Abstract
HIV-1 viral protein R (Vpr) shuttles between the nucleus and the cytoplasm and is believed to contribute to the process of nuclear translocation of the viral preintegration complex, thus facilitating HIV-1 replication in macrophages. In this report, we demonstrate that Hsp70, a heat-shock protein contributing to cellular stress responses, inhibits nuclear translocation of HIV-1 Vpr. In macrophages, Hsp70 is induced shortly after HIV-1 infection. Recombinant Hsp70 or a mild heat shock diminished replication of the wild-type HIV-1, suggesting that Hsp70 might function as an innate antiviral factor. Surprisingly, Hsp70 stimulated nuclear import and replication in macrophages of the Vpr-deficient HIV-1 construct. This finding suggests that Hsp70 and Vpr may function in a similar manner when expressed separately, but they neutralize each other's activity when present together. Consistent with this interpretation, Hsp70 coprecipitated with Vpr from HIV-1–infected cells.
Introduction
The ability of HIV-1 to replicate in nonproliferating cells, such as terminally differentiated macrophages, sets this virus, and the group of lentiviruses to which it belongs, apart from other retroviruses that can productively infect only mitotic cells.1-4 A large body of evidence linked the ability of HIV to replicate in nondividing and slowly dividing cells to the translocation of the viral preintegration complex (PIC) through the intact nuclear envelope.3,5-8 These observations suggested that HIV “hijacks” the host cells' own nuclear import machinery to deliver the viral genome into the nucleus. Since this machinery normally deals with proteins carrying some type of nuclear localization signal (NLS), it is reasonable to assume that a component or components of the PIC contain targeting signals that direct the PIC to the nucleus.
One of the PIC proteins believed to mediate HIV-1 nuclear import is viral protein R (Vpr).9-12 Numerous published reports identified several important features of Vpr that might be related to its activity in HIV-1 nuclear translocation (reviewed in Bukrinsky and Adzhubei13 ). Vpr has been shown to enter nuclei and localize to both nuclear envelope and nucleoplasm.12,14 In addition, Vpr induces dynamic disruptions in nuclear envelope integrity, which may serve as entry points for large nucleoprotein complexes such as HIV-1 PIC.15,16 Vpr also binds to certain nuclear pore proteins (nucleoporins),17 an activity that might mediate binding of the PIC to the nuclear pore.10 Finally, Vpr was found to bind to importin α,11 the cellular receptor for basic-type NLSs,12 and change its affinity for the NLS, suggesting that it might stimulate importin α/β (cellular NLS receptor)–dependent nuclear import of the HIV-1 PIC.11
Surprisingly, the requirement for Vpr in HIV-1 nuclear import appears to be cell-type–dependent. Nuclear import and replication of HIV-1 in certain cells, such as growth-arrested HeLa or T cells, do not require Vpr,3,18,19 and import of the Vpr-deficient HIV-1 PIC can be rescued by HeLa cytosol, but not by the cytosol of other cells (eg, macrophages) where HIV-1 replication is Vpr-dependent.11 This capacity of the HeLa cytosol to support nuclear import of the Vpr-deficient HIV-1 PICs was shown to depend on the presence of heat-shock protein 70 (Hsp70),20 a member of the large family of heat-shock proteins.
Heat-shock proteins are produced in response to stress and function to assist protein folding and as chaperones. One of their stress-response activities is a role in cellular nuclear import. In particular, Hsp70 was shown to facilitate the interaction between the basic type NLS and importin α.21,22 The ectopic expression of human Hsp70 in mouse cells complemented the defective import of a mutant simian virus 40 (SV40) large T antigen,23 and the depletion of Hsp70 from cytosolic extracts prevented import.24,25 In addition, our recent studies demonstrated that Hsp70 stimulates nuclear import of the HIV-1 PIC in a cell-free in vitro assay.20
In this study, we demonstrate that Hsp70 is induced in macrophages by HIV-1 infection. Exogenously added Hsp70, as well as mild heat shock that initiated prolonged Hsp70 expression in macrophages, diminished replication of the Vpr-positive HIV-1 but stimulated replication of the Vpr-deficient virus. These results reveal new details about Hsp70 activity in macrophages.
Materials and methods
Reagents
Recombinant Hsp70, as well as Hsp27 and Hsp70 enzyme-linked immunosorbent assay (ELISA) kits were purchased from Stressgen Biotechnologies (Victoria, BC, Canada). Mouse monoclonal antibody against human Hsp70 was from Calbiochem (San Diego, CA), anti-Hsp70 (Hsp72) rabbit polyclonal antibody was from Stressgen, and anti-Vpr rabbit polyclonal antibody (clone 709) was provided by Dr Josephine Sire (Institut National de la Santé et de la Recherche Médicale, Marseille, France). Synthetic Vpr26 was a kind gift of Dr Ulrich Schubert (Humboldt University, Berlin, Germany). Lactate dehydrogenase (LDH) kit was from Sigma-Aldrich (St Louis, MO). DNA constructs (green fluorescent protein–pyruvate kinase [GFP-PK], nuclear localization signal–GFP-PK [NLS-GFP-PK], and GFP-PK–viral protein R [GFP-PK-Vpr]) have been described previously.27 Briefly, GFP-PK encodes for a fusion protein between GFP and chicken piruvate kinase (an 88-kDa chimera), NLS-GFP-PK adds the classical NLS (PKKKRKV) from the SV40 large T antigen to the amino terminus of GFP, and GFP-PK-Vpr has full-length Vpr fused at the carboxyl-terminus.
Cells, viruses, and infections
Monocyte-derived macrophages (MDMs) were prepared from peripheral blood mononuclear cells (PBMCs) by adherence to plastic as described previously.28 Briefly, human PBMCs were isolated from buffy coats of healthy seronegative donors by Ficoll density gradient centrifugation (Ficoll-Paque PLUS; Pharmacia Biotech, Piscataway, NJ). PBMCs were cultured in Primaria flasks (Becton Dickinson, Franklin Lakes, NJ) in culture medium (Dulbecco modified Eagle medium [DMEM]) supplemented with 10% heat-inactivated normal human serum, 2 mM glutamine, 50 U/mL penicillin, and 50 μg/mL streptomycin (all from Life Technologies, Bethesda, MD) at 37° CinaCO2 incubator at a cell density of 8 × 106 cells/mL. At 2 hours after plating, nonadherent cells were aspirated, and the adherent cells were cultured overnight in DMEM supplemented with macrophage colony-stimulating factor (M-CSF, 2 ng/mL). At 18 hours after initial plating, adherent cells were detached with 10 mM EDTA (ethylenediaminetetraacetic acid)/phosphate-buffered saline (PBS) and plated in 24-well Primaria plates (Becton Dickinson) at a density of 106 cells/mL for 7 days in the presence of recombinant human M-CSF (at 2 ng/mL). After 7 days, final MDM cultures were composed of about 98% macrophages as judged by morphology and nonspecific esterase activity. The following HIV-1 strains were used for infection of MDM: macrophage-tropic strain ADA29 ; Vpr-positive and Vpr-negative NLHADA constructs.11 For experiments involving polymerase chain reaction (PCR) analysis, viral inoculum was treated with 200 U/mL RNAse-free DNAse (Roche, Basel, Switzerland) for 1 hour at room temperature. Cells were infected with viral inoculae equalized according to the reverse transcriptase (RT) activity (5 × 105 counts per minute/106 cells). To increase the efficiency of infection, in some experiments NLHADA constructs were pseudotyped with murine leukemia virus (MLV) envelope as described.30
PCR analysis
PCR analysis of HIV-1 infection was performed 24 hours after infection using primers specific for the pol region of the viral genome (these primers amplify late products of reverse transcription) or 2–long terminal repeat (2-LTR) circle forms of viral DNA (these forms are found exclusively in the nucleus5 ) essentially as described previously.31
Immunoprecipitation
MDMs infected with MLV-pseudotyped NLHADA virus were washed with PBS twice and lysed in the immunoprecipitation buffer containing 5.0 mM CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid), 50 mM NaCl, and 20 mM Tris (tris(hydroxymethyl)aminomethane, pH 7.5), according to Mariani et al.32 Equal amounts of the lysates (according to protein content) were immunoprecipitated with rabbit anti-Hsp70 polyclonal antibody and analyzed by Western blotting using rabbit anti-Vpr polyclonal or mouse monoclonal anti-Hsp70 antibodies.
Analysis of subcellular localization
Analysis of subcellular localization of Vpr constructs was performed in HEK 293T cells. Cells were cotransfected with 10 μg pcDNA3.1-Hsp70/Myc plasmid33 and 2 μg vector DNA expressing GFP-PK, NLS-GFP-PK, or GFP-PK-Vpr using Metafectene (Biontex, Munich, Germany) following the manufacturer's protocol. Cells were washed with phosphate-buffered saline (PBS) 24 hours after transfection, fixed on coverslips for 10 minutes in 4% paraformaldehyde, and rinsed in PBS. The coverslips were then inverted and mounted on glass slides using Mount Medium H1200 containing DAPI (4,6 diamidino-2-phenylindole; Vector Laboratory, Burlingame, CA). To inhibit nuclear export, 400 nM leptomycin B (LMB; Sigma-Aldrich, St Louis, MO) was added to the medium for 2.5 hours prior to cell fixation.
Results
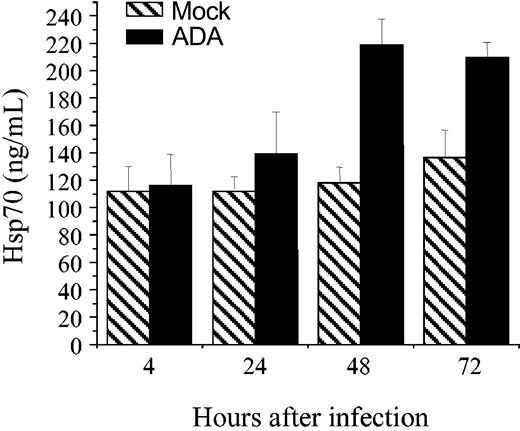
A study by Brenner and colleagues (Wainberg et al34 ) demonstrated induction of Hsp70 in HIV-1–infected T cells. However, quantitative measurements of Hsp70 have not been reported. We thus analyzed Hsp70 in HIV-1–infected monocyte-derived macrophages using ELISA. This analysis demonstrated an increase in Hsp70 levels after HIV-1 infection (Figure 1). Hsp70 expression peaked 48 hours after infection and decreased thereafter. Given that Hsp70 stimulates cellular nuclear import in general,24 and nuclear import of the HIV-1 preintegration complex in vitro,20 it was reasonable to hypothesize that Hsp70 induced by HIV-1 infection may assist Vpr in facilitating HIV-1 replication in macrophages.
HIV-1 infection induces Hsp70 in macrophages. Macrophages (1 × 106 cells) were infected with HIV-1 ADA or mock infected. Intracellular Hsp70 was measured by ELISA before infection (time 0) and at indicated time points after infection. Results are mean ± SE of triplicate cultures.
HIV-1 infection induces Hsp70 in macrophages. Macrophages (1 × 106 cells) were infected with HIV-1 ADA or mock infected. Intracellular Hsp70 was measured by ELISA before infection (time 0) and at indicated time points after infection. Results are mean ± SE of triplicate cultures.
To test this hypothesis, we first analyzed the effect of Hsp70 on subcellular localization of ectopically expressed Vpr. For this analysis, the following constructs were used: GFP-PK-Vpr (its subcellular localization has been shown to be determined by Vpr27 ); GFP-PK (control construct that is excluded from the nucleus27 ); and NLS-GFP-PK, which localizes to the nucleus due to the presence of a basic-type NLS.27 These constructs were cotransfected into HEK 293T cells together with a vector expressing Hsp70 (or an empty vector), and localization of the GFP marker was monitored in the absence or presence of leptomycin B (an inhibitor of the chromosome region maintenance-1 (Crm1)–dependent nuclear export35 ). Similar to the results reported by Greene and colleagues (Sherman et al27 ) in the absence of leptomycin B (LMB), only NLS-GFP-PK, but not GFP-PK-Vpr, localized to the nucleus (Figure 2A), consistent with a presence of a potent Crm1-dependent nuclear export signal in Vpr. When nuclear export was inhibited by LMB, both GFP-PK-Vpr and NLS-GFP-PK (but not GFP-PK) localized to the nucleus (Figure 2B, upper row panels). However, the nuclear localization of GFP-PK-Vpr was abolished when Hsp70-expressing vector was cotransfected together with the GFP-PK-Vpr–expressing plasmid (Figure 2B, bottom row panels). Importantly, cotransfection of Hsp70-expressing plasmid did not affect nuclear localization of the NLS-GFP-PK protein. This result indicates that Hsp70 specifically inhibits nuclear localization of Vpr, without affecting nuclear targeting of nucleophiles carrying a basic-type NLS. It also seems to contradict the initial hypothesis concerning the stimulating effect of Hsp70 on HIV-1 nuclear import, thus providing an unexpected intrigue to our studies.
Hsp70 specifically inhibits nuclear import of Vpr. (A) HEK 293T cells were transfected with vectors expressing indicated constructs.27 At 48 hours after transfection, cells were fixed, mounted in ProLong Antifade Imaging Medium (Molecular Probes, Eugene, OR), and analyzed by fluorescent microscopy. (B) HEK 293T cells were cotransfected with an Hsp70-expressing (or an empty pcDNA) vector and a plasmid encoding indicated constructs. At 48 hours after transfection, cells were exposed to leptomycin B (LMB, 400 nM) for 2.5 hours, fixed, and analyzed by fluorescent microscopy. A 5-fold excess of the Hsp70-encoding vector over the plasmid-encoding Vpr construct was used. All images were acquired using a BX-60 Olympus Epi-fluorescence microscope (Melville, NY) with Evolution MP Digital Camera using Image-Pro Plus v. 4.5 acquisition software (Media Cybernetics, Silver Spring, MD). Images are presented at × 100 original magnification (100 × oil objective lens [NA = 1.30]). Results are shown for 1 representative experiment of 3 performed.
Hsp70 specifically inhibits nuclear import of Vpr. (A) HEK 293T cells were transfected with vectors expressing indicated constructs.27 At 48 hours after transfection, cells were fixed, mounted in ProLong Antifade Imaging Medium (Molecular Probes, Eugene, OR), and analyzed by fluorescent microscopy. (B) HEK 293T cells were cotransfected with an Hsp70-expressing (or an empty pcDNA) vector and a plasmid encoding indicated constructs. At 48 hours after transfection, cells were exposed to leptomycin B (LMB, 400 nM) for 2.5 hours, fixed, and analyzed by fluorescent microscopy. A 5-fold excess of the Hsp70-encoding vector over the plasmid-encoding Vpr construct was used. All images were acquired using a BX-60 Olympus Epi-fluorescence microscope (Melville, NY) with Evolution MP Digital Camera using Image-Pro Plus v. 4.5 acquisition software (Media Cybernetics, Silver Spring, MD). Images are presented at × 100 original magnification (100 × oil objective lens [NA = 1.30]). Results are shown for 1 representative experiment of 3 performed.
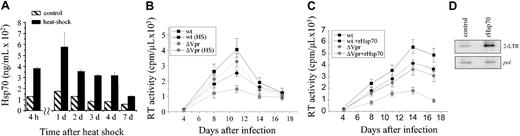
Vpr is believed to be one of the principal factors that regulate nuclear translocation of the HIV-1 preintegration complex in macrophages, and our finding that Hsp70 specifically inhibits nuclear localization of Vpr suggested that it may also inhibit HIV-1 replication in macrophages. To test this premise, we subjected primary human macrophages to a mild heat shock by transferring plates for one hour to an incubator at 43° C. Such treatment did not affect viability of macrophage cultures as evidenced by cell morphology, cell count, and LDH assay performed 1 day or 7 days after the heat shock (not shown). Heat-shocked macrophages, in contrast to control cultures, expressed high levels of Hsp70, which persisted for 5 to 7 days after cells were returned to normal culture conditions (Figure 3A). Heat-shocked and normal macrophages were infected with Vpr-positive or Vpr-deficient HIV-1NLHADA,11 and virus replication was followed by RT activity in culture supernatants. It should be noted here that reports from different groups documented anywhere from 2-fold to more than 100-fold impairment in replication of Vpr-deficient HIV-1 mutants in macrophages.17,37-39 In addition to variations in cell culture conditions and HIV-1 strains, these differences are likely due to different multiplicities of infection.38 Under standard conditions used in our laboratory, we routinely observe a 3- to 10-fold difference in replication between Vpr-positive and Vpr-deficient viruses in monocyte-derived macrophages.19,40 As shown in Figure 3B, replication of the Vpr-positive virus was diminished in heat-shocked cells relative to normal macrophages. Surprisingly, replication of the Vpr-deficient virus was enhanced by heat shock.
Analysis of Hsp70 effects on HIV-1 nuclear import and replication in macrophages. (A) Macrophages (1 × 106 cells) were subjected to mild heat shock (47°C, one hour) and then cultured for indicated time. Intracellular Hsp70 was measured by ELISA. Results are mean ± SE of triplicate cultures. (B) Macrophages subjected or not to heat shock (HS) were infected in triplicates with the wild-type (wt) or Vpr-deficient (ΔVpr) HIV-1 NLHXADA.36 Viral replication was assessed at the indicated time points by measuring RT activity in the culture supernatants. Results are mean ± SE of triplicates. (C) Triplicate macrophage cultures were infected with the wild-type (wt) or Vpr-deficient (ΔVpr) HIV-1 NLHXADA36 and cultured in the absence or presence of 100 ng/mL recombinant human Hsp70. Viral replication was assessed as in panel B, and results are presented as mean ± SE. (D) At 24 hours after infection of macrophage cultures with Vpr-deficient HIV-1 NLHXADAΔVpr,36 cells were lysed and assayed for 2-LTR– and pol-specific (a control for total HIV-1 DNA) DNA products by PCR.31 Results are representative of 2 independent experiments.
Analysis of Hsp70 effects on HIV-1 nuclear import and replication in macrophages. (A) Macrophages (1 × 106 cells) were subjected to mild heat shock (47°C, one hour) and then cultured for indicated time. Intracellular Hsp70 was measured by ELISA. Results are mean ± SE of triplicate cultures. (B) Macrophages subjected or not to heat shock (HS) were infected in triplicates with the wild-type (wt) or Vpr-deficient (ΔVpr) HIV-1 NLHXADA.36 Viral replication was assessed at the indicated time points by measuring RT activity in the culture supernatants. Results are mean ± SE of triplicates. (C) Triplicate macrophage cultures were infected with the wild-type (wt) or Vpr-deficient (ΔVpr) HIV-1 NLHXADA36 and cultured in the absence or presence of 100 ng/mL recombinant human Hsp70. Viral replication was assessed as in panel B, and results are presented as mean ± SE. (D) At 24 hours after infection of macrophage cultures with Vpr-deficient HIV-1 NLHXADAΔVpr,36 cells were lysed and assayed for 2-LTR– and pol-specific (a control for total HIV-1 DNA) DNA products by PCR.31 Results are representative of 2 independent experiments.
In addition to Hsp70, heat shock activates expression of a number of other stress-response genes, any of which could potentially be responsible for the observed effect. To directly analyze the effect of Hsp70 on HIV-1 replication in macrophages, we cultured HIV-1–infected macrophages in the presence or absence of recombinant Hsp70. As demonstrated previously, Hsp70 is efficiently taken up by cells from the culture medium and is transported into the nucleus,41 indicating that its ability to interact with the nuclear import machinery is preserved. Similar to the effect of heat shock, recombinant Hsp70 diminished replication of the Vpr-positive HIV-1 but stimulated an otherwise inefficient replication of the Vpr-deficient virus (Figure 3C). Importantly, this effect could not be attributed to a possible endotoxin contamination of the recombinant Hsp70, as endotoxin is expected to reduce HIV-1 replication in macrophages by down-regulating CCR5 receptor.42,43 This result confirms the role of Hsp70 as a regulator of HIV-1 infection and suggests that Hsp70 is a significant contributor to the effect of heat shock on viral replication.
Enhancement of replication of the Vpr-deficient virus correlated well with increased levels of 2-LTR circle forms (but not total proviral DNA amplified with primers specific for the pol gene) of HIV-1 DNA in cultures treated with recombinant Hsp70 relative to control cultures analyzed 24 hours after infection (Figure 3D). Since production of 2-LTR forms is indicative of the efficiency of nuclear import,5 this result suggests that the observed Hsp70-mediated enhancement of replication of the Vpr-deficient HIV-1 may be due to stimulation of this virus' nuclear import in macrophages.
Our results so far indicate that Hsp70 stimulates nuclear import and replication of the Vpr-deficient virus, but inhibits replication of the Vpr-positive strain, suggesting that the resulting effect of Hsp70 depends on whether Vpr is present in the preintegration complex. To test whether Hsp70 interacts with Vpr in macrophages, we immunoprecipitated Hsp70 from HIV-1– or mock-infected cells and analyzed the precipitated proteins by Western blotting. As shown in Figure 4, Vpr did coprecipitate with Hsp70 (panel C). Hsp70 also coimmunoprecipitated with synthetic Vpr26 added to cell lysates. Importantly, this effect was specific for Vpr, as p24 was not found in immunoprecipitates (Figure 4A). Therefore, when Vpr and Hsp70 are present together in a cell, they bind and may neutralize each other.
Hsp70 coimmunoprecipitates with Vpr from HIV-1– infected cells. Macrophages were infected with macrophage-tropic HIV-1 strain ADA or mock infected. At 48 hours after infection, cells were lysed and the lysates were immunoprecipitated with a polyclonal anti-Hsp70 antibody. Cell lysates and immunoprecipitates were analyzed by Western blotting for the presence of p24 (A), Hsp70 (B), and Vpr (C). In one sample (mock + sVpr), synthetic Vpr26 was added to mock-infected cell lysate prior to immunoprecipitation. WB indicates Western blot; IP, immunoprecipitation.
Hsp70 coimmunoprecipitates with Vpr from HIV-1– infected cells. Macrophages were infected with macrophage-tropic HIV-1 strain ADA or mock infected. At 48 hours after infection, cells were lysed and the lysates were immunoprecipitated with a polyclonal anti-Hsp70 antibody. Cell lysates and immunoprecipitates were analyzed by Western blotting for the presence of p24 (A), Hsp70 (B), and Vpr (C). In one sample (mock + sVpr), synthetic Vpr26 was added to mock-infected cell lysate prior to immunoprecipitation. WB indicates Western blot; IP, immunoprecipitation.
Discussion
Heat-shock proteins are produced in response to physiologic stress, such as heat shock or ultraviolet radiation. Similar stress responses are also initiated upon bacterial or viral infection44 ; in this case, heat-shock proteins, and in particular Hsp70, may contribute to activation of innate and adaptive immunity by activating natural killer cell (NK), gamma delta T-cell, and cytotoxic T-lymphocyte (CTL) activities.45 On the other hand, Hsp70 has been shown to promote increased viral gene expression and cytopathic effect during measles virus infection.46 Results presented in this report show that Hsp70 is induced in macrophages upon infection with HIV-1 and may provide some protection against infection with a Vpr-positive virus.
This protective effect is most evident in macrophages subjected to heat shock, likely because Hsp70 levels induced by the heat shock are significantly higher than after HIV-1 infection (compare Figures 1 and 3A). It is possible that other heat-shock proteins, such as Hsp27, also contribute to the observed protective effect of the heat shock. In addition, the fact that Hsp70 is already induced in a heat-shocked cell when it encounters HIV-1, while under normal infection conditions Hsp70 induction follows cell contact with the virus, has significant bearing on the magnitude of the protective effect. It should be noted that results in Figure 1 do not indicate that Hsp70 induction occurs only after 48 hours of infection of an individual cell; this experiment analyzes spreading unsynchronized infection and measures total accumulation of Hsp70. Normally, HSPs are induced within hours after infection,47 thus leaving sufficient time to neutralize HIV-1 Vpr before the preintegration complex enters the nucleus (10-12 hours after infection31 ). The balance between Vpr and Hsp70 may determine, at least in part, the efficiency of HIV-1 infection. For example, high levels of Hsp70 may contribute to relative resistance to HIV-1 infection of activated macrophages.48
Intriguingly, Hsp70 stimulates nuclear import and replication in macrophages of a Vpr-deficient HIV-1. This effect is consistent with the capacity of Hsp70 to stimulate nuclear import of weak karyophiles23 and suggests that Vpr and Hsp70 might enhance HIV-1 nuclear import through a similar mechanism. In particular, Vpr, similar to the effect of Hsp70, may enhance karyophilic properties of weak NLSs present in the matrix protein,49 thus stimulating nuclear import of the HIV-1 PIC. Intriguingly, the magnitude of the Vpr effect appears to increase when viral inoculum is decreased.38 Therefore, it is likely that the effect of Hsp70 on macrophage infection by Vpr-deficient HIV-1 may be even more pronounced when multiplicity of infection is low. Such conditions occur in vivo, where infection with viruses carrying mutated Vpr has been described.50,51 While infection with these mutant viruses is believed to be associated with a long-term nonprogressor status, the rate of replication of these viruses in macrophages (and replication in these cells may determine the viral load52 ) may depend on expression in these cells of Hsp70, which is induced by environmental stresses and infectious agents. It appears rational to recommend that such patients try to minimize stresses that may stimulate Hsp70 expression.
If both Vpr and Hsp70 stimulate HIV-1 nuclear import, why does Hsp70 reduce replication of the Vpr-positive virus? It should be noted that Vpr is a component of the HIV-1 PIC19,53 and exerts its activity in cis, while the Hsp70 functions in trans with regard to the PIC. Our results demonstrate that Hsp70 and Vpr interact within an infected cell (Figure 4). We cannot exclude the possibility that binding of Hsp70 to Vpr is indirect, mediated by some cellular adaptor protein. This concept is supported by the lack of direct interaction between recombinant Vpr and Hsp70 proteins (S.I., unpublished observation, February 2004). In any case, binding of Vpr and Hsp70 in HIV-1–infected cells may explain the neutralizing effect of Hsp70 on Vpr activity. For example, binding of Hsp70 to Vpr may make the PIC less capable of traversing the nuclear pore (due to excessive size and/or inefficient interaction with the cellular nuclear import machinery).
In this report, we provide evidence that Hsp70 counteracts nuclear import activity of Vpr and reduces HIV-1 replication in macrophages in a Vpr-dependent fashion. This finding extends our recent observations that Hsp70 reverses the G2 arrest induced by Vpr in proliferating cells (S.I., unpublished observation, February 2004). Therefore, Vpr and Hsp70 counteract each other's activity, likely through a direct interaction. These inhibitory effects of Hsp70 on Vpr-dependent G2 arrest and HIV-1 nuclear import are consistent with Hsp70 functioning as an innate anti-HIV factor.
Given that macrophage is the main (and sometimes the only) target cell for lentiviruses,54 the fact that both primate and nonprimate lentiviruses encode a Vpr-like protein55 indicates a critical role that this protein plays in lentiviral infection of macrophages. Therefore, the activity of Hsp70 to bind and neutralize Vpr is likely an important innate antilentiviral mechanism. It is thus not surprising that expression of Hsp70 is activated in HIV-1–infected cells (this report, Wainberg et al,34 and Scheuring et al56 ). Identifying the biochemical and structural basis of Hsp70 interaction with Vpr may provide new targets for future approaches aimed at curtailing HIV-1 replication.
Prepublished online as Blood First Edition Paper, May 27, 2004; DOI 10.1182/blood-2004-01-0081.
Supported by the National Institutes of Health (NIH) grants AI40891 and GM63080 (Y.Z.) and AI33776 (M.B.).
S.I. and Y.Z. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Drs M. Sherman and W. Greene (Gladstone Institute of Virology and Immunology, San Francisco, CA) for providing GFP-PK-Vpr, GFP-PK, and NLS-GFP-PK constructs. We thank Dr Richard Vile for a generous gift of pcDNA3.1-Hsp70/Myc plasmid, Dr Josephine Sire for anti-Vpr antibody, and Dr Ulrich Schubert for synthetic Vpr. pcDNA-Env(MLV) plasmid encoding Env of the amphotropic MLV (from Dr N. Landau) was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases NIH.

![Figure 2. Hsp70 specifically inhibits nuclear import of Vpr. (A) HEK 293T cells were transfected with vectors expressing indicated constructs.27 At 48 hours after transfection, cells were fixed, mounted in ProLong Antifade Imaging Medium (Molecular Probes, Eugene, OR), and analyzed by fluorescent microscopy. (B) HEK 293T cells were cotransfected with an Hsp70-expressing (or an empty pcDNA) vector and a plasmid encoding indicated constructs. At 48 hours after transfection, cells were exposed to leptomycin B (LMB, 400 nM) for 2.5 hours, fixed, and analyzed by fluorescent microscopy. A 5-fold excess of the Hsp70-encoding vector over the plasmid-encoding Vpr construct was used. All images were acquired using a BX-60 Olympus Epi-fluorescence microscope (Melville, NY) with Evolution MP Digital Camera using Image-Pro Plus v. 4.5 acquisition software (Media Cybernetics, Silver Spring, MD). Images are presented at × 100 original magnification (100 × oil objective lens [NA = 1.30]). Results are shown for 1 representative experiment of 3 performed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/6/10.1182_blood-2004-01-0081/6/m_zh80180466790002.jpeg?Expires=1769204969&Signature=Q5kKK7Qo6pntWoOGp3SmZLS8Hd3AemyVRSOrsC9FOXTm09askmdORdpBnHl9aop-7xmqqbhzn3OcU5ATjbKFlYwRy9Ux3YSukXq2GioUhvguQMgrXdTE1Kq2mQWwoaqOWpZpiVc7p6zEK8j7H6Fk5Vf4nqFfc8TsA4o9ppwLMKhI2YfVMu2w1ABYEfQltHzZUa3yTM6qB3ymjndyWY5ZLxctF8c282IFnnGQ5v~wJ-rOXqJgtFke456Zfz0psL1sr5prHYn5B0qaivHENvChZhhoGCc9NzLEQHYLyl9dUkRK6zxxuv~01VsKhKWNrDvCoODiFKhN6QfQeuX5REb6Zw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal