Abstract
The development of immunodeficient mouse xenograft models has greatly facilitated the investigation of some human hematopoietic malignancies, but application of this approach to the myelodysplastic syndromes (MDSs) has proven difficult. We now show that cells from most MDS patients (including all subtypes) repopulate nonobese diabetic-severe combined immunodeficient (scid)/scid-β2 microglobulin null (NOD/SCID-β2m-/-) mice at least transiently and produce abnormal differentiation patterns in this model. Normal marrow transplants initially produce predominantly erythroid cells and later predominantly B-lymphoid cells in these mice, whereas most MDS samples produced predominantly granulopoietic cells. In 4 of 4 MDS cases, the regenerated cells showed the same clonal markers (trisomy 8, n = 3; and 5q-, n = 1) as the original sample and, in one instance, regenerated trisomy 8+ B-lymphoid as well as myeloid cells were identified. Interestingly, the enhanced growth of normal marrow obtained in NOD/SCID-β2m-/- mice engineered to produce human interleukin-3, granulocyte-macrophage colony-stimulating factor, and Steel factor was seen only with 1 of 7 MDS samples. These findings support the concept that human MDS originates in a transplantable multilineage hematopoietic stem cell whose genetic alteration may affect patterns of differentiation and responsiveness to hematopoietic growth factors. They also demonstrate the potential of this new murine xenotransplant model for future investigations of MDS. (Blood. 2004;103:4285-4293)
Introduction
The myelodysplastic syndromes (MDSs) comprise a heterogeneous group of clonal marrow disorders characterized by ineffective hematopoiesis leading to cytopenias and dysplastic changes in one or more of the myeloid lineages.1,2 Prognosis is generally poor and most patients die of complications secondary to their disease either before or after its progression to an acute leukemia. Typically the bone marrow (BM) of MDS patients is of normal cellularity or is hypercellular but often with a reduced number of primitive progenitors. These include cells able to generate colonies of terminally differentiating myeloid cells directly in semisolid media (colony-forming cells [CFCs])3,4 as well as their more primitive precursors referred to as long-term culture-initiating cells (LTC-ICs) because they generate CFCs for at least 5 weeks in long-term cultures containing stromal cell feeder layers.5-8 Progenitor cells from patients with MDS display impaired responses to various growth factors in vitro,9-12 although growth factor administration in vivo can improve the output of mature blood cells.13-15 BM cells from MDS patients, including the more primitive cells that express CD34, also typically contain an increased frequency of apoptotic cells.16-18 MDS cells exhibit a variety of cytogenetic abnormalities, and evidence of a multistep pathogenesis originating in a lymphomyeloid stem cell has been reported.19-24 However, the low frequency of primitive cells in patients' BM and blood has hampered further investigation of these difficult diseases and better models are needed.
Over the last 10 years, recognition of the ability of intravenously injected primitive normal and leukemic human hematopoietic cells to home to and proliferate in the BM of sublethally irradiated immunodeficient mice has offered new approaches to the study of these cells.25-34 However, engraftment of mice by cells from human MDS patients has rarely been obtained with available models.21,35-37 Recently, it was shown that enhanced engraftment of normal human hematopoietic cells could be obtained when the residual natural killer (NK) cell activity present in nonobese diabetic-severe combined immunodeficient (NOD/SCID) mice was eliminated, either by inactivation of the β2 microglobulin (β2m) gene38-40 or by injecting NOD/SCID mice with antibodies against NK cells.41,42 However, it was later shown that the higher numbers of human cells produced in such mice were due to a differential ability of human cells with varying repopulating potential to engraft different types of immunodeficient mice. Human short-term repopulating cells appear able to engraft NOD/SCID-β2m-/- mice much more efficiently than regular NOD/SCID mice, whereas more primitive long-term repopulating human cells engraft both types of mice equally well.40 Another strategy that has been used to amplify the numbers of cells produced by adult human cells transplanted into immunodeficient mice has been to inject the mice with human-specific growth factors26,43 or to engineer the mice to produce them endogenously.35,44-46
Here we investigated whether either of these approaches might enable the development of a more consistent in vivo xenograft model of human MDS. The results show that blood or BM samples from most MDS patients are able to engraft NOD/SCID-β2m-/- mice and regenerate progeny that display phenotypic as well as genotypic abnormalities typical of the original neoplastic clone. However, in contrast to transplants of normal human BM, the levels of engraftment attained in mice that received transplants of some MDS samples appeared insensitive to transgenically produced human interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and Steel factor (SF).
Patients, materials, and methods
Patients and preparation of human cells
Blood and BM cells were obtained from 11 MDS patients undergoing BM and blood examination for diagnostic purposes in Vancouver or Patras prior to the initiation of any treatment and included all disease subtypes as defined by the French-American-British (FAB) classification scheme (Table 1). Normal adult BM cells were from the Northwest Tissue Center (Seattle, WA). All human samples were obtained with informed consent and handled according to procedures approved by the University of British Columbia and Patras Medical School. Low-density (< 1.077 g/mL) cells were isolated by centrifugation on Ficoll-Hypaque (Pharmacia Biotech, Baie d-Urfe, QC, Canada). The normal BM cells were also immunomagnetically depleted of lineage (lin) marker-positive cells using a column according to the manufacturer's instructions (StemCell Technologies, Vancouver, BC, Canada). The depletion cocktail consisted of antibodies to CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, and glycophorin A (gly A). Cells from 2 of the MDS samples were similarly depleted of T cells using antibodies to CD3. All samples were then cryopreserved in 90% fetal calf serum (FCS; StemCell Technologies) plus 10% dimethylsulfoxide (Sigma, St Louis, MO) and stored at -135°C prior to use. Frozen cells were thawed quickly at 37°C and slowly diluted with Iscove modified Dulbecco medium (IMDM) containing 20% FCS and 100 μg/mL deoxyribonuclease I (Sigma). Cells were then washed twice in IMDM with 20% FCS, counted, and the cell viability was determined by trypan blue exclusion. An aliquot from each sample was used for phenotype, cytogenetic, and progenitor assays and the remainder was suspended in a small volume of Hanks balanced salt solution containing 2% FCS (HBSS 2%; StemCell Technologies) for injection into mice.
Clinical characteristics of the MDS patients
. | Age, y/sex . | . | WBC count, × 109/L . | BM cytogenetics [% abnormal at diagnosis] . | Source of injected cells . | Progenitors/2 × 106 cells . | . | No. of human cells injected per mouse, × 106 . | |
|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | . | FAB . | . | . | . | CFCs . | LTC-ICs* . | . | |
| 1 | 67/M | RA | 4 | 47,XY,+8[56];42-47,XY,+8,18q−, −21[42] | BM | 1200 | 15 | 10-17 | |
| 2 | 77/F | RARS | 23 | 46,XX | BM | 30 | ND | 4 | |
| 3† | 85/F | CMML | 196 | 46,XX | PB | 730 | 4 | 12, T depleted | |
| 4 | 65/M | CMML | 16 | 46,XY | BM | 700 | 4 | 10 | |
| 5 | 67/M | CMML | 248 | 46,XY | BM | 1300 | ND | 13 | |
| 6 | 71/M | CMML | 5 | 45,X,-Y[100] | BM | ND | ND | 10 | |
| 7† | 72/M | RAEB | 2 | 46,XY | BM | 520 | 7 | 15, T depleted | |
| 8 | 71/M | RAEB | 7 | 46,XY,del(5q)(q13q33)[100] | PB | 580 | 92 | 4 | |
| 9 | 73/M | RAEBT | 3 | 47,XY,+8[15] | BM | ND | ND | 4 | |
| 10† | 63/M | RAEBT | 10 | 46,XY | BM | 590 | 36 | 15 | |
| 11 | 37/F | RAEBT | 5 | 47,XX,+8[48] | BM | 610 | 5 | 7-10 | |
| Normal cells | lin-BM | 400‡ | 150‡ | 0.4 | |||||
. | Age, y/sex . | . | WBC count, × 109/L . | BM cytogenetics [% abnormal at diagnosis] . | Source of injected cells . | Progenitors/2 × 106 cells . | . | No. of human cells injected per mouse, × 106 . | |
|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | . | FAB . | . | . | . | CFCs . | LTC-ICs* . | . | |
| 1 | 67/M | RA | 4 | 47,XY,+8[56];42-47,XY,+8,18q−, −21[42] | BM | 1200 | 15 | 10-17 | |
| 2 | 77/F | RARS | 23 | 46,XX | BM | 30 | ND | 4 | |
| 3† | 85/F | CMML | 196 | 46,XX | PB | 730 | 4 | 12, T depleted | |
| 4 | 65/M | CMML | 16 | 46,XY | BM | 700 | 4 | 10 | |
| 5 | 67/M | CMML | 248 | 46,XY | BM | 1300 | ND | 13 | |
| 6 | 71/M | CMML | 5 | 45,X,-Y[100] | BM | ND | ND | 10 | |
| 7† | 72/M | RAEB | 2 | 46,XY | BM | 520 | 7 | 15, T depleted | |
| 8 | 71/M | RAEB | 7 | 46,XY,del(5q)(q13q33)[100] | PB | 580 | 92 | 4 | |
| 9 | 73/M | RAEBT | 3 | 47,XY,+8[15] | BM | ND | ND | 4 | |
| 10† | 63/M | RAEBT | 10 | 46,XY | BM | 590 | 36 | 15 | |
| 11 | 37/F | RAEBT | 5 | 47,XX,+8[48] | BM | 610 | 5 | 7-10 | |
| Normal cells | lin-BM | 400‡ | 150‡ | 0.4 | |||||
FAB indicates French-American-British (classification); WBC, white blood cell; RA, refractory anemia; RARS, refractory anemia with ringed sideroblasts; ND, not done; CMML, chronic myelomonocytic leukemia; RAEB, refractory anemia with excess blasts; and RAEBT, refractory anemia with excess blasts in transformation.
Values shown are the number of CFCs produced by 2 × 106 low-density BM or PB cells (as indicated) after 5 weeks in LTCs.
Separate aliquots transplanted on 2 or 3 different occasions.
Values shown are the numbers of CFCs measured directly (CFCs) and after 6 weeks in LTCs (LTC-ICs) per 2 × 103 lin− normal BM cells.
Mice and transplantation procedure
Mice were bred and maintained under microisolation conditions in our animal facility. NOD/SCID-β2m-/- mice were expanded from breeding pairs originally obtained from Dr L Schultz (Jackson Laboratories, Bar Harbor, ME). Transgenic NOD/SCID-β2m-/- mice expressing human IL-3 (1 ng/mL), GM-CSF (> 5 ng/mL), and SF (1.6 ng/mL) were generated by crossing a NOD/SCID mouse producing all 3 of these growth factors45,46 twice with NOD/SCID-β2m-/- mice and then selecting progeny with a β2m-/--3/GM/SF genotype (absence of cell surface β2m and presence of human IL-3, GM-CSF, and SF in the serum). Just prior to use as recipients, each transgenic NOD/SCID-β2m-/--3/GM/SF mouse was bled and the presence of expected levels of the 3 human growth factors in the serum was confirmed by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (R&D Systems, Minneapolis, MN).
Mice that were to receive transplants were irradiated at 8 to 10 weeks of age with 350 cGy of 137Cs γ rays and injected intravenously within 24 hours with human cells (either 4 × 105 lin- normal BM cells plus 106 irradiated [15 Gy] normal human BM cells as carriers or 4 × 106 to 17 × 106 MDS cells per mouse; Table 1). Thereafter the mice received acidified water containing 100 mg/L ciprofloxacin (Bayer, Leverkusen, Germany).40
Analysis of human cells in murine tissues
At various times after transplantation, a sample of BM cells was removed from alternate femurs of anesthetized mice using a syringe and a 22-gauge needle,47 and the cells were then suspended in HBSS 2%. At the end of the experiment, mice were killed and the entire BM contents of both femurs and tibiae were collected in HBSS 2% using a syringe and a 21-gauge needle. Spleen cells were obtained by applying gentle pressure to the splenic capsule with curved scissors and flushing the cells into HBSS 2%.
For phenotype analyses, the red blood cells (RBCs) were lysed with 0.8% ammonium chloride (StemCell Technologies), washed twice, and then suspended in 0.2 to 0.4 mL HBSS containing 5% human serum and an antimouse immunoglobulin G (IgG) receptor antibody (2.4G2; American Type Culture Collection, Rockville, MD) to minimize nonspecific antibody binding. Cells were incubated on ice for 10 minutes and then separate cell aliquots were incubated for another 30 minutes with a panel of monoclonal antibodies against the following human markers: CD34 (8G12), CD71 (OKT9), and gly A (10F7MN; kindly provided by Dr Peter Lansdorp, Terry Fox Laboratory); CD19, CD20, and CD33 (Becton Dickinson, San Jose, CA); and CD45, CD15, CD66b, and CD41a (Pharmingen, Canada Inc, Mississauga, ON, Canada). A separate cell aliquot was stained with irrelevant isotype-matched monoclonal antibodies labeled with the same fluorochromes to establish the levels of nonspecific immunofluorescence. Cells were washed once with HBSS and then once again with the same medium containing 2 μg/mL propidium iodide (PI; Sigma) to eliminate dead (PI+) cells from the analyses. For each analysis, a minimum of 2 × 104 viable (PI-) events were collected. Gates were set to exclude at least 99.9% of the cells labeled with isotype control antibodies. Mice were considered to be engrafted when at least 0.1% of the cells present stained positively for human CD45/71.27 When sufficient human CD45/71+ cells were present, these were isolated using a FACStar+ (Becton Dickinson) equipped with neon and argon lasers and then plated in CFC assays or used for fluorescent in situ hybridization (FISH) or morphologic analyses.
Progenitor cell assays
Patient starting cells or fluorescence-activated cell sorter (FACS)-sorted human CD45/71+ cells were assessed for CFCs and LTC-ICs as previously described.48,49 Briefly, cells were plated at 1 × 105/mL to 2 × 105/mL in 0.9% methylcellulose medium (MethoCult; StemCell Technologies) supplemented with 3 U/mL erythropoietin (Epo; StemCell Technologies), 50 ng/mL SF (Amgen, Thousand Oaks, CA), and 20 ng/mL each of IL-3 and GM-CSF (Novartis, Basel, Switzerland), G-CSF (StemCell Technologies), and IL-6 (Cangene, Mississauga, ON, Canada) and colonies were scored after 14 days at 37°C. For LTC-IC assays, low-density cells were suspended at 2 × 106 cells/mL in LTC medium (MyeloCult; StemCell Technologies) supplemented with freshly dissolved sodium hydrocortisone sodium hemisuccinate (Sigma) and seeded onto pre-established feeder layers of irradiated (80 Gy) murine fibroblast cell lines genetically engineered to produce human SF, G-CSF, and IL-3. LTCs were then incubated at 37°C for 5 or 6 weeks with weekly half-medium changes at the end of which both the adherent and nonadherent cells were harvested, pooled, and assayed for CFCs. In some cases (ie, patients with a known chromosomal abnormality), individual initial or LTC-IC-derived colonies were picked from synchronized methylcellulose cultures, placed into hypotonic KCl solution (0.075 M), fixed in methanol-acetic acid (3:1), and then either G-banded for cytogenetic evaluation or stored for FISH analysis.49-51
FISH
FISH analysis was performed on patients' starting cells (at least 500 cells/sample), individually plucked colonies (at least 5 cells per colony), and human CD45/71+ cells obtained from the mice and deposited directly onto microscopic slides using the FACS (at least 500 cells/sample). Cells were first fixed in methanol-acetic acid (3:1) and then hybridized overnight for 16 hours with denatured DNA probes specific for centromeric repeat sequences on chromosome 8 (clone D8Z2; American Type Culture Collection) or 5q31 (amplified by inter-Alu polymerase chain reaction [PCR]52 from a bacteria artificial chromosome clone [b-IL9]).27,49,51 Both probes were kindly provided by Dr Donna Hogge (Terry Fox Laboratory) and labeled with digoxigenin (DIG; digoxigenin-11-deoxyuridine triphosphate [dUTP]; Boehringer-Mannheim, Laval, QC, Canada) using a nick translation protocol. DIG-labeled cells were detected by staining with sheep anti-DIG-fluorescein isothiocyanate (FITC) antibody (Boehringer-Mannheim) and rabbit antisheep FITC antibody (Vector Labs, Burlington, ON, Canada). Slides were counterstained with 0.5 μg/mL PI in antifade (Vectashield; Vector Labs) and viewed on a Zeiss Axioplan fluorescent microscope (Thornwood, NY). Trisomy 8 was indicated by nuclei containing 3 fluorescent signals and del(5)(q13q33) by nuclei containing a single signal. The frequency of false-positive cells measured for these procedures from an assessment of 500 normal BM cells processed in the same way was 0.4% for trisomy 8 and 2.3% for 5q-.
Morphologic analyses
FACS-sorted human cells were collected in IMDM supplemented with 2% FCS and then used to make cytospin preparations. Slides were stained using Wright-Giemsa and the cells were analyzed using bright-field microscopy.
Calculations
The number of human cells present is shown as the total number per mouse. These values were calculated by multiplying the percentage of viable human cells determined in BM aspirate samples obtained at defined times after transplantation by separately determined estimates of the total BM cellularity at these times, assuming 2 femurs plus 2 tibiae contain 25% of the total BM mass.53 The latter values were obtained by averaging the data from a large number of irradiated NOD/SCID-β2m-/- and NOD/SCID-β2m-/--3/GM/SF mice killed and analyzed at varying times after being injected with 4 × 105 normal human lin- BM cells. All results are expressed as mean ± SEM. The significance of differences between groups was determined using a 2-tailed Student t test.
Results
Enhanced growth of normal human BM cells in NOD/SCID-β2m-/- mice engineered to produce IL-3, GM-CSF, and SF
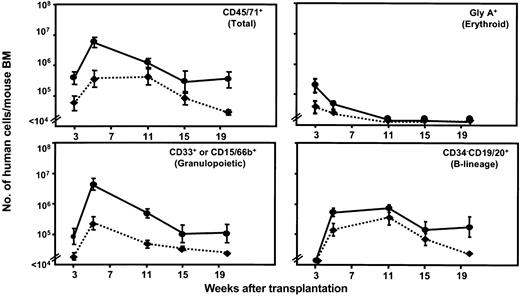
A first series of experiments was undertaken to compare the kinetics of production of different types of normal human hematopoietic cells in regular NOD/SCID-β2m-/- mice and NOD/SCID-β2m-/- mice that we had engineered to produce human IL-3, GM-CSF, and SF by crossing NOD/SCID-3/GM/SF mice45,46 with congenic NOD/SCID-β2m-/- mice. Parallel groups of NOD/SCID-β2m-/- and NOD/SCID-β2m-/--3/GM/SF mice were irradiated and received transplants of 4 × 105 normal adult human lin- BM cells and then serial BM aspirates were obtained from 3 to 20 weeks later. The harvested cells were then stained with antibodies to detect human erythroid (gly A), megakaryocytic (CD41+), granulopoietic (CD33+ or CD15/66b+), and B-lymphoid (CD34-CD19/20+) cells as well as progenitors (CD34+ cells) and total hematopoietic cells (CD45/71+). The proportion of human cells of each type was converted to an absolute (per mouse) value based on independent measurements of the total cellularity of the femurs of such mice over this time period. The results obtained are shown in Figure 1.
Enhancement of normal human hematopoiesis in transgenic NOD/SCID-β2m-/- mice producing human IL-3, GM-CSF, and SF. Each panel shows the time course of changes in the number of different types of human cells present in the BM of NOD/SCID-β2m-/--3/GM/SF (solid lines) and NOD/SCID-β2m-/- mice (dotted lines) from 3 to 20 weeks after being injected with 4 × 105 normal human lin- BM cells. Values are the mean ± SEM of results obtained from individually assessed mice (6 mice/group) and were calculated by multiplying the average proportion of cells with the phenotype shown within aspirated samples by the total number of cells present in the BM of similar mice determined from a large data set from other experiments.
Enhancement of normal human hematopoiesis in transgenic NOD/SCID-β2m-/- mice producing human IL-3, GM-CSF, and SF. Each panel shows the time course of changes in the number of different types of human cells present in the BM of NOD/SCID-β2m-/--3/GM/SF (solid lines) and NOD/SCID-β2m-/- mice (dotted lines) from 3 to 20 weeks after being injected with 4 × 105 normal human lin- BM cells. Values are the mean ± SEM of results obtained from individually assessed mice (6 mice/group) and were calculated by multiplying the average proportion of cells with the phenotype shown within aspirated samples by the total number of cells present in the BM of similar mice determined from a large data set from other experiments.
As predicted from previous studies of the effect of injections of human growth factors in NOD/SCID mice that received transplants,26 the level of human (CD45/71+) cells was consistently elevated several-fold in the transgenic NOD/SCID-β2m-/--3/GM/SF mice by comparison to regular NOD/SCID-β2m-/- hosts. This increase affected all lineages in a time-dependent fashion. The production of human granulopoietic cells was most strongly enhanced, particularly early after transplantation (19-fold at 5 weeks and 10-fold at 11 weeks; P < .03). The production of more primitive (CD34+) cells was also significantly increased between 3 and 5 weeks after transplantation (P < .05). Enhancement of the early wave of human erythropoiesis that peaks at 3 weeks after transplantation40 was also seen in the NOD/SCID-β2m-/--3/GM/SF mice, as was the later output of B-lineage cells seen between 5 and 20 weeks after transplantation, although neither of these latter effects achieved statistical significance at any given time point (P > .05).
Cells from MDS patients consistently repopulate NOD/SCID-β2m-/- mice but generally do not produce more progeny in NOD/SCID-β2m-/--3/GM/SF recipients
Cells from 11 newly diagnosed MDS patients including all conventionally defined subtypes (Table 1) were then transplanted into both NOD/SCID-β2m-/--3/GM/SF and regular NOD/SCID-β2m-/- mice and the production of human cells again followed in serially aspirated BM cells. Overall, 9 of the MDS samples produced detectable levels of human hematopoietic cells (of any type, ie, CD45/71+) in one or both types of recipients at some time point. The 2 MDS samples that did not engraft were both from chronic myelomonocytic leukemia (CMML) patients (4 and 6). However, this was not a feature of CMML, as the 2 other CMML samples tested (from patients 3 and 5) did regenerate detectable progeny in the mice. For 3 samples (including one of the positive CMML samples), different aliquots were thawed at different times and transplanted (Table 1) and consistent results were obtained.
For 7 of the MDS samples, a direct comparison of the repopulation obtained in the 2 types of host could be made (Tables 2, 3). For 3 of these (patients 3, 7, and 9), the number of human cells produced in the NOD/SCID-β2m-/--3/GM/SF mice was either lower or not different than in the regular NOD/SCID-β2m-/- mice. For another 3 (patients 1, 8, and 11), human cells were not detected in any of the NOD/SCID-β2m-/- mice that received a transplant (one recipient per patient), whereas low levels of human cells were seen in some of the NOD/SCID-β2m-/--3/GM/SF recipients. Only in the case of patient 10 was there an obvious marked increase in the output of human cells in the BM of the NOD/SCID-β2m-/--3/GM/SF mice, the greatest difference being seen after 7 weeks (70-fold; P = .01).
Comparison of the percentages of human cells in the BM of NOD/SCID-β2m−/− and NOD/SCID-β2m−/−-3/GM/SF mice at varying times after transplantation
. | . | No. of positive mice/no. of mice injected . | Percentage of human CD45/71+ cells in BM aspirates (range delimited by ± SEM)* . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Recipient genotype . | . | 3wk . | 5-7wk . | 8-12 wk . | 15-23 wk . | |||
| 1 | β2m−/−-3/GM/SF | 1/2 | 0.3 | 0.1 | 6.8, 5.1† | 7, 1.7† | |||
| 1 | β2m−/− | 0/2 | — | — | — | — | |||
| 3 | β2m−/−-3/GM/SF | 2/2 | 1.2, 0.2 | ≤ 0.1, 0.1 | — | — | |||
| 3 | β2m−/− | 5/7 | 1.0 (0.4-3.0) | 0.05 (0.03-0.07) | — | — | |||
| 7 | β2m−/−-3/GM/SF | 3/6 | 39, 0.4, 1.9 | ≤ 0.1, 0.1, ≤ 0.1 | — | — | |||
| 7 | β2m−/− | 7/7 | 1.1 (0.4-3.2) | 0.1 (0.04-0.3) | — | — | |||
| 8 | β2m−/−-3/GM/SF | 1/1 | — | — | 0.1 | 0.1 | |||
| 8 | β2m−/− | 0/1 | — | — | — | — | |||
| 9 | β2m−/−-3/GM/SF | 1/1 | 2.6 | 4.5 | — | — | |||
| 9 | β2m−/− | 1/1 | 43 | 1.5 | — | — | |||
| 10 | β2m−/−-3/GM/SF | 2/2 | 13, 1.0‡ | 48, 9.5‡ | 19, 2.5‡ | 20, 1.9‡ | |||
| 10 | β2m−/− | 7/7 | 0.4 (0.3-0.7) | 0.3 (0.2-0.7) | 0.2 (0.1-0.4) | 0.4 (0.2-0.8) | |||
| 11 | β2m−/−-3/GM/SF | 1/2 | 5.7 | 0.6 | ≤ 0.1 | ≤ 0.1-0.13 | |||
| 11 | β2m−/− | 0/2 | — | — | — | — | |||
| Normal lin− BM | β2m−/−-3/GM/SF | 6/6 | 1.7 (1.0-2.9) | 8.1 (5.7-12)‡ | 1.7 (1.5-2.4) | 0.4 (0.1-0.9) | |||
| Normal lin− BM | β2m−/− | 5/6 | 0.17 (0.1-0.4) | 0.5 (0.3-1.0) | 0.6 (0.3-1.2) | 0.1 (0.06-0.2) | |||
. | . | No. of positive mice/no. of mice injected . | Percentage of human CD45/71+ cells in BM aspirates (range delimited by ± SEM)* . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Recipient genotype . | . | 3wk . | 5-7wk . | 8-12 wk . | 15-23 wk . | |||
| 1 | β2m−/−-3/GM/SF | 1/2 | 0.3 | 0.1 | 6.8, 5.1† | 7, 1.7† | |||
| 1 | β2m−/− | 0/2 | — | — | — | — | |||
| 3 | β2m−/−-3/GM/SF | 2/2 | 1.2, 0.2 | ≤ 0.1, 0.1 | — | — | |||
| 3 | β2m−/− | 5/7 | 1.0 (0.4-3.0) | 0.05 (0.03-0.07) | — | — | |||
| 7 | β2m−/−-3/GM/SF | 3/6 | 39, 0.4, 1.9 | ≤ 0.1, 0.1, ≤ 0.1 | — | — | |||
| 7 | β2m−/− | 7/7 | 1.1 (0.4-3.2) | 0.1 (0.04-0.3) | — | — | |||
| 8 | β2m−/−-3/GM/SF | 1/1 | — | — | 0.1 | 0.1 | |||
| 8 | β2m−/− | 0/1 | — | — | — | — | |||
| 9 | β2m−/−-3/GM/SF | 1/1 | 2.6 | 4.5 | — | — | |||
| 9 | β2m−/− | 1/1 | 43 | 1.5 | — | — | |||
| 10 | β2m−/−-3/GM/SF | 2/2 | 13, 1.0‡ | 48, 9.5‡ | 19, 2.5‡ | 20, 1.9‡ | |||
| 10 | β2m−/− | 7/7 | 0.4 (0.3-0.7) | 0.3 (0.2-0.7) | 0.2 (0.1-0.4) | 0.4 (0.2-0.8) | |||
| 11 | β2m−/−-3/GM/SF | 1/2 | 5.7 | 0.6 | ≤ 0.1 | ≤ 0.1-0.13 | |||
| 11 | β2m−/− | 0/2 | — | — | — | — | |||
| Normal lin− BM | β2m−/−-3/GM/SF | 6/6 | 1.7 (1.0-2.9) | 8.1 (5.7-12)‡ | 1.7 (1.5-2.4) | 0.4 (0.1-0.9) | |||
| Normal lin− BM | β2m−/− | 5/6 | 0.17 (0.1-0.4) | 0.5 (0.3-1.0) | 0.6 (0.3-1.2) | 0.1 (0.06-0.2) | |||
β2m−/−-3/GM/SF indicates NOD/SCID-β2m−/−-3/GM/SF ; β2m−/−, NOD/SCID-β2m−/−; and —, no data available.
Values shown are the percentages of human CD45/71+ cells in BM aspirates of individually analyzed mice or the geometric means when data for at least 3 mice were available.
Data are from a single mouse assessed at different times in the interval shown (the second mouse died 8 weeks after transplantation).
Values in the 2 different types of recipient are significantly different P <.05.
Comparison of the absolute numbers of human cells in the BM of NOD/SCID-β2m−/− and NOD/SCID-β2m−/−-3/GM/SF mice at varying times after transplantation
. | . | No. of positive mice/no. of mice injected . | Percentage of human CD45/71+ cells in BM aspirates (range delimited by ± SEM)* . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Recipient genotype . | . | 3 wk . | 5-7 wk . | 8-12 wk . | 15-23 wk . | |||
| 1 | β2m−/−-3/GM/SF | 1/2 | 0.47 | 0.75 | 32, 15† | 42, 16† | |||
| 1 | β2m−/− | 0/2 | — | — | — | — | |||
| 3 | β2m−/−-3/GM/SF | 2/2 | 4.8, 0.6 | 0.25, 0.5 | — | — | |||
| 3 | β2m−/− | 5/7 | 3.3 (1.2-9.2) | 0.4 (0.3-0.5) | — | — | |||
| 7 | β2m−/−-3/GM/SF | 3/6 | 149, 1.4, 4 | 0.25, 0.3, 0.25 | — | — | |||
| 7 | β2m−/− | 7/7 | 3 (1-11) | 1 (0.5-2.3) | — | — | |||
| 8 | β2m−/−-3/GM/SF | 1/1 | — | — | 0.4 | 0.8 | |||
| 8 | β2m−/− | 0/1 | — | — | — | — | |||
| 9 | β2m−/−-3/GM/SF | 1/1 | 3 | 36 | — | — | |||
| 9 | β2m−/− | 1/1 | 85 | 10 | — | — | |||
| 10 | β2m−/−-3/GM/SF | 2/2 | 24, 2‡ | 387, 77‡ | 134, 16‡ | 138, 12‡ | |||
| 10 | β2m−/− | 7/7 | 1 (0.7-1.5) | 2.6 (1.6-4.4) | 1.8 (1.1-2.9) | 2.8 (1.5-5.2) | |||
| 11 | β2m−/−-3/GM/SF | 1/2 | 8 | 4 | 0.28 | 0.4-0.8 | |||
| 11 | β2m−/− | 0/2 | — | — | — | — | |||
| Normal lin− BM | β2m−/−-3/GM/SF | 6/6 | 3.7 (2.3-6.1) | 56 (37-83)‡ | 12 (8.5-16) | 2.8 (1.2-6.4) | |||
| Normal lin− BM | β2m−/− | 5/6 | 0.6 (0.3-1) | 3.6 (1.9-6.9) | 4.1 (2.2-7.6) | 0.8 (0.5-1.4) | |||
. | . | No. of positive mice/no. of mice injected . | Percentage of human CD45/71+ cells in BM aspirates (range delimited by ± SEM)* . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Recipient genotype . | . | 3 wk . | 5-7 wk . | 8-12 wk . | 15-23 wk . | |||
| 1 | β2m−/−-3/GM/SF | 1/2 | 0.47 | 0.75 | 32, 15† | 42, 16† | |||
| 1 | β2m−/− | 0/2 | — | — | — | — | |||
| 3 | β2m−/−-3/GM/SF | 2/2 | 4.8, 0.6 | 0.25, 0.5 | — | — | |||
| 3 | β2m−/− | 5/7 | 3.3 (1.2-9.2) | 0.4 (0.3-0.5) | — | — | |||
| 7 | β2m−/−-3/GM/SF | 3/6 | 149, 1.4, 4 | 0.25, 0.3, 0.25 | — | — | |||
| 7 | β2m−/− | 7/7 | 3 (1-11) | 1 (0.5-2.3) | — | — | |||
| 8 | β2m−/−-3/GM/SF | 1/1 | — | — | 0.4 | 0.8 | |||
| 8 | β2m−/− | 0/1 | — | — | — | — | |||
| 9 | β2m−/−-3/GM/SF | 1/1 | 3 | 36 | — | — | |||
| 9 | β2m−/− | 1/1 | 85 | 10 | — | — | |||
| 10 | β2m−/−-3/GM/SF | 2/2 | 24, 2‡ | 387, 77‡ | 134, 16‡ | 138, 12‡ | |||
| 10 | β2m−/− | 7/7 | 1 (0.7-1.5) | 2.6 (1.6-4.4) | 1.8 (1.1-2.9) | 2.8 (1.5-5.2) | |||
| 11 | β2m−/−-3/GM/SF | 1/2 | 8 | 4 | 0.28 | 0.4-0.8 | |||
| 11 | β2m−/− | 0/2 | — | — | — | — | |||
| Normal lin− BM | β2m−/−-3/GM/SF | 6/6 | 3.7 (2.3-6.1) | 56 (37-83)‡ | 12 (8.5-16) | 2.8 (1.2-6.4) | |||
| Normal lin− BM | β2m−/− | 5/6 | 0.6 (0.3-1) | 3.6 (1.9-6.9) | 4.1 (2.2-7.6) | 0.8 (0.5-1.4) | |||
β2m−/−-3/GM/SF indicates NOD/SCID-β2m−/−-3/GM/SF ; β2m−/−, NOD/SCID-β2m−/−; and —, no data available.
Values shown are the derived total numbers of human CD45/71+ cells (×105) assuming that 2 femurs and 2 tibiae represent 25% of all mouse BM cells and that the total cellularity of these 4 long bones was as measured in a large set of similarly treated mice.
Data are from a single mouse assessed at different times in the interval shown (the second mouse died 8 weeks after transplantation).
Values in the 2 different types of recipient are significantly different P <. 05.
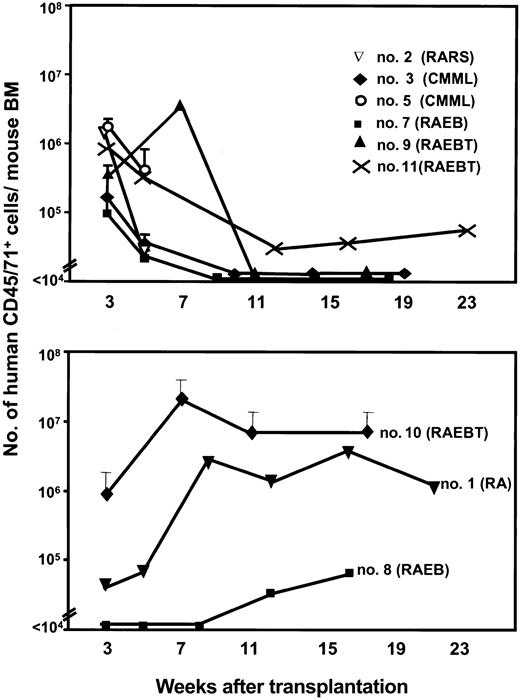
Time course studies revealed 2 general patterns of repopulation in the BM of mice that received transplants of MDS cells, regardless of the type of recipient. Figure 2 shows the results for 7 of the 9 samples transplanted into NOD/SCID-β2m-/--3/GM/SF mice where repopulation levels were assessed at 2 or more different time points and for 2 patients (patients 2 and 5) in NOD/SCID-β2m-/- hosts where such data were available only in these mice. The most common pattern was characterized by an early but transient appearance of human hematopoietic cells 3 weeks after transplantation, which then disappeared or decreased to a much lower level. Six of the MDS patients' samples showed this pattern (patients 2, 3, 5, 7, 9, and 11; Figure 2 top). The other pattern was characterized by a slower appearance of human cells that then increased and were sustained throughout the subsequent 3 to 4 months of follow-up. The other 3 of the MDS patients' samples showed this second pattern (1, 8, and 10; Figure 2 bottom). No association between the FAB disease subtype of the sample transplanted and the pattern of repopulation obtained was noted.
Two patterns of engraftment of MDS cells in NOD/SCID-β2m-/- (and NOD/SCID-β2m-/--3/GM/SF) mice. Values shown are the mean ± SEM of total human hematopoietic (CD45/71+) cells in mice assessed individually at at least 2 time points (2 to 6 mice/patient sample for patients 3, 5, 7, and 10; 1 mouse/patient for patients 1, 2, 8, 9, and 11). Data are for NOD/SCID-β2m-/--3/GM/SF mice except for patients 2 and 5, which are from NOD/SCID-β2m-/- recipients. Data were generated as described in Figure 1.
Two patterns of engraftment of MDS cells in NOD/SCID-β2m-/- (and NOD/SCID-β2m-/--3/GM/SF) mice. Values shown are the mean ± SEM of total human hematopoietic (CD45/71+) cells in mice assessed individually at at least 2 time points (2 to 6 mice/patient sample for patients 3, 5, 7, and 10; 1 mouse/patient for patients 1, 2, 8, 9, and 11). Data are for NOD/SCID-β2m-/--3/GM/SF mice except for patients 2 and 5, which are from NOD/SCID-β2m-/- recipients. Data were generated as described in Figure 1.
Human CD45/71+ cells were detected at a similar frequency in the spleen and BM in the single mouse that received a transplant of cells from patient 1 at the time the experiment was terminated 21 weeks after transplantation. At this time, low numbers of human cells were also detected in the blood of this mouse (data not shown). Human CD45/71+ cells were likewise detected at a similar frequency in the spleen and BM of several mice that received transplants of cells from patient 10 when these were examined 17 weeks after transplantation. At this time, human CFCs were also present in the BM (38/105 human CD45/71+ cells).
Abnormal lineage distributions in mice repopulated with cells from most MDS patients
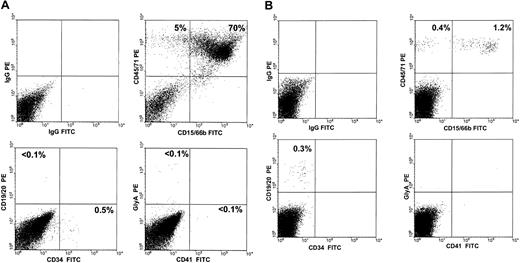
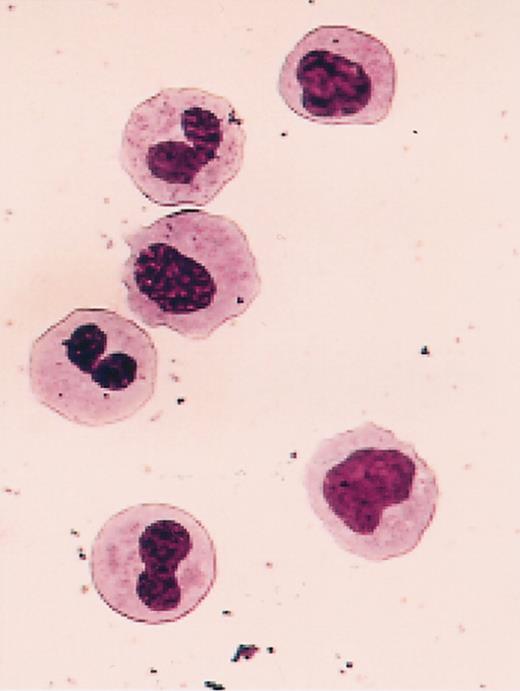
Cells obtained from the BM of mice repopulated with cells from MDS patients were also stained with antibodies to specific human hematopoietic cell types, and the proportions of human cells belonging to the different lineages assessed are summarized in Table 4 together with the corresponding distributions for the normal BM transplants. Overall, there was, again, no consistent difference between the values seen in the 2 types of recipients of MDS cells. In the first 3 weeks after transplantation, samples from 3 of the MDS patients (patients 2, 5, and 10) produced a substantial population of human erythroid cells (gly A+ cells; ranging from 38% to 56% of the total human population present), thus mimicking the early pattern seen in these mice when they received transplants of normal human BM cells (57% ± 5% and 61% ± 14% erythroid cells). However, for all 8 of the MDS samples examined 3 weeks after transplantation, the proportion of granulopoietic (CD15/66b+ or CD33+) cells at this time was already elevated relative to normal BM cell transplants and, in 6 cases (patients 1, 3, 7, 9, 10, and 11), these reached values of at least 80% in one or both types of recipient (see Table 4 and a representative FACS profile in Figure 3A). For recipients of cells from patients 3 and 7, the presence of human cells in the late stages of granulocyte maturation was confirmed in Wright-Giemsa-stained cytospin preparations of FACS-sorted CD45/71+ cells (Figure 4).
Distribution of human phenotypes in the BM of mice that received transplants
. | . | Wk 3 . | . | . | Wk 5-7 . | . | . | Wk 8-12 . | . | . | Wk 15-23 . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Recipient genotype . | %E . | %GM . | %L . | %E . | %GM . | %L . | %E . | %GM . | %L . | %E . | %GM . | %L . | ||||||||
| 1 | β2m−/−-3/GM/SF | 0 | 96 | 0 | 0 | 94 | 0 | 0 | 93 | 0 | 0 | 94 | 0 | ||||||||
| 3 | β2m−/−-3/GM/SF | 0 | 91 ± 3 | 0 | — | — | — | — | — | — | — | — | — | ||||||||
| 7 | β2m−/−-3/GM/SF | 0 | 82 ± 10 | 0 | — | — | — | — | — | — | — | — | — | ||||||||
| 8 | β2m−/−-3/GM/SF | — | — | — | — | — | — | — | — | — | — | 90 | — | ||||||||
| 9 | β2m−/−-3/GM/SF | 0 | 92 | 0 | 0 | 95 | 0 | ||||||||||||||
| 10 | β2m−/−-3/GM/SF | 38 ± 33 | 52 ± 23 | 6 ± 5 | 0 | 92 ± 2 | 3 ± 1 | 0 | 75 ± 1 | 18 ± 2 | 0 | 70 ± 10 | 13 ± 7 | ||||||||
| 11 | β2m−/−-3/GM/SF | 3 | 92 | 0 | 0 | 92 | 0 | 0 | 92 | 0 | 0 | 92 | 0 | ||||||||
| Normal lin− BM | 61 ± 14 | 30 ± 9 | 0 | 1 ± 0.3 | 79 ± 3 | 10 ± 2 | 0 | 41 ± 2 | 62 ± 5 | 0 | 39 ± 16 | 52 ± 21 | |||||||||
| 2 | β2m−/− | 56 | 31 | 0 | — | — | — | —* | — | — | — | — | — | ||||||||
| 3 | β2m−/− | 0 | 96 ± 1 | 0 | 0 | 90 ± 4 | 0 | — | — | — | — | — | — | ||||||||
| 5 | β2m−/− | 52 ± 11 | 45 ± 3 | 0 | 0 | 91 ± 8 | 0 | —* | — | — | — | — | — | ||||||||
| 7 | β2m−/− | 0 | 86 ± 6 | 0 | 0 | 98 ± 1 | 0 | — | — | — | — | — | — | ||||||||
| 9 | β2m−/− | 4 | 90 | 0 | 0 | 78 | 20 | — | — | — | — | — | — | ||||||||
| 10 | β2m−/− | 0 | 80 ± 3 | 10 ± 3 | 0 | 60 ± 10 | 26 ± 9 | 0 | 30 ± 11 | 44 ± 14 | 0 | 11 ± 3 | 86 ± 10 | ||||||||
| Normal lin− BM | β2m−/− | 57 ± 5 | 20 ± 3 | 0 | 3 ± 1 | 57 ± 7 | 33 ± 7 | 0 | 9 ± 3 | 86 ± 4 | 0 | 27 ± 10 | 76 ± 11 | ||||||||
. | . | Wk 3 . | . | . | Wk 5-7 . | . | . | Wk 8-12 . | . | . | Wk 15-23 . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Recipient genotype . | %E . | %GM . | %L . | %E . | %GM . | %L . | %E . | %GM . | %L . | %E . | %GM . | %L . | ||||||||
| 1 | β2m−/−-3/GM/SF | 0 | 96 | 0 | 0 | 94 | 0 | 0 | 93 | 0 | 0 | 94 | 0 | ||||||||
| 3 | β2m−/−-3/GM/SF | 0 | 91 ± 3 | 0 | — | — | — | — | — | — | — | — | — | ||||||||
| 7 | β2m−/−-3/GM/SF | 0 | 82 ± 10 | 0 | — | — | — | — | — | — | — | — | — | ||||||||
| 8 | β2m−/−-3/GM/SF | — | — | — | — | — | — | — | — | — | — | 90 | — | ||||||||
| 9 | β2m−/−-3/GM/SF | 0 | 92 | 0 | 0 | 95 | 0 | ||||||||||||||
| 10 | β2m−/−-3/GM/SF | 38 ± 33 | 52 ± 23 | 6 ± 5 | 0 | 92 ± 2 | 3 ± 1 | 0 | 75 ± 1 | 18 ± 2 | 0 | 70 ± 10 | 13 ± 7 | ||||||||
| 11 | β2m−/−-3/GM/SF | 3 | 92 | 0 | 0 | 92 | 0 | 0 | 92 | 0 | 0 | 92 | 0 | ||||||||
| Normal lin− BM | 61 ± 14 | 30 ± 9 | 0 | 1 ± 0.3 | 79 ± 3 | 10 ± 2 | 0 | 41 ± 2 | 62 ± 5 | 0 | 39 ± 16 | 52 ± 21 | |||||||||
| 2 | β2m−/− | 56 | 31 | 0 | — | — | — | —* | — | — | — | — | — | ||||||||
| 3 | β2m−/− | 0 | 96 ± 1 | 0 | 0 | 90 ± 4 | 0 | — | — | — | — | — | — | ||||||||
| 5 | β2m−/− | 52 ± 11 | 45 ± 3 | 0 | 0 | 91 ± 8 | 0 | —* | — | — | — | — | — | ||||||||
| 7 | β2m−/− | 0 | 86 ± 6 | 0 | 0 | 98 ± 1 | 0 | — | — | — | — | — | — | ||||||||
| 9 | β2m−/− | 4 | 90 | 0 | 0 | 78 | 20 | — | — | — | — | — | — | ||||||||
| 10 | β2m−/− | 0 | 80 ± 3 | 10 ± 3 | 0 | 60 ± 10 | 26 ± 9 | 0 | 30 ± 11 | 44 ± 14 | 0 | 11 ± 3 | 86 ± 10 | ||||||||
| Normal lin− BM | β2m−/− | 57 ± 5 | 20 ± 3 | 0 | 3 ± 1 | 57 ± 7 | 33 ± 7 | 0 | 9 ± 3 | 86 ± 4 | 0 | 27 ± 10 | 76 ± 11 | ||||||||
Values shown are the percentages of detectable glyA+ erythroid (E), CD33+ or CD15/66b+ granulopoietic/macrophage (GM) and CD34−CD19/20+ B-lymphoid (L) cells within the total human cell population measured in highly engrafted mice, where such measurements were possible. The percentage of cells in each subset is expressed as the mean ± SEM except for patients 1, 2, 8, 9, and 11, where data were from single mice. β2m−/−-3/GM/SF indicates NOD/SCID-β2m−/−-3/GM/SF; β2m−/−, NOD/SCID-β2m−/−; and —, no data available.
Experiments were terminated 5 weeks after transplantation.
Altered patterns of differentiation seen in NOD/SCID-β2m-/- mice engrafted with human MDS cells. Representative FACS profiles of cells aspirated from the BM of a mouse that received a transplant 3 weeks previously with cells from patient 3 (A) and from the BM of a mouse that received a transplant 7 weeks previously with cells from patient 9 (B), in both instances showing a predominance of human granulopoietic (CD15/66b+) cells.
Altered patterns of differentiation seen in NOD/SCID-β2m-/- mice engrafted with human MDS cells. Representative FACS profiles of cells aspirated from the BM of a mouse that received a transplant 3 weeks previously with cells from patient 3 (A) and from the BM of a mouse that received a transplant 7 weeks previously with cells from patient 9 (B), in both instances showing a predominance of human granulopoietic (CD15/66b+) cells.
Abnormal morphology of granulocytes produced in mice engrafted with human MDS cells. Shown is a representative Wright-Giemsa-stained cytospin preparation of FACS-sorted human CD45/71+ cells isolated from a mouse injected 3 weeks previously with cells from patient 3. Original magnification, × 63.
Abnormal morphology of granulocytes produced in mice engrafted with human MDS cells. Shown is a representative Wright-Giemsa-stained cytospin preparation of FACS-sorted human CD45/71+ cells isolated from a mouse injected 3 weeks previously with cells from patient 3. Original magnification, × 63.
At later times (≥ 5 weeks) after transplantation, a disproportionate output of granulopoietic cells continued to be seen in recipients of cells from most MDS patients (see data for patients 1, 3, 5, 7, 8, 9, 10, and 11 in Table 4 and example shown in Figure 3B). These results contrast markedly with the large numbers of human B-lineage (CD19/20+) cells consistently seen by week 10 when the same types of mice received transplants of normal human BM cells (Figure 1). However, human B-lineage cells were produced at detectable levels in recipients of cells from 2 MDS patients (9 and 10), and, in the NOD/SCID-β2m-/- recipients of patient 10 cells, these actually reached normal levels by week 17. Primitive (CD34+) human cells were also present in both types of mice that received transplants of cells from patient 10 (2%-25% of the total human CD45/71+ population) and at a very low frequency in 2 recipients of cells from patient 7 but not in any other mice. CD41+ cells were not seen in any of the recipients of MDS cells at any time.
Presence of cytogenetically abnormal cells in mice repopulated with MDS cells
Cells from 4 of the 5 MDS patients whose original BM samples had been found to contain cytogenetically abnormal cells (15% to 100%; Table 1) produced sufficient human cells in mice to allow the regenerated cells to be isolated by FACS and analyzed by interphase FISH. FISH performed on the initial BM cells from patients 1, 9, and 11 showed trisomy 8 in 64%, 11%, and 8% of the cells, respectively (Table 5). All of the CFCs and LTC-ICs in the original sample from patient 1 also contained an extra copy of chromosome 8, whereas all of the CFCs and LTC-ICs in the starting sample from patient 11 were cytogenetically normal (Table 5). FISH analysis of CFCs and LTC-ICs in the initial sample from patient 9 was not performed. Patient 8 had a 5q-clone that accounted for 100% of the metaphases seen in his original BM sample.
Cytogenetic data for 4 MDS samples
. | . | %abnormal cells (abnormal/total nuclei or total colonies) . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | . | Initial sample* . | . | . | FACS-sorted human CD45/71+ cells from individual mice . | . | . | . | ||||||
| . | FISH probe . | Total cells . | CFCs . | LTC-ICs . | Wk 3 . | Wk 5-7 . | Wk 8-12 . | Wk 15-23 . | ||||||
| 1 | 8 centromere | 64 (329/516) | 100† (10/10) | 100 (15/15) | 28 (60/212) | 29 (37/128) | 9 (51/566) | 2.7-1.6 (35/1309)-(12/772) | ||||||
| 8 | 5q31 | 100† (23/23) | 94 (17/18) | 95 (18/19) | — | — | — | 85 (128/150) | ||||||
| 9 | 8 centromere | 11 (56/500) | — | — | 3; 11 (2/63;207/1845) | 0.4; 9 (7, 18)‡ (10/2272; 119/1343) | — | — | ||||||
| 11 | 8 centromere | 8 (66/864) | 0 (0/40) | 0† (0/5) | 1.5 (14/957) | 1 (10/902) | — | 0.6 (3/538) | ||||||
. | . | %abnormal cells (abnormal/total nuclei or total colonies) . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | . | Initial sample* . | . | . | FACS-sorted human CD45/71+ cells from individual mice . | . | . | . | ||||||
| . | FISH probe . | Total cells . | CFCs . | LTC-ICs . | Wk 3 . | Wk 5-7 . | Wk 8-12 . | Wk 15-23 . | ||||||
| 1 | 8 centromere | 64 (329/516) | 100† (10/10) | 100 (15/15) | 28 (60/212) | 29 (37/128) | 9 (51/566) | 2.7-1.6 (35/1309)-(12/772) | ||||||
| 8 | 5q31 | 100† (23/23) | 94 (17/18) | 95 (18/19) | — | — | — | 85 (128/150) | ||||||
| 9 | 8 centromere | 11 (56/500) | — | — | 3; 11 (2/63;207/1845) | 0.4; 9 (7, 18)‡ (10/2272; 119/1343) | — | — | ||||||
| 11 | 8 centromere | 8 (66/864) | 0 (0/40) | 0† (0/5) | 1.5 (14/957) | 1 (10/902) | — | 0.6 (3/538) | ||||||
— indicates no data available.
Initial sample refers to the cells injected.
Analysis of G-banded metaphases.
Numbers in parentheses are the % of cells with trisomy 8 in CD15/66b+ and CD19/20+ populations isolated as separate subsets from the BM of one mouse 7 weeks after transplantation.
The human CD45/71+ cells isolated from mice engrafted with cells from patients 1, 9, and 11 showed readily detectable and sustained levels of trisomy 8+ cells but generally at levels a few-fold lower than in the original suspension transplanted (ie, 0.4% to 29%; Table 5 and Figure 5). Notably, in one of the mice engrafted with cells from patient 9, human granulopoietic (CD15/66b+) and B-lymphoid (CD19/20+) cells were isolated by FACS as distinct subsets prior to FISH analysis and the presence of trisomy 8 was seen in a similar proportion of both of these lineages (7% and 18%, respectively). In the single NOD/SCID-β2m-/- mouse that showed delayed repopulation with cells from patient 8 at 16 weeks after transplantation, 85% of the human cells were found to display the 5q-abnormality.
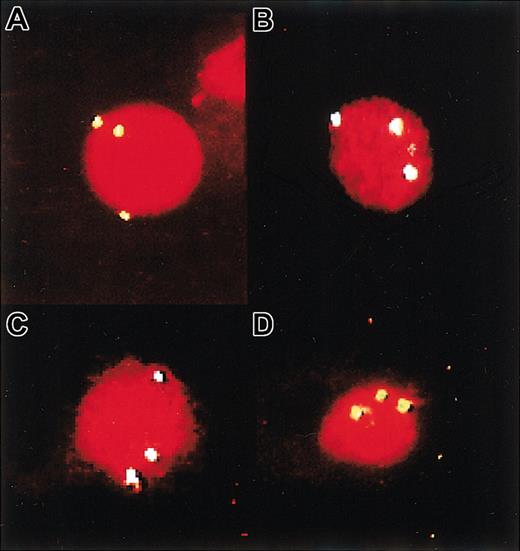
FISH detection of trisomy 8+cells produced in mice that received transplants of MDS cells. Panel A shows a cytospin of starting cells from patient 9, and panel B shows some of the human CD45/71+ cells isolated by FACS from the BM of a mouse injected 3 weeks previously with these cells. (C-D) Examples of cells from a mouse injected 3 and 5 weeks previously with cells from patient 1. Trisomy 8+ cells were identified by the detection of 3 fluorescent signals in single nuclei. Original magnifications, × 100 for all panels.
FISH detection of trisomy 8+cells produced in mice that received transplants of MDS cells. Panel A shows a cytospin of starting cells from patient 9, and panel B shows some of the human CD45/71+ cells isolated by FACS from the BM of a mouse injected 3 weeks previously with these cells. (C-D) Examples of cells from a mouse injected 3 and 5 weeks previously with cells from patient 1. Trisomy 8+ cells were identified by the detection of 3 fluorescent signals in single nuclei. Original magnifications, × 100 for all panels.
Discussion
The use of immunodeficient mice as hosts of human hematopoietic cell transplants has provided powerful in vivo models of human hematopoiesis.54 Subfractionation of the input cells coupled with time course studies of the numbers and types of mature progeny produced has revealed the presence in normal human hematopoietic tissues of a hierarchy of primitive transplantable progenitors that produce different spectra of progeny for varying but predictable periods. Through as yet poorly understood mechanisms, the NOD/SCID mouse supports the selective engraftment and regenerative activity of the most primitive types of these cells that are characterized by a CD34+CD38- phenotype and have self-renewal as well as multilineage lympho-myeloid reconstituting potential.25,42,55-58 In contrast, the NOD/SCID-β2m-/- mouse permits the additional engraftment of a broader spectrum of cells with more restricted proliferative and differentiation potentialities.40 The mature cells generated by these additional types of engrafting cells account for the variably increased levels of hematopoiesis seen in NOD/SCID-β2m-/- mice that receive transplants of human cord blood, adult BM, and mobilized blood cells.
Nevertheless, the total number of mature cells produced in xenografted mice per normal human repopulating cell is relatively low.59 This situation is reminiscent of human LTCs where it is clear that closely related types of primitive cells (LTC-ICs) are responsible for most of the granulocytes produced after 5 to 8 weeks, although the output of mature cells from each LTC-IC is also relatively low.60 One possibility is that the stromal cells present in both scenarios (in the marrow of engrafted mice and in the adherent layer of the LTC system) efficiently activate the most primitive types of human hematopoietic cells but are less supportive of the expansion of their maturing progeny. In the LTC system, the number of CFCs produced per LTC-IC can be increased several-fold if the stromal cells are engineered to produce human IL-3, G-CSF, and SF.48 Similarly, we have previously shown that NOD/SCID mice engineered to produce elevated serum levels of human IL-3, GM-CSF, and SF support increased outputs of human CFCs and granulopoietic cells.46 In the present study, we have extended these observations to NOD/SCID-β2m-/- mice. Of particular note is the observation of an increase in the early transient output in these mice of erythroid as well as granulopoietic cells produced by normal human short-term myeloid-restricted repopulating cells. This finding is consistent with the ability of these growth factors in concert with Epo to stimulate human erythropoiesis in vitro.61,62 Similarly, we have found the later output of normal granulopoietic progeny to be selectively enhanced in NOD/SCID-β2m-/- mice engineered to produce human IL-3, GM-CSF, and SF. These observations demonstrate that the full proliferative potential of many types of human cells able to engraft the BM is not elicited in the sublethally irradiated NOD/SCID or NOD/SCID-β2m-/- mouse and underscore the need for continued caution in interpreting results obtained in either of these models.
A more significant outcome of the present work was the finding that cells from 9 of 11 MDS patients (all FAB subtypes) could generate detectable levels of human hematopoietic progeny in NOD/SCID-β2m-/- (or NOD/SCID-β2m-/--3/GM/SF) mice. For 8 of these patients, the distribution of differentiated progeny obtained in the mice was abnormal. This included a reduced early output of erythroid cells and later output of B-lineage cells, accompanied by a disproportionate increase in the production of granulopoietic cells and maturing myelocytes. Confirmation of the neoplastic origin of the engrafting cell was obtained from the 4 cases where there was a clonal marker in the original BM. In all 4 of these, the same marker was also seen in the regenerated cells. Importantly, in one case, both human lymphoid and myeloid cells with the same cytogenetic abnormality (trisomy 8) present in the original BM cells could be identified in a mouse that received transplants of these cells 7 weeks earlier, demonstrating origin of the MDS clone in this patient in a self-renewing stem cell with lymphoid and myeloid differentiation potential that can also repopulate NOD/SCID-β2m-/- mice. The present findings thus represent a significant advance over previous experience with transplants of primary MDS cells in NOD/SCID hosts.21,36,37
Much evidence points to the stem cell origin of many human malignancies affecting the myeloid system, including MDS, with a multistep pathogenesis leading to the development of sufficiently large and deregulated clones to cause symptomatic disease.23,24 Such a model is compatible with the maintenance of a residual hierarchical structure within the evolving leukemic clone, even though terminal differentiation events may be perturbed.54,63 Accordingly, the BM of different MDS patients would be predicted to contain variable numbers of residual normal stem cells and early progenitors in addition to variable numbers of their neoplastic counterparts, only some of which might carry mutations acquired during the progression of the initial clone. Such variations could explain the different patterns of repopulation characteristic of different samples. For example, in mice that received transplants of cells from many of the cases studied, only an early transient wave of granulopoiesis was seen. Based on previous studies with normal cells, this result with MDS samples could suggest the presence in the BM of these patients of detectable frequencies of a relatively late type of “multilineage but myeloid-restricted” neoplastic “stem” cell with repopulating activity but limited self-renewal ability and skewed differentiation potential. On the other hand, several of the MDS samples tested here also appeared to contain more primitive neoplastic cells, as indicated by their more durable reconstituting activities and, in 2 cases, a capacity to generate lymphoid as well as myeloid progeny. Unfortunately, more prolonged follow-up of the cells produced was not possible because of the reduced lifespan of the mice used in these studies, due to their spontaneous development of lethal thymomas between 6 and 12 months of age.38
Our findings also suggest that the repopulating cells present in MDS patients may include residual normal elements or preneoplastic elements with more normal features that outcompete the neoplastic cells if adequately stimulated. Evidence for such cells was provided most convincingly in mice that received transplants of cells from patient 10 (a patient with refractory anemia with excess blasts in transformation [RAEBT]). In these, the distribution of mature cell types seen was only modestly skewed, as would have been expected from the presence in the original transplant of a mixed population of neoplastic and normal cells with repopulating ability. In addition, it should be noted that in all 4 cases where a cytogenetically abnormal clone could be detected in the original sample, the proportion of these among the cells regenerated in the mice was typically lower.
Finally, the discrepancy observed between the significantly enhanced output of human cells in NOD/SCID-β2m-/--3/GM/SF recipients of normal human BM cells and the lack of such an effect in the recipients of MDS cells is noteworthy. Perhaps this is an indication of the refractory responsiveness of neoplastic progenitors from some patients with MDS to growth factor stimulation, as previously documented both in vitro10,11 and in vivo where administration of pharmacologic doses of G-CSF or GM-CSF can favor the production of mature cells from residual normal (non-clonal) precursors.14
In summary, we have described a new xenograft model applicable to all stages of human MDS and have used this model to characterize the neoplastic cells that are regenerated in vivo. These cells display the expected features of defective erythroid and B-lymphoid differentiation and blunted responsiveness to elevated levels of circulating hematopoietic growth factors. This model should be valuable for more detailed characterization of the neoplastic cells in MDS patients that possess repopulating activity and their in vivo responses to novel therapies.
Prepublished online as Blood First Edition Paper, February 12, 2004; DOI 10.1182/blood-2003-09-3192.
Supported by grants from the National Cancer Institute of Canada with funds from the Terry Fox Run and the Canadian Cancer Society and by research funds from the Hematology Division of Patras University Medical School, Greece.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank the physicians of the Division of Hematology of the BC Cancer Agency, Vancouver Hospital, and Patras University Hospital for assistance in accruing patient material; the members of the Stem Cell Assay and Flow Cytometry Services of the Terry Fox Laboratory for assistance in initial cell processing and operation of the sorter; the staff of the Animal Facility of the BC Cancer Research Center for breeding and care of the mice; and Angela Padilla for secretarial assistance. Thanks are also extended to Dr Peter Lansdorp and Dr Donna Hogge (Terry Fox Laboratory), StemCell Technologies (Vancouver, BC, Canada), Novartis (Basel, Switzerland), and Cangene (Toronto, ON, Canada) for valuable gifts of antibodies and growth factors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal