Abstract
Severe and prolonged cytopenias represent a considerable problem in clinical stem cell transplantations. Cytokine-induced ex vivo expansion of hematopoietic stem and progenitor cells has been intensively explored as a means of accelerating hematopoietic recovery following transplantation but have so far had limited success. Herein, overexpression of D-type cyclins, promoting G0/G1 to S transition, was investigated as an alternative approach to accelerate myeloid reconstitution following stem cell transplantation. With the use of retroviral-mediated gene transfer, cyclin D2 was overexpressed in murine bone marrow progenitor cells, which at limited doses showed enhanced ability to rescue lethally ablated recipients. Competitive repopulation studies demonstrated that overexpression of cyclin D2 accelerated myeloid reconstitution following transplantation, and, in agreement with this, cyclin D2–transduced myeloid progenitors showed an enhanced proliferative response to cytokines in vitro. Furthermore, cyclin D2–overexpressing myeloid progenitors and their progeny were sustained for longer periods in culture, resulting in enhanced and prolonged granulocyte production in vitro. Thus, overexpression of cyclin D2 confers myeloid progenitors with an enhanced proliferative and granulocyte potential, facilitating rapid myeloid engraftment and rescue of lethally ablated recipients.
Introduction
Through their unique ability to self-renew as well as multilineage differentiate into all blood cell lineages, long-term repopulating hematopoietic stem cells (LT-HSCs) have proven to be both required and sufficient to ensure durable multilineage reconstitution following transplantation of myeloablated recipients.1,2 However, LT-HSCs appear less efficient at rapidly reconstituting hematopoiesis on transplantation, and rather distinct populations of short-term HSCs (ST-HSCs) and progenitors are critical for immediate replenishment of short-lived myeloid cells following hematopoietic transplantations.1,3 Severe and prolonged cytopenias continue to represent a major cause of morbidity and mortality following transplantations.4 HSCs and progenitors expand during development and on transplantation,5 and it is, therefore, natural to seek to improve the quality of hematopoietic grafts by ex vivo expansion. However, so far, extensive efforts to ex vivo expand hematopoietic progenitor and stem cells prior to transplantation have been disappointing, resulting in little or no enhanced reconstituting activity, and in many instances in compromised reconstitution.5-12
To date, attempts to ex vivo expand HSCs have almost exclusively focused on the use of cytokines promoting in vitro proliferation of HSCs,8,13,14 but recent studies suggest that modification of other molecular targets, such as the transcription factor HoxB4, might be more suitable for obtaining such expansion.15 D-type cyclins, (cyclin D1, D2, and D3) promote transition through the G1 phase of the cell cycle, and, when overexpressed, shorten the time required for cell cycling and can extend the replicative life span of cells.16-18 Through activation of CDK4 and CDK6, they accelerate G0/G1 to S-phase transition and mediate mitogenic signals, including those signaled through cytokine receptors.19 Cyclin D2 and D3 are known to be expressed in normal lymphocytes and all 3 types of D-cyclins in myeloid progenitors.20,21 D-type cyclins, when overexpressed in the cytokine-dependent 32Dcl3 myeloid progenitor cell line, shorten G1.22,23 However, whether this can be translated into enhanced proliferative activity of primary hematopoietic progenitor and stem cells has not been investigated. Of particular relevance for ex vivo and in vivo expansion of hematopoietic progenitors, it also remains unclear whether overexpression of D-type cyclins would enhance the proliferative activity of progenitor and stem cells beyond that obtained by optimal combinations of already identified cytokines, postulated to also mediate the enhanced proliferative activity of progenitors observed following transplantation.24
In the present studies we explored the potential of overexpressing D-type cyclins to promote ex vivo expansion of hematopoietic progenitor cell activity, as well as in vivo myeloid reconstitution following myeloablation and transplantation. Herein, we demonstrate that cyclin D2 overexpression enhances hematopoietic progenitor cell proliferation and expansion, translating into enhanced and accelerated short-term myeloid reconstitution and rescue of lethally myeloablated recipients.
Materials and methods
Mice and bone marrow cells
Eight- to 12-week-old mice of congenic C57bl/6 strains (CD45.1, CD45.2, and F1-CD45.1/CD45.2) were used. In most experiments, CD45.1 mice were used as bone marrow (BM) donors for transduction, CD45.2 BM for competitors, and F1-CD45.1/CD45.2 mice as recipients. BM cells were collected and counted after crushing of hind limbs in a mortar. Mice were maintained under specific pathogen-free conditions and fed irradiated chow and autoclaved and acidified water. All in vivo experiments were approved by the local ethical committee.
Hematopoietic growth factors
Recombinant rat (rr) stem cell factor (SCF), recombinant human (rh) granulocyte colony-stimulating factor (G-CSF) and rh megakaryocyte growth and development factor (MGDF) for in vitro studies were generously provided by Amgen (Thousand Oaks, CA), recombinant murine (rm) interleukin 3 (IL-3) by PeproTech (Rocky Hill, NJ), rhIL-6 by Genentics Institute (Cambridge, MA), and rhFlt3-ligand (FL) by Immunex (Seattle, WA). Unless otherwise indicated, cytokine concentrations used in this study were as follows: 50 ng/mL rrSCF, 25 ng/mL rhG-CSF, 50 ng/mL rhFL, 50 ng/mL rhMGDF, 10 ng/mL rmIL-3, and 50 ng/mL rhIL-6. For in vivo studies, rhG-CSF was purchased from Amgen.
Antibodies
All antibodies were from PharMingen (San Diego, CA) unless otherwise described. Antibodies used for cell surface staining were 2.4G2 (CD16/32), RA3-6B2 (B220), RB6-8C5 (Gr1), M1/70 (CD11b, Mac1), 145-2C11 (CD3ϵ), H129.19 (CD4), 53-7 (CD5), 53-6.7 (CD8α), Ter-119, E13-161.7 (Sca-1), 2B8 (c-kit), A20 (CD45.1), 104 (CD45.2), and polyclonal goatantirat (Caltag, Burlingame, CA).
Fluorescence activated cell sorting (FACS) of Linlo/–Sca-1+c-kit+ hematopoietic stem cells
Linlo/–Sca-1+c-kit+ (LSK) cells were sorted as described elsewhere.25 Briefly, lineage marker–positive cells were depleted by use of purified antibodies against B220, CD4, CD5, CD8α, CD11b, Gr1, and Ter119, and immunomagnetic beads were conjugated with sheep-antirat Fc (Dynabeads, Dynal, Oslo, Norway). Lineage (Lin)–depleted BM cells were stained with a secondary goat-antirat antibody, conjugated with phycoerythrin-Cy5 (PECy5; Tri-color; Caltag) to visualize residual Lin+ cells, and stained with anti–Sca-1–FITC (fluorescein isothiocyanate) and anti–c-kit–APC (allophycocyanin) antibodies. Stained cells were sorted on a FACSVantageSE (Becton Dickinson, San Jose, CA). Cells with low viability were excluded from the sorting gate by staining with 7-amino-actinomycin D (Sigma-Aldrich, St Louis, MO).
Retroviral constructs
Murine cyclin D2 cDNA, a kind gift from Charles J. Sherr (Memphis, TN)16,23 was inserted in a retroviral construct26 in front of the sequences for internal ribosome entry site (IRES) and enhanced green fluorescence protein (GFP), driven by a murine stem cell virus (MSCV) promoter and named MCd2IG (Figure 1). MCd2IG was stably expressed in the GP+E86 cell line (generously provided by Arthur Bank, New York, NY) and cloned from single colonies. The clone used in this study had a virus titer of around 1 × 107 TU/mL. As a control virus producer, GP+E86 cell line engaged to integrate MGirL22Y construct was used (a generous gift from Derek A. Persons and Arthur W. Nienhuis, Memphis, TN),27 with more than 1 × 107 transducing unit (TU)/mL virus titer. Because the MGirL22Y vector, unlike the MCd2IG vector, contains the L22Y gene (conferring trimetrexate resistance), we compared in some experiments the effects of this vector with that of another control vector, IRES-GFP (green fluorescent protein), containing only IRES and GFP driven by the MSCV promoter, to rule out effects mediated by L22Y expression.
Design of retroviral vectors and overexpression of cyclin D2 in hematopoietic progenitors. (A) The cyclin D2 gene was inserted in front of IRES and GFP into a retroviral construct.26 Cyclin D2 gene expression was driven from a MSCV promoter. The control vector contained GFP-IRES-L22Y. L22Y represents a human dihydrofolate reductase variant containing a leucine to tyrosine substitution in codon 22 of the gene.27 L22Y confers trimetrexate resistance; however, only GFP expression was used as a marker gene in this study. (B) LSK cells transduced with control (left) or cyclin D2 (right) containing vectors were cultured for an additional 3 days after transduction and analyzed by FACS for expression of cyclin D2 and GFP. Inserted in the upper left of the FACS plots are the control stainings for cyclin D2 (goat-antimouse-PE). Note that GFP+ and GFP– control-transduced cells exhibited comparable levels of endogenous cyclin D2 protein. Results are from 1 of 3 experiments with similar results.
Design of retroviral vectors and overexpression of cyclin D2 in hematopoietic progenitors. (A) The cyclin D2 gene was inserted in front of IRES and GFP into a retroviral construct.26 Cyclin D2 gene expression was driven from a MSCV promoter. The control vector contained GFP-IRES-L22Y. L22Y represents a human dihydrofolate reductase variant containing a leucine to tyrosine substitution in codon 22 of the gene.27 L22Y confers trimetrexate resistance; however, only GFP expression was used as a marker gene in this study. (B) LSK cells transduced with control (left) or cyclin D2 (right) containing vectors were cultured for an additional 3 days after transduction and analyzed by FACS for expression of cyclin D2 and GFP. Inserted in the upper left of the FACS plots are the control stainings for cyclin D2 (goat-antimouse-PE). Note that GFP+ and GFP– control-transduced cells exhibited comparable levels of endogenous cyclin D2 protein. Results are from 1 of 3 experiments with similar results.
Transduction and ex vivo culture of transduced progenitors
Hematopoietic cell cultures were performed either in serum-free X-vivo 15 (BioWhittaker, Walkersville, MD), supplemented with 1% bovine serum albumin (BSA; StemCell Technologies, Vancouver, Canada), 2 mM l-glutamine, 100 U/mL penicillin, 100 U/mL streptomycin, and 0.1 mM 2-mercaptoethanol (Sigma-Aldrich), or in Iscove modified Dulbecco medium (IMDM), supplemented with 20% fetal calf serum (FCS), 2 mM l-glutamine, 100 U/mL penicillin, 100 U/mL streptomycin, and 0.1 mM 2-mercaptoethanol (hereafter serum-free medium and serum-containing medium). To maintain the virus-producing cell line, Dulbecco modified Eagle medium (DMEM) supplemented with 10% FCS, 2 mM l-glutamine, 100 U/mL penicillin, and 100 U/mL streptomycin was used.
LSK cells were prestimulated for 2 to 3 days in serum-free medium supplemented with rrSCF, rmIL-3, and rhIL-6 (S36), rrSCF, rhFL, and rhMGDF (SFM), or SFM3, and then cocultured with the virus producer cell line for 2 days in serum-containing medium with the same cytokine combinations. Transduction efficiency was measured by plating cells after coculture in methylcellulose, and 7 days later counting green (GFP+) granulocyte-macrophage colony-forming unit (CFU-GM) colonies under a fluorescent microscope. Transduced cells were always used without any prior sorting for GFP expression and were either transplanted into lethally irradiated mice or used for in vitro experiments.
Hematopoietic progenitor assay
For detecting CFU-GM, methylcellulose (M3134; StemCell Technologies) with 20% FCS, 2 mM l-glutamine, 100 U/mL penicillin, 100 U/mL streptomycin, and 0.1 mM 2-mercaptoethanol was supplemented with 10 ng/mL rrSCF, 25 ng/mL rhFL, 25 ng/mL rhMGDF, 10 ng/mL rmIL-3, and 25 ng/mL rhG-CSF and plated in 35-mm Petri dishes with 2-mm grids to allow estimation and comparison of the size of colonies.
In vivo repopulation assay
Transduced LSK cells (CD45.1) were transplanted into lethally irradiated (975 cGy) mice (CD45.1/CD45.2). Peripheral blood (PB) was analyzed 3 to 7 weeks and 4 months after transplantation. Briefly, red cells were sedimentated in phosphate-buffered saline (PBS) containing 1% Dextran. Residual red cells were lysed in 0.8% ammonium chloride in PBS. White cells were then incubated with purified anti-CD16/CD32 antibody; stained against CD45.1, CD45.2, and Lin marker antigens (B220, CD3ϵ, CD11b, and Gr1); and analyzed on a FACSCalibur (Becton Dickinson) for multilineage donor-derived reconstitution.25 One to 2 weeks after transplantation, PB cellularity was enumerated manually for leukocytes and by automated counter KX-21 (Sysmex Europe GMBH, Norderstedt, Germany) for erythrocytes and platelets.
Detection of cyclin D2 protein
To investigate the level of cyclin D2 protein expression obtained, we used a flow cytometric approach. Cells were fixated and permeabilized using Cytofix/Cytoperm kit (PharMingen), stained with mouse antihuman cyclin D2 antibody (Ab-4; NeoMakers, Fremont, CA; cross-reactive with mouse cyclin D2), for 3 hours at 4° C, and then visualized with goat-antimouse-PE (phycoerythrin). Control stainings were performed either with anti-IgG antibody plus goat-antimouse-PE, or goat-antimouse-PE alone.
Statistics
Student t test was performed for statistical analyses.
Results
Overexpression of cyclin D2 protein in vitro
After FACS, LSK bone marrow cells were transduced with a cyclin D2-IRES-GFP or GFP-IRES-L22Y control vector (Figure 1). Transduction rates were measured by plating transduced cells in methylcellulose and counting GFP+ colonies under a fluorescent microscope 7 days later. The transduction efficiency was on average 60% (40%-100%) for cells transduced with the cyclin D2 vector and 85% (67%-100%) if transduced with the control vector. The number of colonies generated was similar between the 2 conditions (mean [SD] colony count per sorted LSK cell was 2.18 [0.69] for control and 2.13 [0.50] for cyclin D2 transduction, n = 6). Transduced cells were also cultured in liquid medium for an additional 3 days and analyzed for expression of cyclin D2 protein as well as GFP by FACS. Low levels of cyclin D2 expression were detected in GFP-negative and -positive cells in control-transduced cultures. In cyclin D2 gene-transduced cultures, a positive correlation was observed between levels of expression for cyclin D2 and GFP (Figure 1).
Cyclin D2 promotes rescue of lethally ablated recipients by enhancing short-term myeloid reconstitution in vivo
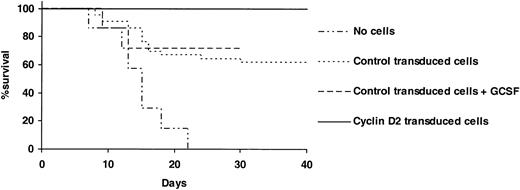
Enhanced cyclin D2 expression could potentially promote proliferation of hematopoietic stem and progenitor cells and accelerate reconstitution in vivo and, thereby, facilitate rescue of recipients subjected to lethal hematopoietic ablation. To investigate this possibility, we transplanted cyclin D2– or control-transduced LSK cells into lethally irradiated mice. Whereas as many as 16 of 42 mice died when 2000 control-transduced LSK cells (containing approximately 4400 CFU-GM) were transplanted per mouse, all 28 mice survived when transplanted with the same number (containing approximately 4300 CFU-GM) of cyclin D2–transduced cells (Figure 2). Thus, overexpression of cyclin D2 in hematopoietic progenitors enhances their ability to rescue lethally irradiated mice.
Overexpression of cyclin D2 in hematopoietic progenitor cells rescues mice from lethal myeloablation. Two thousand LSK cells transduced with control- or cyclin D2–containing vector were transplanted without any support cells into lethally ablated (975 cGy) adult recipients. A total of 42, 14, and 28 mice were transplanted in control-transduced, control-transduced + G-CSF–treated, and cyclin D2–transduced groups, respectively. From day 1 after transplantation, PBS (control transduced) or 100 μg/kg rhG-CSF was subcutaneously injected every other day for a total of 7 times. Twenty-eight mice served as nontransplantation controls.
Overexpression of cyclin D2 in hematopoietic progenitor cells rescues mice from lethal myeloablation. Two thousand LSK cells transduced with control- or cyclin D2–containing vector were transplanted without any support cells into lethally ablated (975 cGy) adult recipients. A total of 42, 14, and 28 mice were transplanted in control-transduced, control-transduced + G-CSF–treated, and cyclin D2–transduced groups, respectively. From day 1 after transplantation, PBS (control transduced) or 100 μg/kg rhG-CSF was subcutaneously injected every other day for a total of 7 times. Twenty-eight mice served as nontransplantation controls.
No previous studies have addressed the ability of G-CSF administration to enhance survival following autologous transplantation of lethally irradiated (BM ablated) mice. Thus, we here investigated whether G-CSF treatment after transplantation could enhance survival to a similar degree as by overexpression of cyclin D2 in progenitors that received transplants. However, unlike overexpression of cyclin D2, G-CSF treatment failed to significantly enhance survival after transplantation (Figure 2).
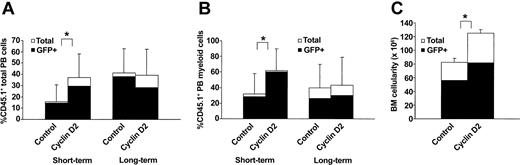
The ability to rescue lethally irradiated recipients is strictly correlated to the capacity of hematopoietic progenitors to rapidly reconstitute mature blood cells and in particular myelopoiesis.3,28 Thus, we next transplanted (CD45.1) LSK cells transduced with control- or cyclin D2–containing vectors together with congenic (CD45.2) competitor cells into lethally irradiated recipients and investigated their ability to rapidly reconstitute mature PB cells. Three to 4 weeks after transplantation, recipients were reconstituted to a considerably higher degree with cyclin D2–transduced than control-transduced cells, both with regard to total (Figure 3A) and myeloid (Figure 3B) reconstitution.
Cyclin D2 enhances short-term reconstitution in vivo. (A-B) Lethally irradiated mice (CD45.2) were transplanted with 3600 LSK (CD45.1) cells transduced with control- or cyclin D2–containing vectors, and 150 000 whole BM (CD45.2) cells. Reconstitution was analyzed in PB 3 to 4 weeks (short term) and 4 months (long term) after transplantation. (A) Total PB reconstitution by transplanted CD45.1 cells. (B) Myeloid (CD11b/Gr1+B220–CD3–) PB reconstitution by transduced (CD45.1) cells. Data in panel A are mean (SD) values from 14 (control) and 12 (cyclin D2) mice in a total of 3 experiments, and in panel B from 8 to 9 mice in 2 experiments. (C) BM cellularity 4 months after transplantation of mice that received transplants with 7000 to 10 000 control- or cyclin D2–transduced LSK cells without competitor cells. BM cells were harvested from femurs and tibias (crushed in a mortar), and nucleated cells were enumerated. Data are mean values (SD) from 3 experiments with 4 to 7 mice that received transplants in each experiment. *P < .05.
Cyclin D2 enhances short-term reconstitution in vivo. (A-B) Lethally irradiated mice (CD45.2) were transplanted with 3600 LSK (CD45.1) cells transduced with control- or cyclin D2–containing vectors, and 150 000 whole BM (CD45.2) cells. Reconstitution was analyzed in PB 3 to 4 weeks (short term) and 4 months (long term) after transplantation. (A) Total PB reconstitution by transplanted CD45.1 cells. (B) Myeloid (CD11b/Gr1+B220–CD3–) PB reconstitution by transduced (CD45.1) cells. Data in panel A are mean (SD) values from 14 (control) and 12 (cyclin D2) mice in a total of 3 experiments, and in panel B from 8 to 9 mice in 2 experiments. (C) BM cellularity 4 months after transplantation of mice that received transplants with 7000 to 10 000 control- or cyclin D2–transduced LSK cells without competitor cells. BM cells were harvested from femurs and tibias (crushed in a mortar), and nucleated cells were enumerated. Data are mean values (SD) from 3 experiments with 4 to 7 mice that received transplants in each experiment. *P < .05.
The transduction efficiency into HSCs (frequency of PB GFP+ cells in long-term reconstituted animals; Figure 3) was high and similar to that of CFU-GM. Importantly, there was no difference in multilineage long-term (4 months) PB reconstitution of primary recipients of transplants with control- and cyclin D2–transduced cells (Table 1). This was not due to gene silencing, because primary and secondary recipients of cyclin D2–transduced cells sustained high levels of GFP+ cells (Table 2; Figure 3), and because cyclin D2–transduced GFP+ BM cells isolated from mice 4 months after transplantation demonstrated high levels of cyclin D2 levels on in vitro culture (Y.S. et al, unpublished observation, July 2002). Furthermore, similar levels of multilineage PB reconstitution of secondary BM recipients were observed in the 2 groups (Table 2). However, the BM cellularity of cyclin D2 recipients was increased by as much as 52% when compared with control recipients 4 months after transplantation (Figure 3B). The phenotype/lineage distribution was comparable in the BM of mice that received transplants 4 months earlier with control- and cyclin D2–transduced cells (80% and 80% Gr1/CD11b+ myeloid cells, and 10% and 8% B220+ B cells for control- and cyclin D2–transduced cells, respectively). Thus, cyclin D2 enhances short-term myeloid reconstitution following HSC transplantation of lethally ablated recipients without affecting long-term HSC multilineage reconstitution.
Balanced long-term multilineage reconstitution of cyclin D2–transduced stem cells
. | % Lineage distribution in GFP+ cells (SD) . | . | . | ||
|---|---|---|---|---|---|
. | B cells . | T cells . | Myeloid cells . | ||
| Control | 54.4 (19.3) | 31.3 (16.3) | 12.6 (9.4) | ||
| Cyclin D2 | 46.2 (16.3) | 38.5 (16.6) | 16.2 (9.0) | ||
. | % Lineage distribution in GFP+ cells (SD) . | . | . | ||
|---|---|---|---|---|---|
. | B cells . | T cells . | Myeloid cells . | ||
| Control | 54.4 (19.3) | 31.3 (16.3) | 12.6 (9.4) | ||
| Cyclin D2 | 46.2 (16.3) | 38.5 (16.6) | 16.2 (9.0) | ||
Data show 4 months of analysis of multilineage PB reconstitution in mice that received transplants of LSK cells transduced with control or cyclin D2–containing vectors (same mice as analyzed in Figure 3A). Donor-revived (GFP+) B cells were identified as B220+ cells, T cells as CD3+ cells, and myeloid cells as CD11b/Gr1+B220-CD3- cells. Data are mean (SD) values from 14 (control) and 12 (cyclin D2) mice in a total of 3 experiments.
Efficient transduction of in vivo repopulating HSCs with cyclin D2–containing vector
. | 1° recipients . | . | 2° recipients . | . | ||
|---|---|---|---|---|---|---|
. | % Donor (CD45.2) reconstitution (SD) . | % GFP+ donor cells (SD) . | % Donor (CD45.2) reconstitution (SD) . | % GFP+ donor cells (SD) . | ||
| Experiment 1 | ||||||
| Control vector | 72.7 (5.2) | 48.5 (7.4) | 60.7 (21.0) | 42.5 (42.8) | ||
| Cyclin D2 vector | 76.2 (3.3) | 33.7 (10.6) | 81.9 (10.6) | 36.8 (33.3) | ||
| Experiment 2 | ||||||
| Control vector | 58.4 (12.1) | 99.4 (0.9) | 34.6 (43.3) | 99.1 (1.2) | ||
| Cyclin D2 vector | 43.7 (15.4) | 97.3 (2.9) | 23.0 (18.9) | 98.1 (3.3) | ||
. | 1° recipients . | . | 2° recipients . | . | ||
|---|---|---|---|---|---|---|
. | % Donor (CD45.2) reconstitution (SD) . | % GFP+ donor cells (SD) . | % Donor (CD45.2) reconstitution (SD) . | % GFP+ donor cells (SD) . | ||
| Experiment 1 | ||||||
| Control vector | 72.7 (5.2) | 48.5 (7.4) | 60.7 (21.0) | 42.5 (42.8) | ||
| Cyclin D2 vector | 76.2 (3.3) | 33.7 (10.6) | 81.9 (10.6) | 36.8 (33.3) | ||
| Experiment 2 | ||||||
| Control vector | 58.4 (12.1) | 99.4 (0.9) | 34.6 (43.3) | 99.1 (1.2) | ||
| Cyclin D2 vector | 43.7 (15.4) | 97.3 (2.9) | 23.0 (18.9) | 98.1 (3.3) | ||
Lethally irradiated mice (1° recipients, CD45.1) received transplants with 5000 (experiment 1) or 1250 (experiment 2) LSK (CD45.2) cells transduced with control- or cyclin D2–containing vectors ('Materials and methods'), and 200 000 (experiment 1) or 100 000 (experiment 2) whole BM (CD45.1) cells. Four months later, half a femur equivalent BM cells from 1° recipients were serially transplanted into 2° (CD45.1) recipients. Reconstitution was analyzed in PB 4 months (1° recipients) and 3 months (2° recipients) after transplantation. As shown for 1° recipients in Table 1, control- and cyclin D2–transduced (GFP+) progenitors showed comparable contributions to B (B220), T (CD3), and myeloid (CD11b/Gr1) lineages also in 2° recipients. Data are mean values (SD) from 4 to 7 mice in each group and experiment.
We next transplanted LSK cells transduced with control- or cyclin D2–containing vectors into lethally irradiated recipients and analyzed early reconstitution in blood, spleen, and BM (Table 3). Recipients of control transduced cells exhibited pancytopenia in PB 7 and 14 days after transplantation. Spleen and BM cellularities were also greatly reduced 7 and 14 days after transplantation. Note, recipients of cyclin D2–transduced cells demonstrated enhanced recovery of PB, spleen, and BM cellularities already 7 days after transplantation, which was further enhanced 14 days after transplantation. Importantly, the enhanced recovery included red blood cells and platelets (Table 3). Furthermore, the number of spleen and BM myeloid progenitors (CFU-GM) was increased more than 5-fold (Table 3).
Overexpression of cyclin D2 in hematopoietic progenitors accelerates erythro-myeloid reconstitution following transplantation of lethally ablated recipients
. | Day 7 . | . | Day 17 . | . | ||
|---|---|---|---|---|---|---|
. | Control . | Cyclin D2 . | Control . | Cyclin D2 . | ||
| WBC, × 109/L (SD) | 0.16 (0.07) | 0.22 (0.07) | 0.24 (0.13) | 0.50 (0.15)* | ||
| RBC, × 109/L (SD) | 5.78 (1.83) | 6.32 (1.56) | 3.37 (0.75) | 5.03 (1.32)* | ||
| PLT, × 109/L (SD) | 48.8 (16.5) | 80.5 (26.9)* | 188 (270) | 358 (390) | ||
| Spleen cellularity × 106 (SD) | 22.2 (9.8) | 50.6 (33.5) | 175 (23.1) | 259 (30.9)* | ||
| Spleen CFU-GM (SD) | 171 (81.6) | 1152 (1053)* | 7850 (3430) | 39 400 (15 900)* | ||
| BM cellularity/hind limbs × 106 (SD) | 7.1 (2.4) | 12.8 (5.4)* | 13.2 (5.1) | 23.3 (8.0)* | ||
| BM CFU-GM/hind limbs (SD) | 122 (85.0) | 276 (248) | 298 (375) | 1 590 (1610)* | ||
. | Day 7 . | . | Day 17 . | . | ||
|---|---|---|---|---|---|---|
. | Control . | Cyclin D2 . | Control . | Cyclin D2 . | ||
| WBC, × 109/L (SD) | 0.16 (0.07) | 0.22 (0.07) | 0.24 (0.13) | 0.50 (0.15)* | ||
| RBC, × 109/L (SD) | 5.78 (1.83) | 6.32 (1.56) | 3.37 (0.75) | 5.03 (1.32)* | ||
| PLT, × 109/L (SD) | 48.8 (16.5) | 80.5 (26.9)* | 188 (270) | 358 (390) | ||
| Spleen cellularity × 106 (SD) | 22.2 (9.8) | 50.6 (33.5) | 175 (23.1) | 259 (30.9)* | ||
| Spleen CFU-GM (SD) | 171 (81.6) | 1152 (1053)* | 7850 (3430) | 39 400 (15 900)* | ||
| BM cellularity/hind limbs × 106 (SD) | 7.1 (2.4) | 12.8 (5.4)* | 13.2 (5.1) | 23.3 (8.0)* | ||
| BM CFU-GM/hind limbs (SD) | 122 (85.0) | 276 (248) | 298 (375) | 1 590 (1610)* | ||
Five thousand LSK cells transduced with control- or cyclin D2–containing vectors were transplanted without any support cells into lethally ablated (975 cGy) adult recipients. A total of 6 mice at day 7, and 8 mice at day 14 in each group were analyzed in 2 separate experiments. Transduction efficiency was more than 90% for both control and cyclin D2 vectors. Data are shown as means (SD). WBC indicates white blood cells; RBC, red blood cells; and PLT, platelet.
P < .05.
Overexpression of cyclin D2 enhances the proliferative capacity of myeloid progenitors and their generation of granulocytes for extended periods of time
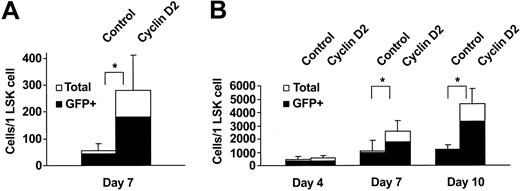
We next investigated whether the enhanced myeloid reconstitution in vivo might be a consequence of enhanced cytokine responsiveness and/or higher proliferative capacity of cyclin D2–overexpressing progenitors. The combination of SCF, FL, and MGDF (SFM) has been demonstrated to efficiently recruit LSK cells into proliferation.8,29 Whereas control-transduced LSK cells expanded 54-fold after 7 days of SFM stimulation, cyclin D2–transduced cells expanded as much as 281-fold (Figure 4A). Interestingly, even with a strong combination of 5 cytokines (SFM3 + G-CSF: SFM3G) promoting extensive proliferation of LSK cells, cyclin D2–overexpression further enhanced the proliferative response (Figure 4B). Although this enhanced proliferative response could be observed already 4 days after transduction, it became more pronounced following 7 and 10 days (Figure 4B). FACS analysis demonstrated that GFP+ cells from control- and cyclin D2–transduced cultures contained similar frequencies of mature myeloid (CD11b+Gr1+) and immature (LSK) cells (Table 4), suggesting that both progenitors and mature myeloid cells are expanded efficiently on overexpression of cyclin D2. Consistent with this finding, the frequency of morphologically identified mature granulocytes was similar at day 7 in control- and cyclin D2–transduced cultures (34% and 37%, respectively; P = .5). Thus, the absolute number of granulocytes was on average 2.6-fold higher in cyclin D2 than in control cultures at day 7 (Figure 5). Of note, following 14 days of culture, at which time the number of mature (short lived) granulocytes was maintained in control cultures (Figure 5), granulocytes increased further in cyclin D2–transduced culture, such that the number of granulocytes was 11-fold higher in cyclin D2–transduced cultures. Thus, overexpression of cyclin D2 in LSK cells enables enhanced and prolonged production of granulocytes in vitro. To rule out possible effects of L22Y expression (conferring trimetrexate resistance) on proliferation of control (MGirL22Y vector) transduced cells, we compared the effects of this vector with that of another control vector, IRES-GFP. Cells transduced with the IRES-GFP vector expanded 2600-fold (mean of 2 experiments) following 11 days of SFM3G stimulation, and cells transduced with the MGirL22Y vector expanded 2400-fold (mean of 2 experiments), demonstrating that L22Y expression does not affect cytokine-induced expansion of LSK cells.
Overexpression of cyclin D2 further enhances proliferation of myeloid progenitors stimulated by a combination of multiple cytokines. LSK cells transduced with control- or cyclin D2–containing vectors were cultured in serum-free medium supplemented with SFM (A), or SFM3 and G-CSF (SFM3G) (B). Cell numbers were scored following 7 days and for SFM3G cultures also following 4 and 10 days of incubation. Data in panels A and B are shown as number of cells produced per plated LSK cell and represent mean (SD) values from 6 and 3 experiments, respectively. *P < .05.
Overexpression of cyclin D2 further enhances proliferation of myeloid progenitors stimulated by a combination of multiple cytokines. LSK cells transduced with control- or cyclin D2–containing vectors were cultured in serum-free medium supplemented with SFM (A), or SFM3 and G-CSF (SFM3G) (B). Cell numbers were scored following 7 days and for SFM3G cultures also following 4 and 10 days of incubation. Data in panels A and B are shown as number of cells produced per plated LSK cell and represent mean (SD) values from 6 and 3 experiments, respectively. *P < .05.
Cyclin D2–overexpressing LSK cells produce enhanced numbers of mature myeloid cells as well as immature progenitors
. | Fold expansion total cells (SD) . | % CD11b+Gr1+ (SD) . | % LSK (SD) . | Fold expansion LSK cells (SD) . |
|---|---|---|---|---|
| Control | 55 (47) | 23.9 (18.0) | 3.4 (1.5) | 1.9 (0.8) |
| Cyclin D2 | 272 (190) | 26.0 (5.5) | 4.7 (3.0) | 12.8 (8.2) |
. | Fold expansion total cells (SD) . | % CD11b+Gr1+ (SD) . | % LSK (SD) . | Fold expansion LSK cells (SD) . |
|---|---|---|---|---|
| Control | 55 (47) | 23.9 (18.0) | 3.4 (1.5) | 1.9 (0.8) |
| Cyclin D2 | 272 (190) | 26.0 (5.5) | 4.7 (3.0) | 12.8 (8.2) |
Control- and cyclin D2–transduced LSK cells were cultured in serum-free medium with SFM for 7 days. Cells were enumerated and analyzed for CD11b/Gr1 and LSK phenotypes by FACS. Data represent total numbers of GFP+ cells and are mean (SD) values from 3 experiments. Mean transduction efficiencies were 87% (70%-98%) and 46% (39%-60%) for control and cyclin D2 vectors, respectively.
Cyclin D2 overexpression confers myeloid progenitors with enhanced and prolonged granulocyte production potential in vitro. Control- and cyclin D2–transduced LSK cells were cultured in serum-free medium with SFM. Following 7 and 14 days of culture, total cell numbers were enumerated, and the frequency of granulocytes was established following May-Grünwald-Giemsa staining of cytospin slides. Data are mean (SD) values from 5 experiments. *P < .05.
Cyclin D2 overexpression confers myeloid progenitors with enhanced and prolonged granulocyte production potential in vitro. Control- and cyclin D2–transduced LSK cells were cultured in serum-free medium with SFM. Following 7 and 14 days of culture, total cell numbers were enumerated, and the frequency of granulocytes was established following May-Grünwald-Giemsa staining of cytospin slides. Data are mean (SD) values from 5 experiments. *P < .05.
Enhanced cyclin D2 expression promotes proliferation and maintenance of myeloid progenitors in vitro
Granulocytes are very-short-lived cells in vitro; therefore, the prolonged production of granulocytes by cyclin D2–transduced LSK cells could either reflect prolonged survival of mature granulocytes or enhanced regenerative capacity of myeloid progenitors. Because mature GFP+ granulocytes were found not to express enhanced levels of cyclin D2 (Y.S. et al, unpublished observation, December 2002), we next investigated the effect of cyclin D2 overexpression on expansion and maintenance of myeloid progenitors (CFU-GM) in vitro.
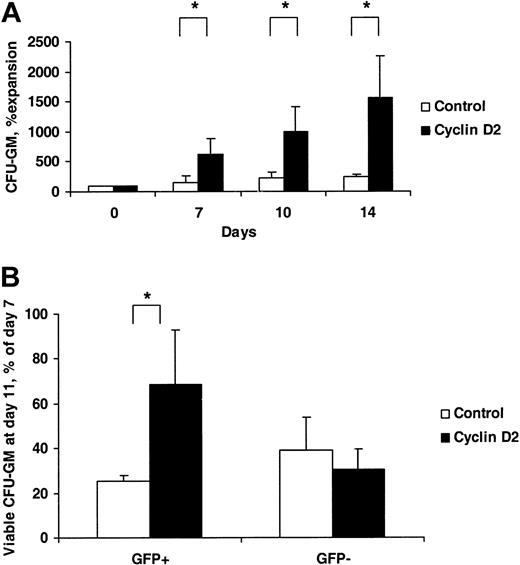
Control- and cyclin D2–transduced LSK cells were stimulated with SFM to investigate their ability to sustain production of CFU-GM in vitro. Whereas the number of GFP+ colonies increased only 2.4-fold in control-transduced cultures from day 0 to day 14, the number of GFP+ cyclin D2 colonies increased as much as 16-fold (Figure 6A). Although the size of CFU-GM generated was reduced over time for both control- and cyclin D2–transduced cells, cyclin D2 colonies sustained a higher proliferative capacity. Specifically, whereas GFP+ colonies derived from control-transduced cultures reached maximum size following 7 days, and at day 11 showed reduced size and viability, most cyclin D2–transduced colonies remained viable and either sustained or increased their size (Figure 6B). Thus, cyclin D2 overexpression results in enhanced and prolonged generation of CFU-GM with enhanced proliferative capacity.
Prolonged and enhanced production of myeloid progenitors by cyclin D2–overexpressing LSK cells. (A) Control- or cyclin D2–transduced LSK cells were cultured in SFM and, at the time points indicated, plated into methylcellulose, and 7 days later evaluated for colony formation. The number of GFP+ colonies generated from day 0 (after transduction) was set as 100% and compared with colony formation at later time points. (B) Control- or cyclin D2–transduced LSK cells were plated in methylcellulose cultures, and colonies were scored after 7 and 11 days. Results are shown as number of GFP+ (or GFP–) colonies at day 11/number of day-7 colonies × 100. Transduction efficiency was 80% (67%-86%) and 50% (42%-55%) (A), and 73% (67%-78%) and 43% (40%-51%) (B) for control- and cyclin D2–transduced colonies, respectively. Data are mean (SD) values from 3 (A) and 4 (B) experiments. * P < .05.
Prolonged and enhanced production of myeloid progenitors by cyclin D2–overexpressing LSK cells. (A) Control- or cyclin D2–transduced LSK cells were cultured in SFM and, at the time points indicated, plated into methylcellulose, and 7 days later evaluated for colony formation. The number of GFP+ colonies generated from day 0 (after transduction) was set as 100% and compared with colony formation at later time points. (B) Control- or cyclin D2–transduced LSK cells were plated in methylcellulose cultures, and colonies were scored after 7 and 11 days. Results are shown as number of GFP+ (or GFP–) colonies at day 11/number of day-7 colonies × 100. Transduction efficiency was 80% (67%-86%) and 50% (42%-55%) (A), and 73% (67%-78%) and 43% (40%-51%) (B) for control- and cyclin D2–transduced colonies, respectively. Data are mean (SD) values from 3 (A) and 4 (B) experiments. * P < .05.
Dysregulated expression of D-type cyclins has been suggested to be involved in the pathogenesis of some types of lymphomas and leukemias.19 Thus, we investigated whether overexpression of cyclin D2 in hematopoietic progenitors might result in leukemic transformation. However, observation of a total of 145 primary, 40 secondary, and 11 tertiary recipients of BM cells overexpressing cyclin D2 (up to 12 months after primary transplantation) failed to show any signs of leukemic development in PB, BM, or spleen (Y.S. et al, unpublished observations, June 2003).
Discussion
These studies demonstrate that overexpression of cyclin D2 in hematopoietic progenitors enhances their ability to rescue lethally ablated recipients. In agreement with this, an improved ability of cyclin D2–overexpressing progenitors to rapidly reconstitute myelopoiesis following transplantation was observed. The fact that not only leukocytes but also erythrocytes and platelets showed accelerated recovery on overexpression of cyclin D2 in hematopoietic progenitor cells was of considerable importance, because recent studies have demonstrated that the ability to rapidly reconstitute these lineages play a key role in rescuing lethally irradiated recipients.3 This could also explain why G-CSF treatment after transplantation failed to significantly protect mice from lethal irradiation.
Transplantation studies in primary and secondary recipients demonstrated that the accelerated short-term myeloid reconstitution observed following transplantation of lethally ablated recipients with cyclin D2–overexpressing progenitors did not occur at the expense of long-term HSC multilineage repopulating ability. Importantly, cyclin D2–transduced cells did not enhance long-term multilineage PB reconstitution in recipients of transplants. This could not be explained by gene silencing because high levels of GFP+ cells were observed long term in primary as well as secondary recipients, and the cyclin D2 levels in these cells were up-regulated on in vitro culture. Rather, it is likely that this observation reflects that, as the transplanted BM cells with time approach steady-state hematopoiesis, the signaling pathways required for cyclin D2 overexpression to enhance reconstitution are no longer activated.
Importantly, following the transduction and immediately before transplantation the levels of myeloid progenitors were comparable in cyclin D2– and control-transduced cultures, whereas as little as 1 week after transplantation they were expanded in recipients of cyclin D2–transduced cells, suggesting that overexpression of cyclin D2 expanded the compartment of myeloid progenitors in vivo following transplantation.
In vitro studies further supported the ability of cyclin D2 to promote myeloid reconstitution, by demonstrating the ability of cyclin D2 to further enhance cytokine-induced proliferation, and an increased proliferative capacity and maintenance of myeloid progenitors. Importantly, this was accompanied by normal myeloid differentiation and as a consequence enhanced and prolonged production of mature granulocytes.
Because combinations of 3 to 5 cytokines have been demonstrated to optimally promote proliferation of hematopoietic stem and progenitor cells,8,30 it was of particular interest that overexpression of cyclin D2 was able to further enhance proliferation of transduced progenitors stimulated simultaneously by high concentrations of 5 cytokines. This suggests that cyclin D2 can promote hematopoietic progenitor proliferation beyond what can be obtained by known cytokines. Importantly, the enhanced short-term reconstitution seen in vivo further supports that the signals efficiently promoting proliferation following transplantation can be further potentiated through overexpression of cyclin D2 in hematopoietic progenitors.
Although the cyclin D2–containing retroviral vector was efficient at transducing HSCs, as evidenced by sustained GFP expression in vivo, we were unable to achieve a detectable overexpression of cyclin D2 expression in donor-derived LSK cells (as determined by FACS; Y.S. et al, unpublished observations, August 2002), in contrast to what was observed in myeloid progenitors. The reason for this remains unclear, but because D-type cyclins are known to have a short half-life as a result of ubiquitin-mediated proteolysis,31 it seems likely that mechanisms are in place in HSCs to tightly regulate cyclin D2 expression through degradation. This could be important because the size of the HSC pool is known to be tightly regulated,32,33 and because HSCs are frequent targets for leukemic transformation.34
These findings clearly demonstrate that the repopulating ability of hematopoietic progenitors can be improved by overexpression of positive regulators of cell cycle progression, and that this might have an effect in clinical transplantation. However, whether overexpression of cyclin D2 could be developed toward clinical application needs to be explored in more depth. Although dysregulated expression of D-type cyclins has not been demonstrated to be involved in leukemic development, and no leukemia was observed in these studies, D-type cyclins have been implicated in the transformation process of solid tumors and lymphomas.19 Regardless, transient overexpression, for instance through adenoviral vectors, might be most desirable and sufficient for enhancing short-term reconstitution.
In conclusion, overexpression of D-type cyclins has a biologic and clinical potential for enhancing the proliferative and regenerative capacity of myeloid progenitors ex vivo as well as following clinical transplantation.
Prepublished online as Blood First Edition Paper, April 22, 2004; DOI 10.1182/blood-2003-07-2277.
Supported by grants from the John and Augusta Persson Foundation, the O and E and Elda Johansson Foundation, the Thelma Zoega's Foundation, the Tobias Foundation, the Greta and Johan Kock's Foundations, Alfred Österlund Foundation, ALF (government public health grant), Skånes Landsting, the Swedish Foundation for Strategic Research, the Swedish Cancer Society, Swedish Society of Pediatric Cancer, and the Medical Faculty, University of Lund. The Lund Stem Cell Center is supported by a Center of Excellence grant from the Swedish Foundation of Strategic Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Charles J. Sherr for generously providing cyclin D2 cDNA, Derek A. Persons and Arthur W. Nienhuis for the MGirL22Y vector, and Arthur Bank for GP+E86 cell line. We also thank Lilian Wittmann, Anna Fossum, Zhi Ma, Gunilla Gärdebring, Ingbritt Å strand-Grundström, Ineke de Jong, Jalal Taneera, Jens M. Nygren, Liping Yang, and Lottie Jansson-Sjöstrand for expert technical assistance, as well as Ewa Sitnicka for critical reading of the manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal