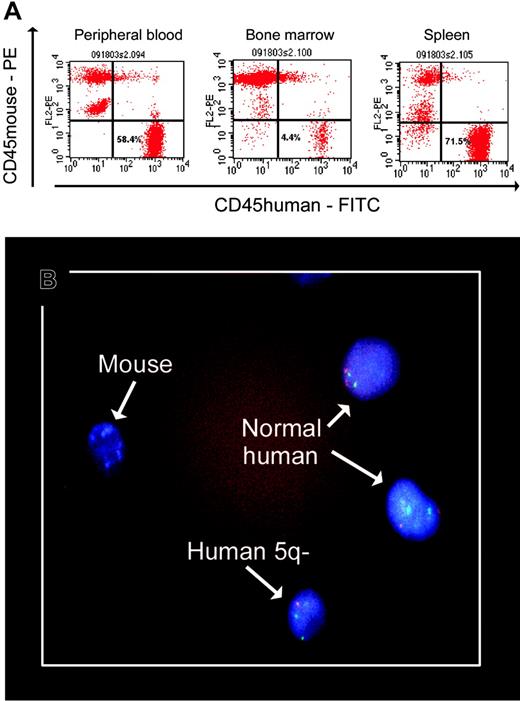
Myelodysplastic syndromes (MDSs) are clonal hematopoietic stem cell disorders. In contrast to leukemic cells, however, propagation of MDS-derived clones in vitro or in vivo has proven difficult.1-3 Thanopoulou et al recently reported the engraftment of human MDS-derived cells in nonobese diabetic-severe combined immunodeficient (NOD/SCID) β2-microglobulin-deficient (β2mnull) mice and NOD/SCID-β2mnull mice transgenically engineered to produce interleukin 3 (IL-3), granulocyte macrophage-colony-stimulating factor (GM-CSF), and SF.4 They observed engraftment from 9 of 11 patients with MDS, and in 4 cases, clonal precursors (identified by chromosomal markers) could be recovered. We previously observed engraftment of normal human progenitors in NOD/SCID mice that received transplants of bone marrow mononuclear cells (BMMCs) from patients with MDS, but failed to show engraftment of identifiable clonal precursors.3 We extended our study using NOD/SCID-β2mnull mice. Cells from 6 patients with MDS were transplanted into 15 NOD/SCID-β2mnull mice. A total of 107 whole BMMCs were injected intramedullarly along with 1 × 105 cells each of the human stroma-derived cell lines HS5 and HS27a, both well characterized in functional studies and with gene expression profiles.5,6 In 11 mice, there was engraftment of human cells in peripheral blood (0.7%-58.4%; median 8.9%). Results from 6 mice, which were followed from 4 to 17 weeks and subjected to complete autopsies, are illustrated in Table 1. The highest proportions of human cells were recovered from the spleens, followed by peripheral blood and marrow. Proportions were higher in this model than in our previous model,3 and higher than in the report by Thanopoulou et al,4 who could not document significant differences in output of MDS-derived cells between the 2 mice strains used in their work. We believe that the higher numbers achieved here are attributable not only to the intramedullary route of transplantation but also to the coinjection of human stroma cells that provide crucial signals facilitating engraftment.
Level of engraftment of human cells in sublethally irradiated NOD/SCID-β2mnull mice
. | . | . | . | . | FISH . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Engraftment† . | . | . | Clonal cells/total . | . | Normal cells/total . | . | ||||
| Wks after transplantation . | Clonal marker (% of cytogenetically abnormal cells*) . | Blood . | Bone marrow . | Spleen . | Bone marrow (%) . | Spleen (%) . | Bone marrow . | Spleen . | ||||
| 4 | del(5q)/trisomy8 (95) | 58.37 | 4.44 | 71.54 | NA | 24/272 (8.8)§ | NA | 248/272 | ||||
| 6 | del(5q)/trisomy8; (95‡) | 26.06 | 37.7 | 27.0 | 6/61 (9.8)§ | 3/44 (6.8)§ | 55/61 | 41/44 | ||||
| 5 | −Y (75) | 0.73 | 2.11 | 14.91 | 0 (0.0) | 0 (0.0) | 100 | 100 | ||||
| 17 | −Y (75) | 0.61 | 1.19 | 0.09 | 22/213 (10.3) | 0 | 191/213 | 4 | ||||
| 6 | del(7q) (90) | 7.77 | 0.71 | 6.1 | 1/2 (50) | 21/218 (9.6) | 1/2 | 197/218 | ||||
| 13 | del(5q) (68) | 1.74 | 0.71 | 34.19 | 1/5 (20) | 13/201 (6.4) | 4/5 | 188/201 | ||||
| Mean ± SEM | — | 15.9 ± 9.4 | 7.8 ± 6.0 | 25.6 ± 10.5 | — | — | — | — | ||||
. | . | . | . | . | FISH . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Engraftment† . | . | . | Clonal cells/total . | . | Normal cells/total . | . | ||||
| Wks after transplantation . | Clonal marker (% of cytogenetically abnormal cells*) . | Blood . | Bone marrow . | Spleen . | Bone marrow (%) . | Spleen (%) . | Bone marrow . | Spleen . | ||||
| 4 | del(5q)/trisomy8 (95) | 58.37 | 4.44 | 71.54 | NA | 24/272 (8.8)§ | NA | 248/272 | ||||
| 6 | del(5q)/trisomy8; (95‡) | 26.06 | 37.7 | 27.0 | 6/61 (9.8)§ | 3/44 (6.8)§ | 55/61 | 41/44 | ||||
| 5 | −Y (75) | 0.73 | 2.11 | 14.91 | 0 (0.0) | 0 (0.0) | 100 | 100 | ||||
| 17 | −Y (75) | 0.61 | 1.19 | 0.09 | 22/213 (10.3) | 0 | 191/213 | 4 | ||||
| 6 | del(7q) (90) | 7.77 | 0.71 | 6.1 | 1/2 (50) | 21/218 (9.6) | 1/2 | 197/218 | ||||
| 13 | del(5q) (68) | 1.74 | 0.71 | 34.19 | 1/5 (20) | 13/201 (6.4) | 4/5 | 188/201 | ||||
| Mean ± SEM | — | 15.9 ± 9.4 | 7.8 ± 6.0 | 25.6 ± 10.5 | — | — | — | — | ||||
Results determined by flow cytometry (% CD45+ human cells) and FISH analysis for individual mice that underwent transplantation and which are illustrated in the insert. NA indicates not available; —, not applicable.
Clonal abnormality examined by FISH.
Percent of CD45+ human cells (illustrated in Figure 1A).
FISH results from spleen cells were based on CD45+CD33+ sorted samples; in the other mice FISH results were based on whole bone marrow and spleen cells.
Only human cells containing an isolated del(5q) were detectable; no cells containing trisomy 8 were identified (illustrated in Figure 1B).
We detected clonal cells in 5 of 6 mice injected with human cells containing fluorescence in situ hybridization (FISH)-recognizable markers, including del(5q), del(7q), and loss of the Y chromosome; proportions in this patient sample were lower than in the original patient sample (Table 1), in agreement with Thanopoulou et al's findings.
There were remarkable results in 2 mice that received transplants of marrow from a patient with del(5q) and trisomy 8 in 95% of the cells analyzed. Recovered cells showed only del(5q), and no cells with trisomy 8, alone or in combination with del(5q), were detected (Table 1 and Figure 1). This observation in vivo supports the in vitro findings by Nilsson et al whose studies on marrow cells with del(5q)/trisomy 8 suggested a competitive advantage of cells with the 5q deletion only.2 The findings presumably reflect the engraftment of early precursors containing del(5q) with a loss of more-mature cells that had acquired trisomy 8 as an additional marker.
Human MDS marrow cells in mouse blood, bone marrow, and spleen. The slide was mounted on Vectashield from Vector Laboratories (Burlingame, CA). A Nikon E600 microscope (Nikon, Melville, NY) was used, equipped with epifluorescence with appropriate double and triple band pass filters for visualization of FITC, Texas Red, Spectrum Orange, Spectrum Green, or DAPI (Nikon Plan Fluor 100×/1.30 oil immersion oil from Criterion Sciences, Riverdale, NJ). Cells were photographed using a Nikon FDX-35 camera with Kodak Professional Ektachrome P 1600 film. The film was developed by Prolab. The slide was scanned on a Nikon Super Coolscan 4000 with no corrections at 2000 dpi and adjusted for levels, color, and size in Photoshop (Adobe, San Jose, CA).
Human MDS marrow cells in mouse blood, bone marrow, and spleen. The slide was mounted on Vectashield from Vector Laboratories (Burlingame, CA). A Nikon E600 microscope (Nikon, Melville, NY) was used, equipped with epifluorescence with appropriate double and triple band pass filters for visualization of FITC, Texas Red, Spectrum Orange, Spectrum Green, or DAPI (Nikon Plan Fluor 100×/1.30 oil immersion oil from Criterion Sciences, Riverdale, NJ). Cells were photographed using a Nikon FDX-35 camera with Kodak Professional Ektachrome P 1600 film. The film was developed by Prolab. The slide was scanned on a Nikon Super Coolscan 4000 with no corrections at 2000 dpi and adjusted for levels, color, and size in Photoshop (Adobe, San Jose, CA).
Thus, clonal hematopoietic precursors from MDS marrow engraft in NOD/SCID-β2mnull mice that undergo transplantation via intramedullar injection of BMMCs along with human stromal cells. Our results are consistent with engraftment of early progenitor cells that sustain long-term hemopoiesis. Ongoing studies are determining the contribution of the various components of the model to transplantation success. In vivo findings derived from these experiments should improve our understanding of the biology and pathophysiology of MDSs.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal