Abstract
During extravasation, neutrophils migrate through the perivascular basement membrane (BM), a specialized extracellular matrix rich in laminins. Laminins 8 (LN-8) (α4β1γ1) and 10 (LN-10) (α5β1γ1) are major components of the endothelial BM, but expression, recognition, and use of these laminin isoforms by neutrophils are poorly understood. In the present study, we provide evidence, using a panel of novel monoclonal antibodies against human laminin α4 (LNα4) chain, that neutrophils contain and secrete LN-8, and that this endogenous laminin contributes to chemoattractant-induced, αMβ2-integrin–dependent neutrophil migration through albumin-coated filters. Phorbol ester–stimulated neutrophils adhered to recombinant human (rh) LN-8, rhLN-10, and mouse LN-1 (mLN-1) (α1β1γ1) via αMβ2-integrin, and these laminin isoforms strongly promoted chemoattractant-induced neutrophil migration via the same integrin. However, only rhLN-8 enhanced the spontaneous migration. In addition, recruitment of neutrophils into the peritoneum following an inflammatory stimulus was impaired in LNα4-deficient mice. rhLN-8 also protected isolated neutrophils from spontaneous apoptosis. This study is the first to identify a specific laminin isoform in neutrophils and provides evidence for the role of LN-8 in the adhesion, migration, extravasation, and survival of these cells.
Introduction
Neutrophils are highly migratory phagocytes with pivotal roles in host defense against infections and numerous inflammatory disorders.1 These leukocytes predominate in the early cellular infiltrate at inflammatory sites, and their migration from the circulation into tissues involves a sequential engagement of adhesion receptors.2,3 Although considerable efforts have been made to understand the initial steps of neutrophil extravasation (rolling and tight adhesion), little is known about the migration of neutrophils through the vessel wall and the interaction of these cells with the underlying extracellular matrix proteins of the perivascular basement membrane, such as laminins, and the interstitium.3,4
Laminins are a family of large heterotrimeric proteins that promote cell adhesion and migration via integrins and other cell-surface receptors.5-7 They are major components of basement membranes of blood vessels and other tissue compartments, and are synthesized by numerous cell types. Through combination, 5 α, 3 β, and 3 γ laminin chains constitute more than 12 laminin isoforms.6,7 Laminin isoforms, particularly their α chain, are expressed in a cell- and tissue-specific manner, and are differentially recognized by integrins (INTs).6,7 Laminin 1 (LN-1) (α1β1γ1), the prototype laminin originally isolated from a mouse tumor more than 20 years ago, has been extensively characterized, and its in vitro effects have been studied in numerous cell types.5 However, expression of LN-1 in newborn and adult stages is mostly restricted to a subtype of epithelial cells,8,9 thus making questionable the role of this laminin isoform in leukocyte extravasation.
Laminin 8 (LN-8) (α4β1γ1) and laminin 10 (LN-10) (α5β1γ1), identified in 1997,10 are major laminin isoforms of blood vessels.11,12 LN-8 is also found in platelets, monocytes, and lymphocytes, and mediates their adhesion via α6β1 integrin.13-15 Moreover, this laminin isoform promotes migration of endothelial and bone marrow progenitor cells, monocytes, and lymphocytes.14-17 Immunohistochemical studies have demonstrated localization of the laminin α4 (LNα4) chain in tissues of mesodermal origin, particularly basement membranes of vessels, and adipose and perineural tissues.10,18,19 Accordingly, LNα4-deficient (knock out [KO]) mice display transient hemorrhages at birth, reflecting impaired microvessel maturation, and a subtle motor defect as adults.20
Rabbit peritoneal exudate neutrophils, but not human blood neutrophils, have been found to express laminin epitopes on their cell surface.21,22 However, these studies were performed with antisera to mouse LN-1 (mLN-1), and identification of the immunoreactive chains was not reported. On the other hand, laminin has been described as mediating adhesion of neutrophils through both integrin and nonintegrin receptors.21,23-27 It has also been reported that blood neutrophils, unlike monocytes, migrate indiscriminately on laminin, fibronectin, and collagen I substrates, and that laminin delays neutrophil apoptosis.28,29 In all of these functional studies, however, mLN-1 was used as the exogenous laminin, though this laminin isoform is not expressed in hematopoietic and lymphoid tissues or by vascular endothelial cells.8,9,30 Blood neutrophils are known to express α6β1 integrin,26,31 but expression, recognition, and use of vascular endothelial laminin isoforms, such as LN-8, by these cells are poorly understood.
Recently, we reported constitutive adhesion of monocytoid cells to LN-8 via α6β1, and to a lesser extent β2 integrin(s), and strong promotion of monocyte migration by this laminin isoform.14 In the present study, we have investigated the expression, recognition, and use of LN-8 by blood neutrophils by using novel monoclonal antibodies (mAbs) to LNα4 chain, recombinant human (rh) LN-8, and LNα4 KO mice.
Materials and methods
Monoclonal antibodies to the human LNα4 chain and to other laminin chains, and purified proteins
There were 7 novel mAbs to the human LNα4 chain generated by hybridoma technology following immunization of Balb/c mice with immunoaffinity-purified LN-8 (α4β1γ1) from platelets. Their specificity was determined by enzyme-linked immunosorbent assay (ELISA) comparing both rhLN-8 (α4β1γ1) and rhLN-10 (α5β1γ1). rhLN-8 and rhLN-10 were produced in a mammalian expression system and affinity purified using anti–FLAG M2 matrix (Sigma, St Louis, MO) and ion-exchange chromatography as described.16,32,33 No contaminants were seen in the purified preparations by silver staining of the protein gels (sodium dodecyl sulfate–polyacrylamide gel electrophoresis [SDS-PAGE]). Of each recombinant protein, 3 different preparations were used, and they behaved similarly in functional assays. rhLN-8 lacking chondroitin sulfate modification was used throughout the study, and these preparations at 4 μg/mL were found negative for endotoxin (limulus amebocyte lysate endosafe assay with sensitivity of 0.06 EU/mL; Charles River Labs, Charleston, SC). Mouse LN-1 (α1β1γ1), isolated from Engelbreth-Holm-Swarm tumor, was obtained from Chemicon (Chemicon International, Temecula, CA). Reactivity of the mAbs with platelet LN-8 (α4 chain) in immunoblotting under reducing and nonreducing conditions was determined, as well as their ability to immunoprecipitate LN-8 from platelet lysate. The LNα4 mAbs used in this study were affinity chromatography–purified immunoglobulin G (IgG). Isotyping with Iso-2 kit (Sigma) indicated that all novel antibodies were mouse IgG1, except 3D7, which was IgG2a. In addition, the following antilaminin mAbs, most of them characterized in Geberhiwot et al,34 were also used: 4C7 (LNα5) and P3H9-2 (LNα3; Chemicon); DG10 (LNβ1; kindly provided by Prof Ismo Virtanen, University of Helsinki); 2G6 (LNβ1; Sera-Lab, Crawley Down, United Kingdom); 22 (LNγ1; BD Biosciences, Heidelberg, Germany); LN-41 (LNγ1; Takara Shuzo, Kyoto, Japan); and CAF-2 (LNγ1; generous gift from Prof Harold P. Erickson, Duke University, Durham, NC). All of these previously characterized antibodies were mouse IgG. Heparitinase (Heparinase III) and chondroitinase (Chondroitinase ABC) were obtained from Sigma. Experiments were approved by the regional ethics committees for experimental research.
ELISA, gel electrophoresis, immunoblotting, immunoprecipitation, enhanced chemiluminescence, and purification of laminin by immunoaffinity chromatography
Characterization of the novel mAbs by ELISA was performed as previously described.19 Protein samples from platelet lysates were prepared and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).13 Immunoprecipitation of LN-8 from platelet lysate was performed with several antibodies and detected with mAb 6C3 (LNα4) by immunoblotting. Laminin was purified from either neutrophil lysate (1 × 109 cells) or neutrophil secreted material as supernatant from 20 × 106 cells/mL stimulated with 200 nM tetradecanoyl phorbol acetate (TPA, also known as PMA; Sigma) for 20 minutes at 37° C by immunoaffinity chromatography in a laminin β1 mAb (DG10)–Sepharose column, as previously described in platelets.13 The purified material was then concentrated and analyzed by SDS-PAGE and immunoblotting.
Neutrophil isolation and immunofluorescence flow cytometry
Neutrophils were isolated from citrated blood of healthy donors by discontinuous Percoll gradient centrifugation (Amersham Biosciences, Uppsala, Sweden).35 More than 97% of the cells were neutrophils as determined morphologically and by immunostaining with mAb C3D-1 (Dako, Glostrup, Denmark) to CD15, a neutrophil marker. Practically platelet-free neutrophil preparations were obtained by washing of the cells at low speed (160g), as demonstrated by lack of reactivity with mAb SZ.22 (Beckman Coulter, Bromma, Sweden) to CD41, a platelet marker.
Isolated neutrophils were washed with phosphate-buffered saline (PBS) and used both nonpermeabilized and permeabilized for immunofluorescence flow cytometry as previously described.14,36 mAbs 5D8, 2G6, and CAF-2 were used to detect the LN-8 chains α4, β1, and γ1, respectively. After staining, neutrophils were gated, according to forward- and side-scatter properties, and analyzed in a FACScan flow cytometer using CellQuest software (BD Biosciences, San Jose, CA).
Neutrophil adhesion and migration assays
To analyze neutrophil adhesion to laminin isoforms, 96-well flat-bottomed polystyrene plates (BD Biosciences, Stockholm, Sweden) were coated with 50 μL/well PBS or 20 μg/mL human serum albumin (HSA), mLN-1, rhLN-8, or rhLN-10 in PBS for 3 hours at 37° C. After rinsing with PBS, free sites were blocked with 2% polyvinylpyrrolidone (PVP; molecular weight, 360 kDa; Sigma) for one hour at room temperature. Isolated neutrophils were resuspended at 2 × 106 cells/mL in RPMI-1640 and incubated separately with 20 μg/mL of either mIgG (negative control), IB4 (integrin β2 chain, CD18),37 60.1 (integrin αM, CD11b),38 2LPM (integrin αM, CD11b), 6S6 (integrin β1, CD29), or GoH3 (integrin α6, CD49f) at room temperature for 20 minutes. mAbs IB4 and 60.1 were generous gifts from Profs Samuel Wright (Merck Research Labs, Rahway, NJ) and Patrick Beatty (University of Utah, Salt Lake City, UT), respectively. Mouse IgG and GoH3 were from BD Biosciences, Stockholm; 2LPM, from Dako; and 6S6, from Chemicon. All selected antibodies to integrins were function blocking and used as purified IgG. Following PVP blocking, the plates were washed twice with PBS and once with RPMI-1640. Thereafter, the cells were plated and preincubated for 10 minutes at 37° C before stimulation with 200 nM TPA for 20 minutes. Nonadherent cells were removed by washing with medium, and adherent cells were fixed with 4% formaldehyde in PBS for 15 minutes and stained overnight with 0.5% toluidine blue (Sigma) in PBS at room temperature. The plate was finally washed with copious amounts of distilled water, and adherent cells were quantified in a plate reader (Multiskan MS; Labsystems, Helsinki, Finland) at 620 nm by releasing the blue dye with 100 μL of 2% SDS (Bio-Rad Laboratories, Richmond, CA).
To investigate the effect of exogenous laminin isoforms on neutrophil migration, transmigration of the cells through a protein-coated filter which separates upper and lower chambers was determined microscopically. Briefly, isolated neutrophils (250 × 103) in 100 μL RPMI-1640 medium were added to a 6.5-mm diameter, 3-μm pore polycarbonate Transwell culture insert (Costar, Cambridge, MA) and incubated at 37° C for 1½ hours in the absence (spontaneous migration) or presence (chemoattractant-stimulated migration) of either 10 nM (4.4 ng/mL) formyl-methionyl-leucyl-phenylalanine (FMLP; Sigma) or 11 nM (100 ng/mL) interleukin-8 (IL-8; R&D Systems, Abingdon, United Kingdom) in 600 μL medium in the lower chamber. The filters had been initially coated and blocked as in the cell adhesion assay, and the neutrophils had been similarly treated with the mAbs. In experiments concerning endogenous laminins, filters were coated with 20 μg/mL HSA and blocked with 0.5% HSA, and cells were treated with mIgG or mAbs to integrins or laminins. mAb G46-2.6 to a common frame epitope of major histocompatibility complex (MHC) class I molecules (BD Biosciences, Stockholm) and mAbs ASC-3 and ASC-8 to integrin β4 (CD104) (Chemicon), which is not expressed by neutrophils, were also used as controls. To efficiently remove all transmigrated cells from the lower chamber, a final concentration of 10 mM ethylenediaminetetraacetic acid (EDTA) was added to each well and cells were vigorously resuspended and collected. Thereafter, the cells were fixed with 1% formaldehyde, and the cell number was determined microscopically using a hemocytometer at × 400 magnification. All mAbs, either homemade, gift, or commercial ones against integrin or laminin chains, used in the functional assays were IgG and affinity purified by protein A or protein G chromatography.
Proteose peptone–induced peritonitis in LNα4-deficient mice
LNα4 KO mice were generated by gene targeting in embryonic stem cells, as previously described.20 The LNα4 KO mice were viable and fertile but displayed transient hemorrhages at birth, reflecting impaired microvessel maturation, and a subtle motor defect as adults. Wild-type (WT, +/+) and knock-out (KO, –/–) adult male mice were used to analyze leukocyte recruitment into the peritoneal cavity. The animals were temporarily anesthetized with isoflurane, and 3 mL of either phosphate-buffered saline (PBS) or 3% proteose peptone (PP; Unipath, Basingstoke, United Kingdom) was injected intraperitoneally. After 6 hours, the mice were killed, and 4 mL cold PBS (containing 0.25% bovine serum albumin and 2 mM EDTA) was injected intraperitoneally. Following gentle abdominal massage, the peritoneal lavage was harvested. Cells from 0.5-mL samples were centrifuged and stained with 10 μg/mL ethidium bromide to gate out nucleus-free debris. After 3 washes, the cells were resuspended in 0.5 mL PBS and analyzed by flow cytometry in a FACSort (BD Biosciences, San Jose). Forward- and side-scatter properties were used to identify the neutrophils.
Measurement of neutrophil apoptosis by annexin V staining and caspase-3–like enzyme activity
Flat-bottom, 24-well culture plates (Costar) were coated overnight with 20 μg/mL of either HSA, mLN-1, rhLN-8, or rhLN-10 at 4° C. In each well, 2 million neutrophils at 1 × 106 cells/mL in RPMI-1640 (Sigma) were plated and incubated for 12 hours at 37° C in a humidified atmosphere containing 5% CO2. Thereafter, cell samples were collected for annexin V staining (flow cytometry) and caspase enzyme activity (fluorometric assay), as previously described.39
Statistics
Results were expressed as mean ± standard deviation (SD). Statistical significance was assessed by paired Student t test when single comparisons were made (in vitro studies) and by unpaired Student t test for unpaired groups (in vivo studies). Differences between means were considered significant when P < .05.
Results
Characterization of novel mAbs to the human LNα4 chain
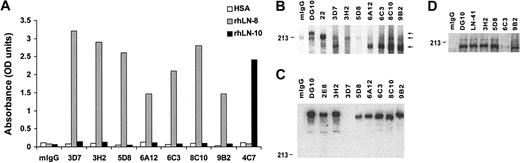
To analyze expression and function of α4 laminins, such as LN-8 (α4β1γ1), in neutrophils, 7 novel mAbs (3D7, 3H2, 5D8, 6A12, 6C3, 8C10, and 9B2) to the human LNα4 chain were generated by hybridoma technology following immunization of mice with purified human platelet LN-8 (Figure 1). The specificity of these antibodies was first established by reactivity with rhLN-8 (α4β1γ1), but not with rhLN10 (α5β1γ1), in ELISA (Figure 1A). Thereafter, reactivity of the antibodies in Western blotting under reducing (Figure 1B) and nonreducing (Figure 1C) conditions was determined against platelet lysate and purified human platelet LN-8, respectively. Under reducing conditions, mAbs 3D7, 6A12, 6C3, 8C10, and 9B2 reacted with 180-kDa (major) and 200-kDa (minor) polypeptides, albeit with variable intensities, as previously reported for platelets with a polyclonal antibody to LNα4.13 Reactivity of mAbs 3H2 and 5D8 was negligible. Control mAbs DG10 (LNβ1) and 22 (LNγ1) reacted with 230- and 220-kDa bands, respectively. Under nonreducing conditions, all mAbs, except 3D7, reacted with an approximately 630-kDa band. This band was also detected with mAbs DG10 and 2E8, indicating the disulfide bonding of LNα4 with LNβ1 and LNγ1 chains in the heterotrimer. Finally, the ability of mAbs 3H2, 5D8, 6C3, and 9B2 to immunoprecipitate α4 laminins (mainly LN-8) from platelet lysate was tested, and detected by the reactivity of the immunoprecipitate with mAb 6C3 (180- and 200-kDa polypeptides) (Figure 1D). mAbs 3H2, 5D8, and 9B2, as well as control antibodies DG10 (LNβ1) and LN-41 (LNγ1), performed well in immunoprecipitation assay. In contrast, mAb 6C3, which reacts well in Western blotting, was a poor immunoprecipitating antibody.
Reactivity of novel monoclonal antibodies to human laminin α4 chain. ELISA (A), Western blotting under reducing (B) and nonreducing (C) conditions, and immunoprecipitation (D). In ELISA, human serum albumin (HSA), recombinant human laminin-8 (rhLN-8, α4β1γ1), and recombinant human laminin-10 (rhLN-10, α5β1γ1) were coated on plastic, and mIgG and mAb 4C7 to human laminin α5 were used as controls. Seven mAbs (3D7, 3H2, 5D8, 6A12, 6C3, 8C10, and 9B2) were classified as specific for LNα4 chain. In Western blotting, antibody reactivity against LN-8–containing platelet lysate (B) and purified platelet LN-8 (C) are shown. mAbs DG10 (LNβ1), 22 (LNγ1), and 2E8 (LNγ1) were also included. (B) Arrows indicate bands of 230, 220, and 180 kDa, corresponding to LNβ1, LNγ1, and LNα4 chains, respectively. Under nonreducing conditions (C), most antibodies react with a band of nearly 630 kDa. (D) The ability of mAbs to (co)immunoprecipitate the LNα4 chain from platelet lysate is shown. LNα4 chain was visualized with mAb 6C3 as a major band of 180 kDa. In addition to 4 mAbs to LNα4 (3H2, 5D8, 6C3, and 9B2), mAbs DG10 (LNβ1) and LN-41 (LNγ1) were used for immunoprecipitation. OD indicates optical density.
Reactivity of novel monoclonal antibodies to human laminin α4 chain. ELISA (A), Western blotting under reducing (B) and nonreducing (C) conditions, and immunoprecipitation (D). In ELISA, human serum albumin (HSA), recombinant human laminin-8 (rhLN-8, α4β1γ1), and recombinant human laminin-10 (rhLN-10, α5β1γ1) were coated on plastic, and mIgG and mAb 4C7 to human laminin α5 were used as controls. Seven mAbs (3D7, 3H2, 5D8, 6A12, 6C3, 8C10, and 9B2) were classified as specific for LNα4 chain. In Western blotting, antibody reactivity against LN-8–containing platelet lysate (B) and purified platelet LN-8 (C) are shown. mAbs DG10 (LNβ1), 22 (LNγ1), and 2E8 (LNγ1) were also included. (B) Arrows indicate bands of 230, 220, and 180 kDa, corresponding to LNβ1, LNγ1, and LNα4 chains, respectively. Under nonreducing conditions (C), most antibodies react with a band of nearly 630 kDa. (D) The ability of mAbs to (co)immunoprecipitate the LNα4 chain from platelet lysate is shown. LNα4 chain was visualized with mAb 6C3 as a major band of 180 kDa. In addition to 4 mAbs to LNα4 (3H2, 5D8, 6C3, and 9B2), mAbs DG10 (LNβ1) and LN-41 (LNγ1) were used for immunoprecipitation. OD indicates optical density.
Expression and function of endogenous LN-8 (α4β1γ1) in neutrophils
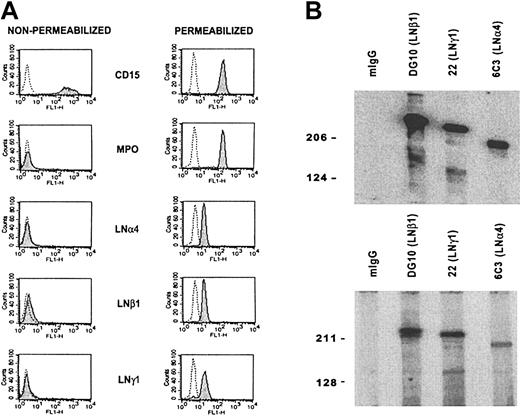
To determine the presence of α4 laminin(s) in blood neutrophils, immunofluorescence flow cytometry of purified cells was performed (Figure 2A). Consistent reactivity of mAbs 5D8 (LNα4), 2G6 (LNβ1), and CAF-2 (LNγ1) with practically all neutrophils was observed following permeabilization. Successful cell permeabilization was confirmed by detection of myeloperoxidase, an intracellular marker of myelomonocytic cells. Nonpermeabilized cells reacted slightly with some laminin antibodies, but only in a few experiments (data not shown).
Expression and secretion of LN-8 by isolated blood neutrophils. (A) Detection of laminin α4, β1, and γ1 chains in neutrophils by immunofluorescence flow cytometry. Reactivity of mAbs with nonpermeabilized and permeabilized cells is shown. Mouse IgG (dotted line) and mAbs (shaded peak) C3D-1 (CD15), MPO-7 (myeloperoxidase), 5D8 (LNα4), 2G6 (LNβ1), and CAF-2 (LNγ1) were used. CD15 and myeloperoxidase were used as neutrophil and cell permeabilization markers, respectively. (B) Western blot analysis of laminin β1 mAb (DG10) immunoaffinity-purified protein from neutrophil lysate (top panel) and neutrophil secretion (bottom panel). Secreted material as supernatant was obtained after stimulating neutrophils with 200 nM TPA for 20 minutes. mIgG (negative control), mAb DG10 (LNβ1), mAb 22 (LNγ1), and mAb 6C3 (LNα4) were used. Bands of 230, 220, and 180 kDa, corresponding to LNβ1, LNγ1, and LNα4 chains, respectively, were detected.
Expression and secretion of LN-8 by isolated blood neutrophils. (A) Detection of laminin α4, β1, and γ1 chains in neutrophils by immunofluorescence flow cytometry. Reactivity of mAbs with nonpermeabilized and permeabilized cells is shown. Mouse IgG (dotted line) and mAbs (shaded peak) C3D-1 (CD15), MPO-7 (myeloperoxidase), 5D8 (LNα4), 2G6 (LNβ1), and CAF-2 (LNγ1) were used. CD15 and myeloperoxidase were used as neutrophil and cell permeabilization markers, respectively. (B) Western blot analysis of laminin β1 mAb (DG10) immunoaffinity-purified protein from neutrophil lysate (top panel) and neutrophil secretion (bottom panel). Secreted material as supernatant was obtained after stimulating neutrophils with 200 nM TPA for 20 minutes. mIgG (negative control), mAb DG10 (LNβ1), mAb 22 (LNγ1), and mAb 6C3 (LNα4) were used. Bands of 230, 220, and 180 kDa, corresponding to LNβ1, LNγ1, and LNα4 chains, respectively, were detected.
To examine whether α4, β1, and γ1 chains were physically associated to form LN-8, the blood neutrophil lysate was immunopurified in a laminin β1 (DG10) antibody column, and the isolated material was analyzed by Western blotting. Under reducing conditions, mAbs DG10 (LNβ1), 22 (LNγ1), and 6C3 (LNα4) recognized polypeptides of 230, 220, and 180 kDa, respectively (Figure 2B, upper panel). Similar bands were obtained in the material isolated from the supernatant of neutrophils stimulated with TPA for 20 minutes (Figure 2B, lower panel), indicating that neutrophils were able to secrete LN-8.
As an initial approach to determine the function of the endogenous LN-8, the effect of mAbs to the LNα4 chain on FMLP-induced migration of neutrophils through human serum albumin (HSA)–coated filters was studied (Figure 3). When blocked with HSA, the HSA-coated filters allowed extensive migration of the cells. mAb 8C10 alone exerted partial inhibition (32%, P < .05) and, when combined with mAb P3H9-2 to the LNα3 chain, reduced the cell migration by 60% (P < .01). Other mAbs to the LNα4 chain were less inhibitory or without effect (data not shown). The neutrophil transmigration was largely dependent on αMβ2 integrin, as demonstrated with function-blocking mAbs 60.1 (INTαM, CD11b) and IB4 (INTβ2, CD18). mAb G46-2.6 to MHC class I antigen, a widely expressed cell surface molecule, and mAbs ASC-3 and ASC-8 to integrin β4, which is not expressed by neutrophils, were without effect, either alone or in combination (data not shown). These results suggested participation of endogenous LN-8, together with an α3 laminin, in neutrophil migration via αMβ2 integrin.
Effect of mAbs to integrins (INT) and laminins (LN) on neutrophil migration through human serum albumin (HSA)–coated filters. Neutrophil transmigration assays were performed by using 3-μm pore insert filters precoated and blocked with HSA, and the number of transmigrated cells was determined microscopically. mIgG (negative control), mAb IB4 (INTβ2, CD18), mAb 60.1 (INTαM, CD11b), mAb 8C10 (LNα4), and mAb P3H9-2 (LNα3) were used. Starting with 250 × 103 neutrophils in the upper chamber, migration to the lower chamber was assessed after incubating the cells for 1.5 hours at 37° C in the presence of FMLP (10 nM) as chemoattractant in the lower chamber. Mean and SD of 4 experiments are shown. P values (*P < .05; **P < .01) compare mAbs with mIgG.
Effect of mAbs to integrins (INT) and laminins (LN) on neutrophil migration through human serum albumin (HSA)–coated filters. Neutrophil transmigration assays were performed by using 3-μm pore insert filters precoated and blocked with HSA, and the number of transmigrated cells was determined microscopically. mIgG (negative control), mAb IB4 (INTβ2, CD18), mAb 60.1 (INTαM, CD11b), mAb 8C10 (LNα4), and mAb P3H9-2 (LNα3) were used. Starting with 250 × 103 neutrophils in the upper chamber, migration to the lower chamber was assessed after incubating the cells for 1.5 hours at 37° C in the presence of FMLP (10 nM) as chemoattractant in the lower chamber. Mean and SD of 4 experiments are shown. P values (*P < .05; **P < .01) compare mAbs with mIgG.
Exogenous LN-8 and other laminin isoforms mediate adhesion and promote migration of isolated neutrophils via αMβ2 integrin
To further analyze interactions between neutrophils and LN-8 and other laminin isoforms, neutrophil adhesion to and migration on mLN-1, rhLN-8, and rhLN-10 substrates were studied, and participation of integrin receptors in these interactions was determined by using function-blocking mAbs. PBS (ie, nonprotein)– and HSA-coated substrates were used for comparison, and blocking of all coated surfaces with polyvinylpyrrolidone (PVP) was performed to minimize “nonspecific” interactions.
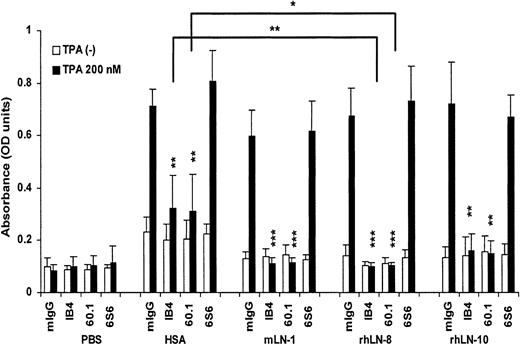
In the absence of stimulus, neutrophils adhered poorly to laminins (Figure 4). However, following stimulation with 200 nM TPA, neutrophils adhered to a considerable degree to all 3 laminin isoforms, as well as to HSA, but not to uncoated surfaces. This adhesion was mainly mediated by αMβ2 integrin. Under these experimental conditions, the blocking mAb 6S6 to β1 integrin was not inhibitory. Moreover, mAb GoH3 to α6 integrin, a major laminin receptor, was without effect, and the inhibitory effect of mAb 60.1 was reproduced with 2LPM, another mAb to αM integrin (data not shown). Interestingly, mAbs IB4 and 60.1 were more inhibitory on rhLN-8 than on HSA substrates (P = .009 and P = .015, respectively). This difference was also statistically significant for mLN-1, but not for rhLN-10 (data not shown). Altogether, the results demonstrated adhesion of stimulated neutrophils to LN-8 and to other laminin isoforms, and participation of αMβ2 integrin in this process.
Adhesion of neutrophils to laminin 8 and other laminin isoforms and participation of integrins. Adhesion of neutrophils to plastic surfaces coated with phosphate-buffered saline (PBS), human serum albumin (HSA), mouse laminin 1 (mLN-1), recombinant human laminin 8 (rhLN-8), and recombinant human laminin 10 (rhLN-10) in the absence or presence of 200 nM TPA was tested. mIgG (negative control), mAb IB4 (INTβ2, CD18), mAb 60.1 (INTαM, CD11b), and mAb 6S6 (INTβ1, CD29). Plastic surfaces were coated with proteins at 20 μg/mL and blocked with 2% polyvinylpyrrolidone (PVP). Adhesion-blocking mAbs were used at 20 μg/mL. Adhesion was measured after incubating the cells (100 × 103 cells/well) in the coated plates for 30 minutes at 37° C, and it is shown as optical density (OD). Mean and SD of 4 to 6 experiments are shown. P values (*P < .05; **P < .01; ***P < .001) compare mAbs with mIgG in each substrate protein. Horizontal lines compare the effect of mAbs IB4 and 60.1 on HSA and rhLN-8 in the presence of 200 nM TPA.
Adhesion of neutrophils to laminin 8 and other laminin isoforms and participation of integrins. Adhesion of neutrophils to plastic surfaces coated with phosphate-buffered saline (PBS), human serum albumin (HSA), mouse laminin 1 (mLN-1), recombinant human laminin 8 (rhLN-8), and recombinant human laminin 10 (rhLN-10) in the absence or presence of 200 nM TPA was tested. mIgG (negative control), mAb IB4 (INTβ2, CD18), mAb 60.1 (INTαM, CD11b), and mAb 6S6 (INTβ1, CD29). Plastic surfaces were coated with proteins at 20 μg/mL and blocked with 2% polyvinylpyrrolidone (PVP). Adhesion-blocking mAbs were used at 20 μg/mL. Adhesion was measured after incubating the cells (100 × 103 cells/well) in the coated plates for 30 minutes at 37° C, and it is shown as optical density (OD). Mean and SD of 4 to 6 experiments are shown. P values (*P < .05; **P < .01; ***P < .001) compare mAbs with mIgG in each substrate protein. Horizontal lines compare the effect of mAbs IB4 and 60.1 on HSA and rhLN-8 in the presence of 200 nM TPA.
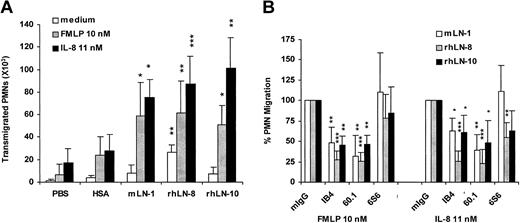
When tested in migration assays with PVP as a blocker, mLN-1, rhLN-8, and rhLN-10, but not PBS or HSA, were strong promoters of chemoattractant-induced neutrophil migration (Figure 5A). On all the substrates, FMLP and IL-8 enhanced cell migration and, when compared with HSA, the migration-promoting activity of the 3 laminin isoforms in the presence of either of the chemoattractants was statistically significant (P < .05-.001). However, in the absence of stimulus, only neutrophil migration on rhLN-8 compared with HSA was statistically significant (P < .05). Chemoattractant-enhanced neutrophil migration on rhLN-8 was not affected by pretreatment of the laminin with either heparitinase or chondroitinase (data not shown), indicating no participation of heparan sulfate or chondroitin sulfate in the preparation. The effect of function-blocking mAbs to integrins indicated that, in the presence of either FMLP or IL-8, migration of neutrophils on mLN-1, rhLN-8, and rhLN-10 was largely mediated by αMβ2 integrins (P < .05-.001) (Figure 5B). mAb 6S6 to β1 integrin was inhibitory to some extent on both rhLN-8 and rhLN-10 substrates, independent of the chemoattractant, but its effect was statistically significant only in the case of IL-8–enhanced neutrophil migration on rhLN-8.
Promotion of neutrophil migration by laminin 8 and other laminin isoforms and participation of integrins. (A) Neutrophil migration through 3-μm pore filters precoated with phosphate-buffered saline (PBS), human serum albumin (HSA), mouse laminin 1 (mLN-1), recombinant human laminin 8 (rhLN-8), and recombinant human laminin 10 (rhLN-10). (B) Effect of mAbs to integrins on neutrophil migration through filters precoated with mLN-1, rhLN-8, and rhLN-10. mIgG (negative control), mAb IB4 (INTβ2, CD18), mAb 60.1 (INTαM, CD11b), and mAb 6S6 (INTβ1, CD29) were used. Transmigration was assessed microscopically after incubating the cells (250 × 103) for 1.5 hours at 37° C without chemoattractant or in the presence of either FMLP (10 nM) or IL-8 (11 nM). Filters were coated with proteins at 20 μg/mL and blocked with 2% polyvinylpyrrolidone (PVP). Adhesion-blocking mAbs were used at 20 μg/mL. Mean and SD are shown. P values (*P < .05; **P < .01; ***P < .001) compare laminin isoforms with HSA (A), and mAbs with mIgG (defined as 100% in all experiments) on each laminin isoform (B). Each substrate protein was tested 4 to 5 times.
Promotion of neutrophil migration by laminin 8 and other laminin isoforms and participation of integrins. (A) Neutrophil migration through 3-μm pore filters precoated with phosphate-buffered saline (PBS), human serum albumin (HSA), mouse laminin 1 (mLN-1), recombinant human laminin 8 (rhLN-8), and recombinant human laminin 10 (rhLN-10). (B) Effect of mAbs to integrins on neutrophil migration through filters precoated with mLN-1, rhLN-8, and rhLN-10. mIgG (negative control), mAb IB4 (INTβ2, CD18), mAb 60.1 (INTαM, CD11b), and mAb 6S6 (INTβ1, CD29) were used. Transmigration was assessed microscopically after incubating the cells (250 × 103) for 1.5 hours at 37° C without chemoattractant or in the presence of either FMLP (10 nM) or IL-8 (11 nM). Filters were coated with proteins at 20 μg/mL and blocked with 2% polyvinylpyrrolidone (PVP). Adhesion-blocking mAbs were used at 20 μg/mL. Mean and SD are shown. P values (*P < .05; **P < .01; ***P < .001) compare laminin isoforms with HSA (A), and mAbs with mIgG (defined as 100% in all experiments) on each laminin isoform (B). Each substrate protein was tested 4 to 5 times.
Impairment of neutrophil extravasation after an inflammatory stimulus in LNα4-deficient mice
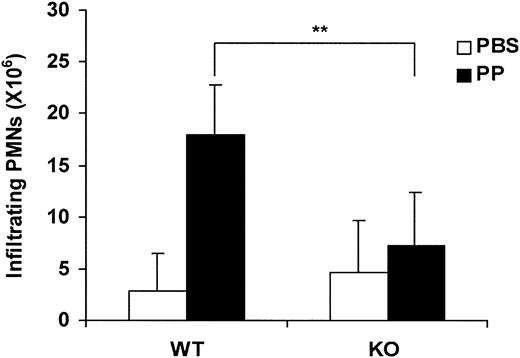
To assess the role of LN-8 in neutrophil migration to extravascular sites in response to an inflammatory stimulus, wild-type (WT) and LNα4 knock-out (KO) mice were compared in the proteose peptone–induced peritonitis model. Mice were killed 6 hours after injection with either phosphate-buffered saline (PBS) or proteose peptone (PP), and neutrophil migration into the peritoneal cavity was quantified. In WT animals, PP elicited significant extravasation of neutrophils (around 80% of total leukocytes) compared with animals injected with PBS (Figure 6). Nearly 18 million neutrophils were recruited into the peritoneum following stimulation, whereas the corresponding figure in LNα4 KO mice was approximately 7 million (60% fewer neutrophils) (Figure 6). This difference was statistically significant (P < .01). In contrast, only a few million neutrophils were collected from PBS-treated mice, and the minor difference between WT and KO animals was not statistically significant. Thus, the results indicated that recruitment of neutrophils into the peritoneal cavity after an inflammatory stimulus is impaired in LNα4-deficient mice.
Proteose peptone–induced neutrophil accumulation in the peritoneal cavity in wild-type and laminin α4–deficient mice. Harvested neutrophil count in wild-type (WT) and LNα4-deficient (KO) mice treated with either phosphate-buffered saline (PBS) or proteose peptone (PP). Neutrophil peritoneal infiltration was induced by intraperitoneal injection of 3% proteose peptone and measured 6 hours after stimulation. Neutrophils accumulated in the peritoneal cavity were harvested and counted by flow cytometry. There were 8 WT and 8 KO mice analyzed (3 with PBS and 5 with PP for each group). Mean, SD, and P value (**P < .01) are shown. PMN indicates polymorphonuclear neutrophils.
Proteose peptone–induced neutrophil accumulation in the peritoneal cavity in wild-type and laminin α4–deficient mice. Harvested neutrophil count in wild-type (WT) and LNα4-deficient (KO) mice treated with either phosphate-buffered saline (PBS) or proteose peptone (PP). Neutrophil peritoneal infiltration was induced by intraperitoneal injection of 3% proteose peptone and measured 6 hours after stimulation. Neutrophils accumulated in the peritoneal cavity were harvested and counted by flow cytometry. There were 8 WT and 8 KO mice analyzed (3 with PBS and 5 with PP for each group). Mean, SD, and P value (**P < .01) are shown. PMN indicates polymorphonuclear neutrophils.
Exogenous LN-8 protects neutrophils from apoptosis
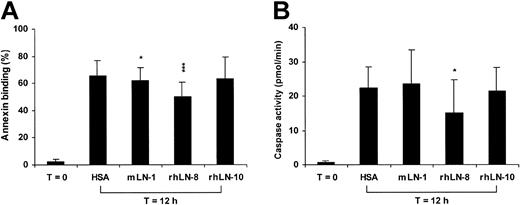
To investigate the role of LN-8 in neutrophil survival, the effect of various laminin isoforms on the degree of constitutive apoptosis of these cells was determined. Incubation of freshly isolated neutrophils on HSA substrate for 12 hours resulted in apoptosis as evidenced by both annexin V staining and caspase-3–like enzyme activity (Figure 7A and B, respectively). Of 3 laminin isoforms, rhLN-8 substrate afforded protection from apoptosis, with 25% (P < .001) and 32% (P < .05) reduction in annexin V binding and caspase activity, respectively. A small but statistically significant inhibition (P < .05) in annexin V binding was observed with mLN-1. rhLN-8 was also protective at 24 hours (data not shown). These results indicate that exogenous LN-8 can protect neutrophils from constitutive apoptosis.
Exogenous laminin 8 protects neutrophils from apoptosis. The percentage of annexin V–binding cells (A) and the degree of caspase-3–like enzyme activity (B) were used as markers of apoptosis. Neutrophils were incubated for 12 hours at 37° C on plates coated with human serum albumin (HSA), mouse laminin 1 (mLN-1), recombinant human laminin 8 (rhLN-8), and recombinant human laminin 10 (rhLN-10). (A) Cells were incubated with both fluorescein isothiocyanate–annexin V and propidium iodide and analyzed by flow cytometry. Cells stained with only annexin V were considered apoptotic. (B) Caspase-3–like activity was determined by the in vitro cleavage of the fluorescent peptide substrate, DEVD-AMC. Mean and SD of 7 experiments for each assay are shown. P values (*P < .05; ***P < .001) compare laminin isoforms with HSA at 12 hours.
Exogenous laminin 8 protects neutrophils from apoptosis. The percentage of annexin V–binding cells (A) and the degree of caspase-3–like enzyme activity (B) were used as markers of apoptosis. Neutrophils were incubated for 12 hours at 37° C on plates coated with human serum albumin (HSA), mouse laminin 1 (mLN-1), recombinant human laminin 8 (rhLN-8), and recombinant human laminin 10 (rhLN-10). (A) Cells were incubated with both fluorescein isothiocyanate–annexin V and propidium iodide and analyzed by flow cytometry. Cells stained with only annexin V were considered apoptotic. (B) Caspase-3–like activity was determined by the in vitro cleavage of the fluorescent peptide substrate, DEVD-AMC. Mean and SD of 7 experiments for each assay are shown. P values (*P < .05; ***P < .001) compare laminin isoforms with HSA at 12 hours.
Discussion
In the present study, a laminin isoform is identified in neutrophils, migration-promoting and antiapoptotic activities of an endothelial laminin isoform are described for normal neutrophils, and a role of laminin in leukocyte extravasation is demonstrated for the first time.
Neutrophil LN-8 could be used by the neutrophil itself or, when secreted, by other leukocytes, vascular endothelial cells, or other cell types. Notably, neutrophils migrated considerably on a surface coated and blocked with albumin. Participation of endogenous laminin(s) was suspected from early studies describing inhibition of FMLP-induced in vitro migration of rabbit peritoneal exudate neutrophils through uncoated filters by affinity-purified antibodies to mLN-1.21 Similarly, keratinocyte motility is promoted by deposition of endogenous LN-5 (α3β3γ2).40 Our present results indicate that endogenous α4 laminin(s), most likely as LN-8, contributes to neutrophil migration on albumin. Since higher inhibition was obtained when antibodies to LNα4 and LNα3 chains were combined, at least 2 laminin isoforms appear to be involved in this process. This may also explain why the inhibition with mAb to LNα4 is only partial. Indeed, we have recently found α3 and α5 laminins in blood cells (M.P. et al, unpublished data, March 1999). Concomitant participation of αMβ2 integrin in the neutrophil migration was intriguing and suggested recognition of the endogenous laminin(s) by this integrin. Since mAbs were always compared with mIgG control in functional assays and several mAbs to LNα4 chains, MHC class I molecule, and β4 integrin were without effect, the possibility that blocking mAbs exert their inhibitory effect by interacting with Fc receptors is most unlikely.
In contrast to uncoated plastic, LN-8 as well as LN-1, LN-10, and albumin were highly adhesive for neutrophils following stimulation of the cells with phorbol ester. Interestingly, the cell adhesion to all 3 laminin isoforms and albumin was largely mediated by αMβ2 integrin. Under the present experimental conditions, participation of (α6)β1 integrin(s) could not be demonstrated, probably because of the dominant role of αMβ2 integrin. Lack of adhesion to mLN-1 and mLN-8 was recently reported with WT and β2 integrin KO neutrophils by Sixt et al.41 The cause of the apparent discrepancy between their and our results is presently unknown. Differences in experimental designs, reagents, and/or species may explain the discrepancies. The authors used leukocytes from β2 integrin–deficient mice to avoid “nonspecific” adhesion to plastic and albumin. In our adhesion assay, blocking with PVP inhibited the adhesion to plastic, but the neutrophil adhesion to albumin was still evident. αMβ2 integrin (CD11b/CD18, Mac-1), the most abundant neutrophil integrin, is known as a rather promiscuous receptor able to recognize iC3b, intercellular adhesion molecule 1 (ICAM-1), and fibrinogen, as well as denatured proteins such as albumin.42,43 Although the degree of adhesion was similar, neutrophil adhesion to LN-8 was statistically more αMβ2 integrin dependent than neutrophil adhesion to HSA, indicating specificity in the interaction. Attachment of both phorbol ester–activated human neutrophils and monocyte chemoattractant protein 1 (MCP-1)–stimulated human monocytes to mLN-1 has also been reported to be mediated by αMβ2 integrin.26,44
In the present study, exogenous LN-1, LN-8, and LN-10 strongly enhanced neutrophil migration in the presence of chemoattractants, when compared with either uncoated or albumin-coated filters. However, only LN-8 significantly promoted spontaneous migration. As in the adhesion studies, αMβ2 integrin largely contributed to the cell migration on the 3 laminin isoforms. A lesser degree of participation of β1 integrin(s) was observed on both LN-8 and LN-10, but the inhibition was statistically significant only on LN-8 when IL-8 was used as chemoattractant. Sixt et al41 reported that LN-8 was a poor promoter of migration for β2-integrin–deficient neutrophils, when compared with LN-10, and that this low migration was partially dependent on α6β1 integrin. In view of our observations that both LN-8 and LN-10 exert strong migration-promoting activities on normal neutrophils, the results reported by Sixt et al41 indirectly suggest a major contribution of β2 integrin(s) to the neutrophil migration on LN-8. The authors were unable to demonstrate specific migration-promoting activities of LN-1, LN-8, and LN-10 for WT neutrophils because, under their experimental conditions, high background binding of the cells masked differences between substrates. Interestingly, blocking antibodies to αM integrin inhibit neutrophil transmigration through blood vessels in αL integrin–deficient mice,45 and both MCP-1–stimulated migration of monocytes on mLN-1 and tumor necrosis factor α (TNFα)–induced respiratory burst of neutrophils on mLN-1 substrate require β2 integrin(s).28,46 Altogether, the results indicate that αMβ2 integrin may function as a receptor for several laminin isoforms, including LN-8.
The in vitro cell migration studies with LN-8, a major vascular endothelial laminin, suggested contribution of this isoform in neutrophil extravasation, and the in vivo studies with LNα4 KO mice demonstrated the critical role of LN-8 in the process. Preliminary intravital microscopy experiments have shown impaired leukocyte migration through the vessel wall in LNα4-deficient mice (L.L. et al, unpublished data, October 2001). Since both WT and KO animals have similar numbers of neutrophils in the blood20 (Z.W. et al, unpublished data, April 2003), neutropenia cannot explain the decreased number of accumulated neutrophils in the peritoneum. Vascular endothelial cells and their basement membranes are by far a richer source of LN-8 than neutrophils, and hence, the impairment of neutrophil extravasation in the LNα4KO mice is most likely due to absence of LN-8 in the vascular basement membrane. Predominant participation of the exogenous (vascular) LN-8 in neutrophil extravasation is supported by the finding that neutrophil-rich cell populations isolated from blood of both WT and KO mice adhere to rhLN-8 to a similar extent following stimulation of the cells with phorbol ester (Z.W. et al, unpublished data, March 2004). It has been reported recently that, in experimental autoimmune encephalomyelitis, inflammatory cuffs of T cells occurred exclusively around endothelial basement membranes containing LN-8, whereas in the presence of LN-10, no infiltration was detectable.47 Moreover, mAb to integrin α6, a major laminin receptor, has been found to inhibit neutrophil extravasation in mice, primarily at the level of the perivascular basement membrane.48 Interestingly, transendothelial migration appears to up-regulate α6β1 integrin on neutrophils, apparently by homophilic engagement of neutrophil CD31 (platelet-endothelial cell adhesion molecule 1 [PECAM-1]).48,49 Although neutrophils were not similarly “primed” in our in vitro transmigration assays, the inhibitory effect of mAb 6S6 on the migration through filters coated with LN-8 and LN-10 may reflect participation of the α6β1 integrin.
Blood neutrophils are short-lived cells with a circulating half-life span of less than a day. These cells die as a consequence of constitutive activation of apoptosis,50,51 and expression of this cell death program is regulated by the inflammatory microenvironment.52 Interestingly, rates of apoptosis are known to be delayed by extravasation of neutrophils, indicating that the vessel wall provides survival signals.53 Indeed, in vitro studies have shown that transendothelial migration and engagement of β2 integrins are able to reproduce these signals.53 Our present results demonstrating protection from apoptosis by LN-8, as evidenced by reduction of both externalization of phosphatidyl-serine residues and activation of caspase-3–like enzymes, suggest that this endothelial laminin isoform may provide such a survival signal. In contrast, the effect of LN-1 was less significant. Although β2 integrins can play a dual role in leukocyte apoptosis,54 clustering of αMβ2 integrin by immobilized LN-8 may mediate the protective effect reported herein.
Altogether, the present results suggest that during neutrophil extravasation, endothelial basement membrane LN-8 contributes to neutrophil migration through the vessel wall, and that this cell-matrix interaction prolongs survival of the extravasated cells. In tissue compartments that lack laminins, neutrophils may use endogenous, secreted LN-8 for migration. Expression of LN-8, but not of LN-1, by all leukocyte subsets, vascular endothelium, and hematopoietic and lymphoid tissues suggests a major role of LN-8 in leukocyte physiology.
Prepublished online as Blood First Edition Paper, June 1, 2004; DOI 10.1182/blood-2004-01-0396.
Supported by grants from the Swedish Cancer Society, Karolinska Institutet, the Swedish Research Council, and the Estonian Science Foundation.
K.T. has declared a financial interest in BioStratum whose products (recombinant laminins) were studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Benedict Chambers for language revision.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal