Imatinib has become standard therapy for patients with chronic myelogenous leukemia (CML). A complete cytogenetic response can be achieved in 75% to 90% of patients treated in early chronic phase,1,2 and a fraction of patients have undetectable levels of BCR-ABL by polymerase chain reaction (PCR).1,3,4 An important unanswered question for patients who achieve a complete molecular response is whether imatinib therapy may be discontinued safely. We herein report on 3 patients who achieved a molecular response (ie, undetectable levels of BCR-ABL) and relapsed shortly after discontinuation of therapy.
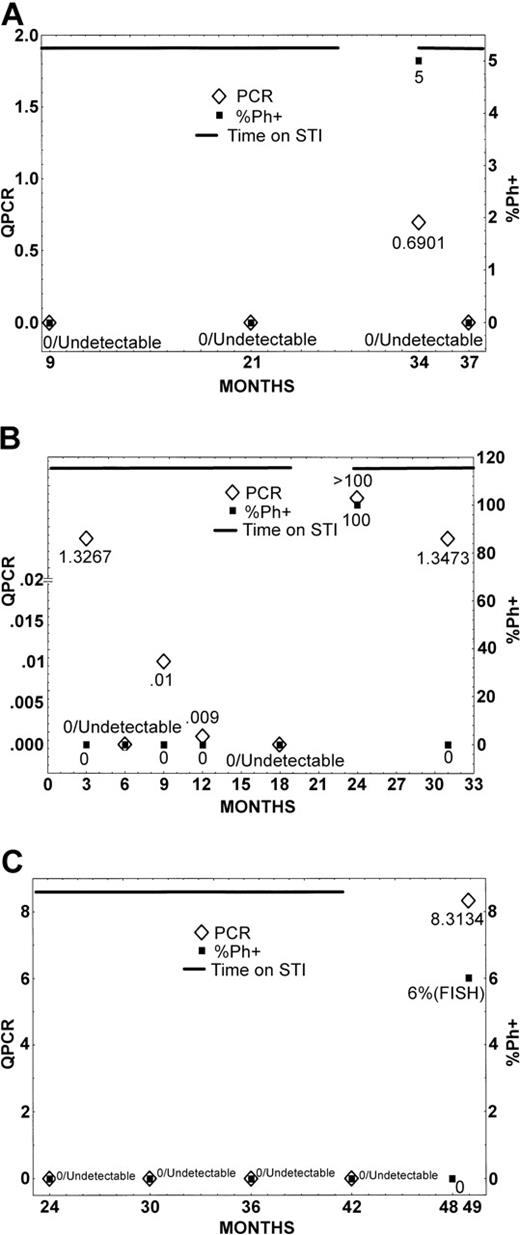
The first patient (Figure 1A) is a 34-year-old woman who was treated with interferon-α (IFN) when first diagnosed with CML. After loss of major cytogenetic response, she started imatinib (400 mg) orally daily. She rapidly achieved a complete cytogenetic response, and after 9 months of therapy she had undetectable levels of BCR-ABL. After 21 months she had identical results, and 9 months later she decided to stop therapy (12 months' total duration of undetectable BCR-ABL), as she wanted to become pregnant. Three months after discontinuation of therapy she had 5% metaphases positive for the Philadelphia chromosome (Ph), and therapy was immediately restarted. Three months after restarting therapy, all 100% metaphases were diploid, and BCR-ABL was again undetectable.
Cytogenetic and molecular analysis during and after therapy with imatinib. Patients 1 (A), 2 (B), and 3 (C).
Cytogenetic and molecular analysis during and after therapy with imatinib. Patients 1 (A), 2 (B), and 3 (C).
The second patient (Figure 1B) is a 70-year-old man who started therapy with imatinib (800 mg) daily for newly diagnosed CML in chronic phase. He achieved a complete cytogenetic remission 3 months after therapy was started. After 6 months he had undetectable levels of BCR-ABL. Subsequent evaluations at 9 and 12 months from the start of therapy showed low levels of BCR-ABL/ABL (0.01 and 0.0009, respectively), but they were again undetectable at the 18-month evaluation. The patient decided to discontinue therapy because of grade 2 tearing of his eyes. Six months later, the patient had recurrence of chronic-phase disease with 100% Ph-positive metaphases. Imatinib therapy was restarted, and 6 months later the patient again achieved a complete cytogenetic remission, with a 2-log reduction in BCR-ABL transcripts.
The third patient (Figure 1C) is a 40-year-old woman who had failed prior IFN therapy and had progressed to the accelerated phase. Imatinib therapy was initiated at 600 mg daily, and she achieved a major cytogenetic response (Ph 10%) after 3 months. This improved to a complete cytogenetic response 3 months later with low levels of BCR-ABL/ABL (0.37 to 0.39). After 24 months of therapy, BCR-ABL became undetectable and remained undetectable for 18 months. The patient decided to discontinue therapy, as she desired to become pregnant. Seven months after discontinuation of therapy she had 6% Ph-positive metaphases by fluorescence in situ hybridization (FISH) (100% diploid by cytogenetic analysis), and the BCR-ABL/ABL ratio had increased to 8.31%. At the time of this letter's composition, the patient had not elected to restart imatinib therapy.
These 3 patients illustrate the need for continued therapy with imatinib after achieving molecular remission. Despite undetectable BCR-ABL levels on imatinib that were sustained for up to 21 months, relapses rapidly occurred in all 3 patients. In all 3 patients the relapse was both molecular and cytogenetic, but no changes in peripheral blood counts occurred. The rapid recurrence of disease despite having been undetectable by PCR suggests that the tempo of CML may be more rapid than previously thought. Also, the 2 patients who restarted therapy responded rapidly, suggesting no acquired resistance to imatinib, but rather representing the need for continued therapy. There is one other patient reported who experienced a rapid relapse after discontinuation of therapy despite a molecular response that had been sustained for 8 months.5 Thus, it cannot be recommended at the present time to discontinue therapy for patients who achieve a molecular remission with imatinib.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal