (21.6-29.6) | 8.95 (7.31-11.0) | 0.26 |
| 3 | 4-7 | 2.30 (2-2.80) | 21.8 (21.1-22.5) | 28.8 (27.7-30.0) | 0.22 |
| 3 | 8-15 | 3.30 (3-3.90) | 9.42 (7.83-11.0) | 120 (88.0-165) | 0.27 |
| Total | 1-15 | 0.83 (0-3.90) | 100 (90.5-110) | 7.31 (5.53-9.66) | 0.47 |
The mean ± 1 SD absolute number of each MK clone size per 96-well Terasaki plate (based on 3 experiments with quadruplicate determinations for each of 8-10 TPO concentrations) was: 1 MK, 26.1 ± 12.6; 2 to 3 MKs, 10.9 ± 3.20; 4 to 7 MKs, 10.4 ± 2.89; 8 to 15 MKs, 3.92 ± 0.63; 1 to 15 MKs, 51.3 ± 20.1. Among 1 MK and 1 to 15 MK clones, the mean absolute number of clones that survived in absence of TPO was 3.83 ± 0.65. In each experiment, four 96-well Terasaki plates were used for each concentration of TPO (total of at least 32 plates).
CV refers to the coefficient of variation (SD/mean) of TPO thresholds.
Total percentage of plateau no. of clones does not equal 100% because the total dose-response curve has been fitted independently of the individual size classes.
Responsiveness to TPO and number of residual doublings (NbDr) are negatively correlated
The overall curve was then resolved into size-class dependent curves by fitting regression equations to the frequencies of clones with 1 (0 doubling; db), 2 to 3 (1-1.58 db), 4 to 7 (2-2.8 db), or 8 to 15 (3-3.9 db) megakaryocytes (Table 1; Figure 2). Again, in each experiment, the gaussian cumulative model provided the best fit to the data points when compared with 29 transition regression equations (r2 ≥ 0.99; P < .001 in all cases). The curves showed highly significant differences in TPO50. Overall, in 3 experiments, TPO50 was 1.89 ± 0.51 pg/mL (mean ± 1 SEM) for 1 MK clones, 7.75 ± 0.81 for 2 to 3 MK clones, 38.5 ± 5.04 for 4 to 7 MK clones, and 91.8 ± 16.0 for 8 to 15 MK clones (P < .001). Figure 3 demonstrates the shift to decreasing responsiveness (ie, increasing TPO50) as the clone size increased.
Dose-response curves for CD34+CD41+ progenitors of megakaryocyte clones of various sizes. In the 5 top panels, dose-response curves have been fitted to the absolute frequencies of clones with the indicated numbers of megakaryocytes. In the bottom panel, the curves have been normalized to a plateau frequency of 100% and superimposed to facilitate comparison. Data points are the mean ± 1 SEM of quadruplicate determinations of the number of clones at day 5 per 96-well Terasaki plate. R2 values for the gaussian cumulative model were 0.99 or more (P < .001) for all curves. Counts obtained for wells with no TPO were assigned a concentration of 10–2 pg/mL to preclude entering the log of zero values in regressions. For 1 MK clones, the mean absolute number of clones per 96-well plate that survived in absence of TPO was 4.28 ± 0.72 (ie, 5.92% of the total clones). The dotted drop lines indicate TPO50 (ie, the TPO dose at which 50% of the plateau number of clones were counted). Results are representative of 3 experiments.
Dose-response curves for CD34+CD41+ progenitors of megakaryocyte clones of various sizes. In the 5 top panels, dose-response curves have been fitted to the absolute frequencies of clones with the indicated numbers of megakaryocytes. In the bottom panel, the curves have been normalized to a plateau frequency of 100% and superimposed to facilitate comparison. Data points are the mean ± 1 SEM of quadruplicate determinations of the number of clones at day 5 per 96-well Terasaki plate. R2 values for the gaussian cumulative model were 0.99 or more (P < .001) for all curves. Counts obtained for wells with no TPO were assigned a concentration of 10–2 pg/mL to preclude entering the log of zero values in regressions. For 1 MK clones, the mean absolute number of clones per 96-well plate that survived in absence of TPO was 4.28 ± 0.72 (ie, 5.92% of the total clones). The dotted drop lines indicate TPO50 (ie, the TPO dose at which 50% of the plateau number of clones were counted). Results are representative of 3 experiments.
Distributions of TPO thresholds for progenitors of 1-, 2-, 3-, and 4-cell MK clones and the total population. The bell-shaped curves are the gaussian analytical derivatives of the cumulative dose-response curves for 4 clone sizes and the total population. The peak of each curve indicates the median TPO threshold for the indicated class and corresponds to the respective TPO50 shown in Figure 2. The solid line represents the TPO threshold distribution for the total MK progenitor population. The 95% confidence intervals (95% CIs) of TPO50 were 2.68 to 9.04 pg/mL (total MK), 0.62 to 2.55 pg/mL (1 MK), 3.36 to 9.32 pg/mL (2 MKs), 13.9 to 21.7 pg/mL (3 MKs), and 32.3 to 35.7 pg/mL (4 MKs). Because the CIs did not overlap, TPO50 values differed significantly from each other at the 5% level. A representative experiment is shown.
Distributions of TPO thresholds for progenitors of 1-, 2-, 3-, and 4-cell MK clones and the total population. The bell-shaped curves are the gaussian analytical derivatives of the cumulative dose-response curves for 4 clone sizes and the total population. The peak of each curve indicates the median TPO threshold for the indicated class and corresponds to the respective TPO50 shown in Figure 2. The solid line represents the TPO threshold distribution for the total MK progenitor population. The 95% confidence intervals (95% CIs) of TPO50 were 2.68 to 9.04 pg/mL (total MK), 0.62 to 2.55 pg/mL (1 MK), 3.36 to 9.32 pg/mL (2 MKs), 13.9 to 21.7 pg/mL (3 MKs), and 32.3 to 35.7 pg/mL (4 MKs). Because the CIs did not overlap, TPO50 values differed significantly from each other at the 5% level. A representative experiment is shown.
To quantitatively estimate the association between TPO responsiveness (1/TPO50) and clone size, a correlation analysis was performed. Separate dose-response curves for clones of 1 to 8 MKs were used without grouping to benefit from maximum resolution of plateau and NbD values. When responsiveness to TPO was plotted versus NbDr, there was a strong negative correlation between these variables (Figure 4 left; P < .001). By using the overall values of the 3 experiments, the slope of the regression line indicated that TPO responsiveness decreased by a factor of 3.5 (95% confidence interval [CI], 2.9-4.1) per doubling.
Interrelationships between TPO responsiveness and NbD undergone by megakaryocyte progenitors in vivo and in vitro. Relative responsiveness was defined as the absolute responsiveness (1/TPO50) at each data point divided by the responsiveness of the least responsive MK progenitor. NbD have been calculated as detailed in “Materials and methods.” Inverse correlations were obtained between responsiveness and numbers of residual doublings undergone in vitro (NbDr; left), and positive correlations were obtained between responsiveness and number of previous doublings undergone in vivo (NbDp; middle). As a result, a reciprocal relationship was found between NbDr and NbDp, indicating that a high number of previous doublings were associated with a low number of residual doublings. The converse was also true (right). The P values for the correlation coefficients were less than .005.
Interrelationships between TPO responsiveness and NbD undergone by megakaryocyte progenitors in vivo and in vitro. Relative responsiveness was defined as the absolute responsiveness (1/TPO50) at each data point divided by the responsiveness of the least responsive MK progenitor. NbD have been calculated as detailed in “Materials and methods.” Inverse correlations were obtained between responsiveness and numbers of residual doublings undergone in vitro (NbDr; left), and positive correlations were obtained between responsiveness and number of previous doublings undergone in vivo (NbDp; middle). As a result, a reciprocal relationship was found between NbDr and NbDp, indicating that a high number of previous doublings were associated with a low number of residual doublings. The converse was also true (right). The P values for the correlation coefficients were less than .005.
Responsiveness to TPO and number of previous doublings (NbDp) are positively correlated
For each size class, the plateau number of clones per 96-well Terasaki plate reflected the relative number of progenitors of that class that were present in the sample of sorted CD34+CD41+ progenitors cultured. Therefore, under the generation-age assumption, the log2 of the plateau percentages of each size class provided an estimate of the number of previous doublings (NbDp) undergone in vivo by the progenitors of the various classes. Because a valid estimate of NbDp depended on plateau estimates, separate dose-response curves for clones of 1 to 8 MKs were used without grouping. There was a strong positive correlation between relative responsiveness and NbDp (Figure 4 middle; P < .005).
Reciprocal relationship between NbDp and NbDr
Because TPO responsiveness was negatively correlated with NbDr and positively correlated with NbDp, a reciprocal relationship between NbDp and NbDr was expected. Indeed, Figure 4 (right panel) demonstrates that those progenitors that had previously undergone many doublings in vivo (NbDp) had the lowest proliferative potential in vitro (NbDr) and vice versa (P < .001). As a result of the reciprocal relationship, the total NbD, that is, NbDp + NbDr, calculated as the sum of the abcissa and the ordinate at any point on the correlation slope in the right panel of Figure 4, tended to be constant with an approximate value of 3 to 5 doublings under the conditions of these experiments. Because these curves were based on clones of 1 to 8 MKs rather than 1 to 15 MKs, the best approximation for the total NbD would be 5 to 7 doublings.
Variations in the number of doublings account for most of the heterogeneity in progenitor responsiveness
In the above-mentioned analyses, the log TPO50 represented the average response threshold for a given clone size class but did not contain any information about the heterogeneity in response threshold within that class. To introduce the missing information into the correlation analysis, 4 samples of 500 progenitors undergoing 0, 1, 1.58, and 2 db's (corresponding to clones with 1, 2, 3, and 4 MKs, respectively) were simulated by using a random number generator so that their TPO50 and the coefficient of variation of their TPO thresholds were those in Table 1. The 2000 simulated values and the corresponding NbDr were then entered into the computation of a Pearson product moment correlation (Figure 5). The R2 value was 0.78 (P < .001), indicating that an estimated 78% of the total variation in NbDr and log TPO was explained by their mutual association and that the remaining 22% reflected the effect of factors that influenced clone size and TPO responsiveness independently of the other.
Analysis of correlation between TPO threshold of individual CD34+CD41+ progenitors and clone size achieved, expressed as NbDr. Samples of 500 TPO thresholds (□) have been simulated so that the corresponding TPO50 and coefficients of variation were those in Table 1. The 4 horizontal groupings represent the sets of simulated values obtained for the 4 NbD size classes, from top to bottom: 8 to 15 MKs; 4 to 7 MKs; 2 to 3 MKs; and 1 MK. Solid and dashed lines indicate the regression line through the total set of points and through percentiles 2.5% and 97.5% of each of the 4 sets of simulated TPO threshold values, respectively. R2 is the squared Spearman rank order correlation (P < .001).
Analysis of correlation between TPO threshold of individual CD34+CD41+ progenitors and clone size achieved, expressed as NbDr. Samples of 500 TPO thresholds (□) have been simulated so that the corresponding TPO50 and coefficients of variation were those in Table 1. The 4 horizontal groupings represent the sets of simulated values obtained for the 4 NbD size classes, from top to bottom: 8 to 15 MKs; 4 to 7 MKs; 2 to 3 MKs; and 1 MK. Solid and dashed lines indicate the regression line through the total set of points and through percentiles 2.5% and 97.5% of each of the 4 sets of simulated TPO threshold values, respectively. R2 is the squared Spearman rank order correlation (P < .001).
Fractions of CD34+CD41+ megakaryocyte progenitors differ in responsiveness and NbDr
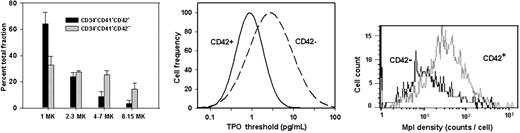
In view of the association between high responsiveness and loss of proliferative potential, a comparison was made of the CD34+CD41+CD42a+ and CD34+CD41+CD42a– fractions separated by cell sorting from the CD34+CD41+ megakaryocyte progenitor population. Figure 6 (left panel) shows that compared with the CD42a– fraction, the CD42a+ fraction was shifted toward the generation of smaller-sized clones (chi-square = 108, P < .001) and produced twice as many single megakaryocyte clones (64% versus 33%). Correspondingly, the CD42a+ fraction had a significantly lower TPO50 (1.13 pg/mL; 95% CI, 0.427-1.82 pg/mL) than the CD42a– fraction (2.75 pg/mL; 95% CI, 1.91-3.96 pg/mL; Figure 6 middle; P < .001). Again, progenitors that responded best to TPO underwent fewer NbDr.
Size of megakaryocyte clonal progeny, TPO responsiveness, and Mpl expression recorded for CD42a+ and CD42– fractions of CD34+CD41+ megakaryocyte progenitors. (Left) Histogram columns indicate the percentages of progenitors of each fraction that generated clones of the sizes indicated. Data are the means of 3 duplicate experiments ± 1 SD. Distributions of percentages in the 2 fractions differed by chi-square analysis (P < .001). (Middle) The bell-shaped curves are TPO threshold distributions for the CD42a+ (left) and CD42a– (right) fractions. The means and 95% confidence intervals (95% CIs) of the corresponding TPO50 were 1.13 pg/mL (95% CI, 0.427-1.82 pg/mL) and 2.75 pg/mL (95% CI, 1.91-3.96 pg/mL), respectively. Because the CI did not overlap, TPO50 values differed significantly from each other at the 5% level. (Right) Flow cytometric analysis of Mpl expression on CD42a+ (right) and CD42a– (left) megakaryocyte progenitors. Mononuclear bone marrow cells were labeled with anti-Mpl followed by antimouse IgG-PE, washed with mouse serum, and further labeled with anti-CD34–PC5, anti-CD41–APC, and anti-CD42a–FITC. Median Mpl expression was 2.8-fold higher on CD42a+ than on CD42a– progenitors (P < .001).
Size of megakaryocyte clonal progeny, TPO responsiveness, and Mpl expression recorded for CD42a+ and CD42– fractions of CD34+CD41+ megakaryocyte progenitors. (Left) Histogram columns indicate the percentages of progenitors of each fraction that generated clones of the sizes indicated. Data are the means of 3 duplicate experiments ± 1 SD. Distributions of percentages in the 2 fractions differed by chi-square analysis (P < .001). (Middle) The bell-shaped curves are TPO threshold distributions for the CD42a+ (left) and CD42a– (right) fractions. The means and 95% confidence intervals (95% CIs) of the corresponding TPO50 were 1.13 pg/mL (95% CI, 0.427-1.82 pg/mL) and 2.75 pg/mL (95% CI, 1.91-3.96 pg/mL), respectively. Because the CI did not overlap, TPO50 values differed significantly from each other at the 5% level. (Right) Flow cytometric analysis of Mpl expression on CD42a+ (right) and CD42a– (left) megakaryocyte progenitors. Mononuclear bone marrow cells were labeled with anti-Mpl followed by antimouse IgG-PE, washed with mouse serum, and further labeled with anti-CD34–PC5, anti-CD41–APC, and anti-CD42a–FITC. Median Mpl expression was 2.8-fold higher on CD42a+ than on CD42a– progenitors (P < .001).
In one experiment that used quadruple labeling and sorting of megakaryocyte progenitors, the expression of Mpl by CD34+ CD41+CD42a+ and CD34+CD41+CD42a– fractions was compared. The expression of Mpl by the total CD34+CD41+ population, expressed in arbitrary fluorescence units, covered a 3-log range with a median of 25.5 units. As shown in Figure 6 (right panel), the most responsive CD42a+ cells had a 2.8-fold greater median expression of Mpl (32.5; 95% CI, 22.6-42.4 units) than their CD42a– counterparts (11.6 units; 95% CI, 7.7-15.5 units; P < .001), suggesting that TPO receptor expression is a significant factor in megakaryocyte progenitor responsiveness to TPO.
NbDr distributions are derived from TPO dose-response curves
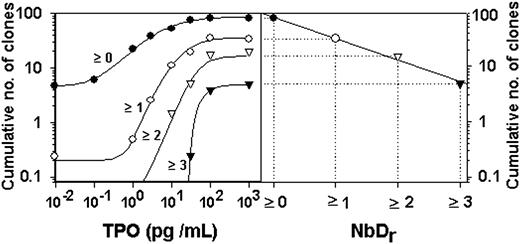
In the previous sections, the overall TPO dose-response curve was resolved into curves for clones having undergone 0 to 3 doublings. We wondered whether there was any relationship between these dose-response curves and the exponential NbDr distributions referred to in “Introduction.” Therefore, dose-response curves were plotted on semilog coordinates for the number of clones that had undergone 0 or more, 1 or more, 2 or more, or 3 or more doublings as a function of TPO concentration (Figure 7 left). With the use of the plateau values of these curves, the frequencies (on log scale) of the clones that had undergone these NbDr were plotted (Figure 7 right). The resulting straight line documented an exponential NbDr distribution, which was derived from the set of size-class dose-response curves.
Relationship between dose-response curves and clone size distributions expressed as NbD. (Left) Dose-response curves for clones with 0 or more, 1 or more, 2 or more, or 3 or more doublings. (Right) Normalized plateau values versus the corresponding NbDr.
Relationship between dose-response curves and clone size distributions expressed as NbD. (Left) Dose-response curves for clones with 0 or more, 1 or more, 2 or more, or 3 or more doublings. (Right) Normalized plateau values versus the corresponding NbDr.
Discussion
In this investigation, TPO dose-response curves have been determined for normal human CD34+CD41+ progenitors sorted into 96-well Terasaki plates and cultured for 5 days in serum-deprived medium supplemented with TPO at a concentration range of 0.1 to 103 pg/mL. Only CD41+ megakaryocyte clones (defined as entities containing at least 1 megakaryocyte2 ) are present at day 5 under these conditions.27,28 TPO acts preferentially on late, CD41+ megakaryocyte progenitors29 by binding to the Mpl receptor, which is expressed at low levels in CD34+ cells and at high levels during the late stages of human megakaryocyte differentiation.30 The CD42a antigen (glycoprotein Ibα) appears later than CD41 (glycoprotein IIb)31,32 and was found in this study to be associated with the expression of greater numbers of Mpl receptors (Figure 6). It had been established that GATA and cis-acting sequences coregulate the megakaryocyte progenitor expression of Mpl,33 CD41,34 and CD42a.35
TPO responsiveness was recorded both as distributions of TPO thresholds (ie, the TPO concentrations necessary and sufficient to ensure the survival of megakaryocyte progenitors, either as single cells or as colonies) and as the medians of these distributions, termed TPO50 (ie, the TPO concentration corresponding to the half-plateau number of clones). TPO responsiveness was defined as 1/TPO50. Whereas TPO thresholds had a lognormal distribution, TPO50 fitted exponential lines when plotted versus NbDr. The significance of these distributions is discussed in the following 3 sections.
Range of responsiveness of individual progenitors covers 4 orders of magnitude
Responsiveness to stimulators depends on the dynamics of ligand-receptor interaction, the combined efficiency of the signal transduction pathways activated by these stimulators, and the pattern of gene expression. The diversity in megakaryocyte progenitor responsiveness is well shown by the bell-shaped frequency distribution of log TPO thresholds that covers a 4-log range. This distribution was recorded, not only for the overall megakaryocyte progenitor population (Figure 1 bottom) but also for clones of different sizes (Figure 3). This distribution, which is the derivative of the dose-response curve (Figure 1), obeyed the gaussian or the very similar sigmoid model. Lognormality of progenitor TPO thresholds has straightforward implications for the process that generates diversity of responsiveness, because the genesis of lognormal distributions is well understood.36 Briefly, a variable (for example, the activity of a TPO-responsive signaling pathway) tends to assume a lognormal distribution when its value at each step of a process is a random proportion (ie, a random multiplier) of its value at the preceding step. In the case of TPO responsiveness, multiplicative variations (ie, variations whose effects multiply each other) must originate in the chain of amplifying signaling events triggered by TPO receptor binding. TPO receptor expression itself may be a significant factor in CD34+CD41+ progenitor responsiveness to TPO because it covered a 3-log range in membrane density and because its median value was 2.8-fold greater in the most responsive CD42a+ fraction than in the CD42a– fraction (Figure 6 right). Lognormality arises because random multiplicative variations in the multistep signaling process translate as random additive variations of the log of this variable; therefore, log TPO thresholds, rather than TPO thresholds, have a gaussian distribution. Heterogeneity in the number of cycles undergone in vivo before sampling may contribute another mechanism generating lognormality, insofar as these additional variations are also multiplicative.
Interrelationships between TPO responsiveness and NbD undergone by megakaryocyte progenitors in vivo and in vitro are compatible with a generation-age structure
When clones were grouped as a function of the size they achieve in vitro, expressed as number of doublings (NbDr), it was found that TPO concentrations at half plateau values (TPO50) increased, and accordingly that TPO responsiveness, defined as 1/TPO50, decreased with clone size (Table 1; Figures 2, 3). Furthermore, when TPO responsiveness was plotted versus NbDr, there was a strong negative correlation between these variables (Figure 4 left), and 78% of the variance in TPO responsiveness of individual progenitors was accounted for by its association with NbDr (Figure 5). (The remaining 22% appear to be generated by factors that influence TPO responsiveness and NbDr independently of each other. Because in each size class, the distribution of TPO thresholds was lognormal [Figures 2, 3], the diversification processes inherent in the cell signaling system discussed earlier provide the most likely mechanism for the NbD-independent variance in these TPO thresholds.)
Comparisons of the 42a+ and 42a– fractions of CD34+CD41+ progenitors, separated by cell sorting, confirmed the inverse relationship between TPO responsiveness and proliferative potential (Figure 6). Compared with the CD42a– fraction, the corresponding CD42a+ fraction had lower TPO50 (P < .001) and underwent fewer NbDr (P < .001). Furthermore, the CD42a+ fraction expressed a 2.8-fold greater median Mpl receptor density than the CD42a– fraction (P < .001). These data, therefore, established a link between TPO responsiveness and Mpl receptor density. A similar relationship between factor responsiveness and receptor density had been found in other systems.37-39
Two main hypotheses can account for the negative correlation between TPO responsiveness and NbDr, depending on which of these 2 variables is seen as determining the other. In the first, differences in TPO responsiveness, generated by variations in the TPO signaling systems, determine the number of cycles undergone by megakaryocyte progenitors before arrest of mitosis and onset of polyploidization. In that view, the differentiative effects of TPO are dominant over its mitogenic action, so that only the less-sensitive progenitors undergo several divisions in culture. In the other view, those megakaryocyte progenitors that accomplish no or few divisions in vitro are those that had undergone several cell cycles in vivo and have thus exhausted all or most of their proliferative capacity. Progenitors of 1 MK clone are, therefore, very responsive to TPO because they have previously completed a significant part of their proliferative program and because the latter entails a stepwise, generation-linked increase in responsiveness. The latter presumably leads to polyploidization and cytoplasmic maturation.
To discriminate between the 2 models, additional data were required concerning the proliferative past of 1 MK to 8 MK clones. Determination of the number of previous doublings (NbDp) approximated this requirement. Assuming that the percentages of 1 MK,2MK,...8MK clones at plateau TPO concentration reflects the number of doublings their ancestral progenitors had previously accomplished in vivo, it was possible to correlate, first, TPO responsiveness with NbDp (Figure 4 middle) and, second, NbDr with NbDp (Figure 4 right). A highly significant positive relationship was obtained between TPO responsiveness and NbDp and a highly significant reciprocal relationship was shown between NbDp and NbDr. A posteriori, the high correlations validate the estimation of NbDp from the log2 percentages of plateau values. The results provided evidence that (1) the number of residual doublings (NbDr) achieved in vitro is determined by the past mitotic history of the progenitor so that those progenitors that had previously undergone many doublings in vivo had the lowest proliferative potential in vitro, and vice versa; (2) with each doubling in vitro, TPO responsiveness decreases by a factor of 3.5 per doubling as a result of the recruitment of progressively more ancestral progenitors. Although the value of 3.5 depends on the culture conditions used and cannot be extrapolated to the doublings undergone in vivo, the data of Figure 4 (middle panel) confirm that responsiveness increases significantly at each doubling in vivo. Increase in factor responsiveness together with a decrease in proliferative potential has been shown previously for hematopoietic progenitors during in vivo lineage maturation.14-18,40 In megakaryocyte cultures, the progenitors of CFU-MK colonies with 3 or more megakaryocytes, of 2 megakaryocyte clones, and of single megakaryocytes display progressively increasing responsiveness to thrombopoietin.18 Similarly, in oligodendrocyte precursor cells, maturation is associated with an increasing number of thyroid hormone receptors and increasing sensitivity to the hormone.39 Increase in receptor level associated with megakaryocyte progenitor maturation was also demonstrated in the present study (Figure 6 right). Of interest, the expression level of the PLZF gene progressively increases through megakaryocytic development, and, when it is transduced into the erythromegakaryocytic TF1 cell line, its overexpression induces Mpl and up-modulates megakaryocytic-specific glycoproteins as well as platelet factor 4 (PF4).41 The observation that 7.62% of single megakaryocyte clones survived in the absence of added TPO may even suggest that at least some postmitotic thrombopoietic cells are TPO independent. However, this observation may also be explained by the effects of contaminants that have been demonstrated in Cohn fraction V, and the insulin and transferrin present in the medium.42
Exponential distribution of NbDr also favors the generation-age structure model
Ascribing the heterogeneity in proliferative potential to the differences in individual progenitor responsiveness (as reflected in the lognormal distribution of TPO thresholds) does not suggest an immediate explanation for the exponential NbDr distribution. However, the exponential NbDr distribution naturally arises from the age structure model. At each doubling in vitro, the number of remaining progenitors capable of additional doublings will decrease according to the reciprocal of the in vivo geometric distribution, with the slope of the exponential distribution of NbDr modulated by the culture conditions used.2 This simplified reasoning can account for the exponential distribution of NbDr and for the reciprocal relationship between NbDr and NbDp. Variations in cell cycle times would not necessarily alter the exponential nature of the doubling distribution.
Recruitment as a mechanism for increased production
The effects of stimulators of hematopoiesis are mediated by suppression of apoptosis and promotion of survival,43-48 shortening of the cell cycle,13,49,50 and retardation of differentiation.50 The results described in the present investigation suggest that recruitment provides an additional mechanism of response to stimulators. In Mpl-transduced BaF-3 cells, TPO promotes survival in cells expressing low levels of the receptor but triggers proliferation in those expressing high levels of Mpl.47 This mechanism ensures that when low stimulator concentration and/or receptor density support survival but not proliferation of the least responsive, most ancestral progenitors, other progenitors with a longer proliferative history and greater factor responsiveness will be preferentially induced to proliferation. The more responsive progenitors may also be able to produce the all-or-none, switchlike responses of the MAPK cascade7,8 that is required for the induction of polyploidization.9-11 Furthermore, under increasing stimulator concentration (eg, high concentrations of TPO that have resulted from decreased numbers of megakaryocytes and platelets), progenitors that are progressively less responsive and less mature, but endowed with greater proliferative potential, will be triggered to proliferate. Subsequently, their more responsive progeny will be driven to terminal maturation.15 Such a pattern of regulation, based on the strong link between past proliferation and factor responsiveness, provides a coherent framework for the interpretation of the generation-age model of progenitor differentiation.
Prepublished online as Blood First Edition Paper, June 1, 2004; DOI 10.1182/blood-2003-05-1745.
Supported by grants from the Fonds National de la Recherche Scientifique (FNRS), Brussels, Belgium; the Institut National de la Santé et de la Recherche Médicale, France; and the US Department of Veterans Affairs. J.L. was the recipient of a fellowship from the FNRS during the performance of some of these investigations. The INSERM U362 received a grant from la Ligue Nationale contre le Cancer (Equipe labellisée 2004). J-M.P. is a Director of Research of the FNRS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal