Abstract
Immune responses to the therapeutic gene product are a potentially serious complication in treatment of genetic disease by gene therapy. Induction and maintenance of immunologic hypo-responsiveness to the therapeutic antigen is therefore critical to the success of gene-based treatment of inherited protein deficiency. Here, we demonstrate induction of antigen-specific CD4+ T-cell tolerance to a secreted transgene product (ovalbumin, ova) in ova-specific T-cell receptor (TCR) transgenic mice by hepatic adeno-associated virus (AAV)–mediated gene transfer. Transduced mice maintained stable circulating ova levels without evidence of an immune response. Lymph node cells and splenocytes were hypo-responsive to ova as early as day 10 after gene transfer. Numbers of TCR+CD4+ cells were reduced in secondary lymphoid organs and in the thymus by 1 to 2 months after vector administration. The remaining TCR+CD4+ cell population was anergic to ova antigen in vitro and enriched for CD25+ cells. These data provide direct evidence that transgene expression following in vivo viral gene transfer can induce CD4+ T-cell tolerance to the transgene product, involving anergy and deletion mechanisms.
Introduction
Gene replacement therapy has become a realistic possibility for genetic disease as efficacy of gene transfer to different target tissues is steadily being increased by improvement of vector design. Viral gene transfer can direct high levels of transgene expression and is currently being tested in a number of clinical trials for treatment of inherited protein deficiencies such as the bleeding disorder hemophilia (coagulation factor VIII or IX deficiency).1,2 A serious concern in expression of a circulating protein is the potential for formation of an antibody response to the therapeutic antigen, which could neutralize gene therapy and interfere with conventional protein therapy.3 The therapeutic antigen is by definition different from the mutated protein expressed by the recipient of gene transfer, whose immune system may therefore not be tolerant to the functional protein. Antibody formation to coagulation factors, α1-antitrypsin, lysosomal enzymes, and other antigens has been demonstrated in animal models of gene transfer and genetic disease.4-9 These antibody responses are typically T-helper cell dependent and are thus not observed in CD4-deficient animals.10 A number of recent studies have demonstrated absence of antibodies to secreted proteins after hepatic gene transfer or hepatocyte-restricted expression following in vivo viral gene transfer, while other treatment strategies (eg, muscle-directed gene transfer) often caused such immune responses.6,8,10-18 However, if sustained expression after hepatic gene transfer was due to ignorance to the therapeutic antigen rather than tolerance induction, this could have dangerous consequences should the immune system be activated at a later time point. It is therefore critical that sustained expression is associated with tolerance induction to the therapeutic neoantigen to avoid potential subsequent immunologic complications.
In a recent study, we documented induction of immune tolerance to coagulation factor IX (FIX), deficiency of which causes the severe X-linked bleeding diathesis hemophilia B.19 Adult mice of different strain backgrounds that had received hepatic adeno-associated virus (AAV)–mediated gene transfer failed to respond to immunization by administration of FIX in complete Freund adjuvant. Absence of T-helper cell–mediated responses to FIX in tolerized mice after immunologic challenge was demonstrated by failure to produce anti-FIX of various immunoglobulin subclasses and lack of in vitro lymphocyte proliferation.19 Ziegler et al20 have recently shown similar results on tolerance induction to α–galactosidase A by hepatic AAV gene transfer in Fabry mice. Induction of immune tolerance to FIX was associated with generation of CD4+ cells that partially suppressed anti-FIX immunoglobulin G (IgG) formation after adoptive transfer to syngeneic mice.19 However, definitive evidence that in vivo gene transfer can induce CD4+ T-cell tolerance has been elusive.
Detailed and more direct studies on mechanisms of tolerance induction were hampered by the inability to physically identify FIX-specific lymphocytes in the experimental animal and because of the low frequency of antigen-specific T cells. In order to overcome these limitations, we chose to perform gene transfer experiments in BALB/c mice transgenic for DO11.10 T-cell receptor (TCR). This TCR (encoded by rearranged Vα13 and Vβ8.2 genes) is specific for a chicken ovalbumin (ova) peptide, amino acids 323-339, presented by the major histocompatibility complex (MHC) class II molecule I-Ad to CD4+ T cells and is expressed in 80% to 90% of T cells in the thymus of transgenic animals.21 Because of the high proportion of CD4+ T cells expressing the TCR, lymphocyte cultures derived from lymphoid organs of naive mice exhibit robust ova-specific in vitro proliferation. Moreover, DO11.10 TCR+CD4+ cells can be identified by means of fluorescence-activated cell sorter (FACS) analysis using dual antibody stain (antimurine CD4 and clonotypic KJ1-26 monoclonal antibody specific to the DO11.10 TCR). DO11.10 transgenic mice represent one of the best-characterized immunologic model systems for studies on CD4+ T-cell differentiation and tolerance induction.22-24 In particular, these mice have been extensively used to elucidate mechanisms of T-cell tolerance following oral administration of protein antigen.25 We rationalized that AAV-mediated transfer of an ova gene construct should allow us to determine whether viral in vivo gene transfer can induce CD4+ T-cell tolerance and, if so, to identify mechanisms responsible for tolerance induction.
Materials and methods
Viral vectors
AAV vectors harboring the ova or green fluorescence protein (GFP) transgene under the control of the human elongation factor-1α (EF1α) enhancer/promoter (AAV-EF1α-ova or AAV-EF1α-GFP) were constructed as previously described.26 Both expression cassettes contain the first intron of the human EF1α gene and a human growth hormone polyadenylation signal and are flanked by AAV serotype 2 inverted terminal repeats (ITRs). Vector production was carried out using the triple transfection protocol of HEK-293 cells, purification by CsCl gradient centrifugation, and quantitation by dot blot hybridization as described.27 All vectors were AAV serotype 2.
Animal studies
Male DO11.10 mice (BALB/c background, 4-6 weeks of age) and wild-type BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were housed in The Children's Hospital of Philadelphia's Animal Laboratory Facility with 12-hour light and dark cycles along with unlimited access to food and water. AAV vectors (25-50 μL per injection) were delivered into the portal vein via splenic capsule injection with a Hamilton syringe following a ventral midline incision as described.19,26 Blood samples were collected from the mice via the retro-orbital plexus using heparinized capillary tubes. Immunization was carried out by subcutaneous administration of 25 μg ovalbumin (Sigma, St Louis, MO) formulated in complete Freund adjuvant.
Ovalbumin transgene expression and antibody formation
Plasma levels of the ova antigen were measured by an ova-specific enzyme-linked immunosorbent assay (ELISA). Briefly, microtiter plates were coated with affinity-purified rabbit antiova (Chemicon, Temecula, CA; 1:2000 dilution) and detected with a rabbit antiova conjugated to horseradish peroxidase (1:2000 dilution; Rockland Immunochemicals, Gilbertsville, PA). Ova protein for standards was obtained from Sigma. Immunofluorescence stain of ova antigen was carried out on frozen liver sections using a rabbit antiova antibody as the primary antibody and a goat antirabbit IgG conjugated to fluorescein isothiocyanate (FITC; American Qualex Antibodies, San Clemente, CA). Stained sections were viewed with the Eclipse E800 microscope (Nikon, Tokyo, Japan) using a Plan Fluor × 40/0.75 objective and epifluorescent light (FITC filter). Images were captured with a Cool Snap-Pro camera and analyzed with Image Pro-Plus Software (both from Media Cybernetics, Silver Spring, MD). Immunocapture assay for determination and quantitation of antiova immunoglobulin subclasses (IgG1, IgG2a, IgG2b, and IgA) were performed as previously published using antibodies from Roche Molecular Biochemicals (Indianapolis, IN). Ova protein for antibody capture and immunoglobulin standards were from Sigma.6,10 The sensitivity of this assay was 100 to 200 ng antiova IgG/mL plasma.
Flow cytometry
Animals were killed in accordance with National Institutes of Health (NIH) guidelines. Spleens, inguinal nodes, and portal nodes were harvested and processed to produce single-cell suspensions as previously described.10 Viable splenocytes and lymphocytes were counted using a hemacytometer and trypan blue. Cells were then placed in culture or subjected to FACS analysis. Fixation and antibody staining of cell surface markers were carried out using standard protocols. DO11.10 TCR specific to ova 323-339 peptide was detected by using the monoclonal KJ1-26 antibody conjugated to the FITC fluorochrome (CALTAG, Burlingame, CA).28 Other monoclonal antibodies used were anti-CD4 phycoerythrin–cyanin 5.5 (PE-Cy5.5), anti-CD25 PE, and anti–natural killer 1.1 (anti-NK1.1) conjugated to allophycocyanin (BD Bioscience, San Jose, CA). Flow cytometry was performed on a FACSCalibur (Becton Dickinson, Mountain View, CA), and data were analyzed with CellQuest software (BD Bioscience). Analysis of cells from AAV2-EF1α-GFP–transduced DO11.10 mice after staining with anti-CD4 PE-Cy5.5 only showed a signal in the FITC channel in less than 0.02% of total cells (ie, there was no evidence for CD4+ cells expressing GFP). The DO11.10 T-cell receptor was also not detected in nontransgenic BALB/c mice (< 0.05% of total lymphocytes). Apoptotic CD4+TCR+ T cells were identified using the annexin-V–FLUOS Staining Kit (Roche Diagnostics, Mannheim, Germany). Briefly, cells were stained with FITC-labeled annexin V (AV), a phospholipids-binding protein that can be used to identify apoptotic cells, and propidium iodide (PI, staining of DNA of leaky necrotic cells) to exclude necrotic cells. Percent apoptotic CD4+TCR+ cells (ie, TCR+AV+PI– cells/TCR+ cells × 100) was determined by flow cytometry (DO11.10 TCR and CD4 were stained with KJ1-26, PE-Cy5.5 conjugate, and antimurine CD4, allophycocyanin conjugate, respectively).
Cytokine release assay
Purified splenocytes or lymphocytes from treated and control mice were plated at 5 × 106 cells/well in a 6-well dish and cultured in 2-MLC (mixed leukocyte culture media including 2% fetal bovine serum) media at 37° Cin 10% CO2 as published.19 Cells were stimulated with 100 μg/mL ova, while mock-stimulated cells were used as negative controls. Samples were collected every 24 hours for 3 days and tested for interleukin 2 (IL-2) and transforming growth factor β (TGF-β). IL-2 ELISA used specific antibodies purchased from Pharmingen (San Diego, CA) and TGF-β detection kit was purchased from Promega (Madison, WI). All ELISA assays were carried out according to manufacturers' instructions.
Proliferation assay
Splenocytes and lymphocytes were harvested 60 days after gene delivery. Cells (quadruplicate, 5 × 105 cells per well in 96-well plates) were incubated with or without 100 μg/mL ova as described for cytokine release assay. After 24 hours, cultured cells were labeled with 3H-thymidine for 12 hours. Thymidine uptake was measured with a scintillation counter. The stimulation index (SI) was calculated as the ratio of thymidine uptake in the ova-stimulated versus mock-stimulated cells.
Adoptive T-cell transfer and TUNEL assay
Spleens and lymph nodes were harvested from DO11.10 TCR transgenic BALB/c mice, pooled, and processed to produce single-cell suspensions. Viable lymphocytes were counted using a hemacytometer and trypan blue. CD4+ T cells were further purified buy the magnetic-activated cell separation (MACS)–positive selection system following manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Purified CD4+ T cells were injected intravenously into naive BALB/c recipient mice (5 × 106 CD4+ cells/mouse).29 These mice received hepatic gene transfer (AAV-EF1α-ova, 3 × 1012 vector genomes [vg's]) or were injected with vehicle control (HEPES-buffered saline [HBS]/5% sorbitol) 24 hours later. Analysis of lymphocytes by flow cytometry was carried out 10 days after gene transfer as described for flow cytometry with the addition of anti-CD95 monoclonal antibody conjugated to PE (BD Bioscience). Furthermore, pooled splenocytes (5 × 106 cells/well) were cultured in a 6-well dish under the above conditions. Cells were stimulated with either TCR-specific ova peptide (ISQAVHAAHAEINEAGR, ova fragment 323-339; 20 μg/mL) or were mock stimulated. Media samples were collected every 12 hours for 3 days to determine IL-2 cytokine release. In a parallel experiment, splenocytes were cultured in 6-well plates for 36 hours for TUNEL (terminal deoxynucleotidyl transferase [TdT]–mediated deoxyuridine triphosphate [dUTP] nick end labeling) assay. Cells were mock stimulated, peptide stimulated, ova stimulated, or incubated with dexamethasone (2 μg/mL; positive control for induction of apoptosis). TUNEL assay was preformed using antibody stains for extracellular markers (CD4 and DO11.10 TCR) and terminal transferase reaction (kit from Roche, Indianapolis, IN) to attach Cy5-labeled dUTP (Amersham, Piscataway, NJ). Again, cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson) and CellQuest software.
Purification of CD4+ T-cell subpopulations
CD4+CD25+ and CD4+CD25– cells from treated mice (AAV-EF1α-ova and AAV-EF1α-GFP) were carried out via the MACS system following manufacturer's instructions (Miltenyi Biotec). Antigen-presenting cells (APCs) were prepared from naive DO11.10 mice using the MACS CD90 separation system (Miltenyi Biotec). Cells were cultured as described for cytokine release assay. CD25+/– coculture experiments were maintained in microtiter wells with 1 × 105 naive APCs, 5 × 104 CD4+CD25–, and varying amounts of CD4+CD25+ (0.85-5 × 104 cells) in the presence of 100 μg/mL ova using a protocol published by others.30
Statistical analysis
Results are expressed as mean ± SEM. Comparisons among groups were made by analysis of variance (ANOVA) followed by Fisher protected least significant difference (PLSD) or by unpaired Student t test. Differences were considered significant at P less than or equal to .05.
Results
Sustained systemic expression of ova by AAV gene transfer
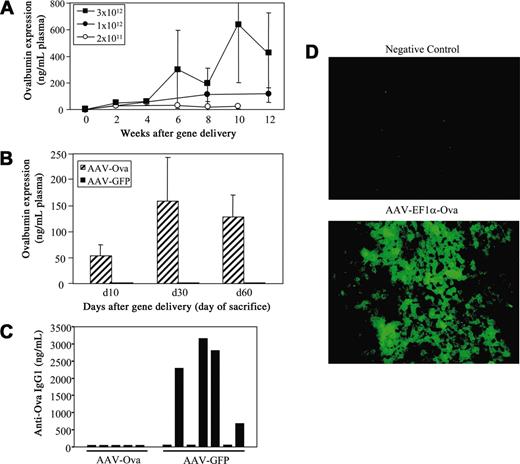
An AAV serotype 2 vector expressing ova cDNA from the EF1α enhancer/promoter was constructed. A similar construct has been successfully used in the past for hepatocyte-derived expression of FIX.26 In preliminary experiments, AAV-EF1α-ova vector introduced into the portal circulation of nontransgenic BALB/c mice directed sustained, vector-dose–dependent systemic levels of ova (Figure 1A). Animals did not produce antiova after gene transfer (10-12 week follow-up, data not shown). Subsequently, we injected DO11.10 TCR transgenic BALB/c mice with 3 × 1012 vector genomes (vg's) of AAV-EF1α-ova (control mice received an equal dose of AAV-EF1α-GFP). Levels of expression of approximately 50 ng ova/mL plasma by day 10 and 100 to 200 ng/mL (2-4 nM; Figure 1B) by 1 to 2 months after AAV-EF1α-ova administration were measured. There was no evidence for antibody formation to ova as determined by immunocapture assay (data not shown). Immunofluorescence stain demonstrated transgene expression by transduced hepatocytes (Figure 1D; data not shown). Histochemical staining of transduced livers gave no evidence for inflammation (not shown). Since transgenic mice had no evidence for cellular or humoral immune responses to the ova transgene product after hepatic gene transfer, we could now ask the question of whether sustained systemic expression resulted in induction of ova-specific CD4+ T-cell tolerance.
Transgene expression. (A) Vector dose-dependent systemic ova expression in BALB/c mice as a function of time after AAV-EF1α-ova administration. Mice were injected with varying doses of AAV-EF1α-ova at day 0 and were followed for 12 weeks (n = 5-6/dose). Mice received 2 × 1011 (○), 1 × 1012 (•) or 3 × 1012 (▪) vector genomes (vg's)/animal. (B) Ova expression levels of DO11.10 transgenic BALB/c mice at the time point that they were killed. Mice received 3 × 1012 vg's of AAV-EF1α-ova (▨) or 3 × 1012 vg's of AAV-EF1α-GFP (▪). Note that none of the mice injected with AAV-EF1α-ova shown in panels A and B formed antibodies to ova. Error bars (A-B) represent SD. (C) Antibody formation to ova 2 weeks after subcutaneous administration of 25 μg ova in cFA is graphed for individual DO11.10 transgenic BALB/c mice that had been injected with AAV-EF1α-ova or AAV-EF1α-GFP 2 months earlier (3 × 1012 vg's/animal). Shown are IgG1 responses (ova-transduced mice did not form IgG1 or IgG2a antiova after immunization). (D) Immunofluorescence staining of ova in a liver section from a nontransduced (top) or from an AAV-EF1α-ova–transduced DO11.10 mouse (bottom, 2 months after gene transfer). Original magnification, × 400.
Transgene expression. (A) Vector dose-dependent systemic ova expression in BALB/c mice as a function of time after AAV-EF1α-ova administration. Mice were injected with varying doses of AAV-EF1α-ova at day 0 and were followed for 12 weeks (n = 5-6/dose). Mice received 2 × 1011 (○), 1 × 1012 (•) or 3 × 1012 (▪) vector genomes (vg's)/animal. (B) Ova expression levels of DO11.10 transgenic BALB/c mice at the time point that they were killed. Mice received 3 × 1012 vg's of AAV-EF1α-ova (▨) or 3 × 1012 vg's of AAV-EF1α-GFP (▪). Note that none of the mice injected with AAV-EF1α-ova shown in panels A and B formed antibodies to ova. Error bars (A-B) represent SD. (C) Antibody formation to ova 2 weeks after subcutaneous administration of 25 μg ova in cFA is graphed for individual DO11.10 transgenic BALB/c mice that had been injected with AAV-EF1α-ova or AAV-EF1α-GFP 2 months earlier (3 × 1012 vg's/animal). Shown are IgG1 responses (ova-transduced mice did not form IgG1 or IgG2a antiova after immunization). (D) Immunofluorescence staining of ova in a liver section from a nontransduced (top) or from an AAV-EF1α-ova–transduced DO11.10 mouse (bottom, 2 months after gene transfer). Original magnification, × 400.
Evidence for ova-specific CD4+ T-cell anergy and deletion
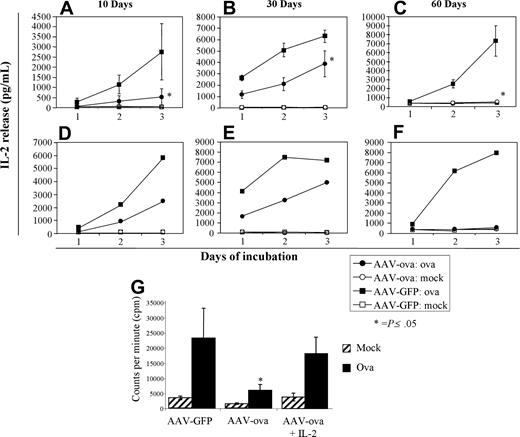
Mice were killed at days 10 (n = 4 per group), 30, and 60 (n = 8 per group and time point) for lymphocyte analyses, including analysis by 4-color flow cytometry for CD4, DO11.10 TCR, CD25, and NK1.1 cell surface markers, as well as ova-specific in vitro proliferation and cytokine release. Secretion of IL-2 cytokine occurs concurrently with proliferation of DO11.10 CD4+ clonal T cells upon in vitro stimulation with ova antigen. However, when lymphocyte cultures derived from spleens or inguinal lymph nodes (ILNs) of AAV-EF1α-ova–transduced mice were stimulated in vitro with ova antigen, IL-2 secretion as a function of time of stimulation was substantially reduced compared with controls (Figure 2A,D). A similar difference in response was measured for animals killed at day 30 (Figure 2B,E).
IL-2 cytokine release from cultured splenocytes and lymph node cells isolated from DO11.10 mice 10, 30, or 60 days after gene delivery. Individual spleens (A-C, n = 4-8) or pooled inguinal nodes (D-F) from GFP- or ova-transduced mice were cultured in the presence of 100 μg ova/mL media for 3 days. Error bars represent SD. *P less than .05 for ova-versus GFP-transduced mice. • indicates cells from ova-transduced mice stimulated with ova antigen; ▪, cells from GFP-transduced mice stimulated with ova; ○ and □, mock-stimulated controls. (G) Proliferation of cultured splenocytes as determined by measurement of 3H-tymidine incorporation (day 60 after vector administration, n = 4/group). Splenocyte proliferation was measured in the presence or absence (mock) of ova in conditioned media. For cells from AAV-ova–transduced mice, cells were also cultured in the presence of murine IL-2 (50 U/mL) in a parallel experiment. Error bars represent SD. *P ≤ .05.
IL-2 cytokine release from cultured splenocytes and lymph node cells isolated from DO11.10 mice 10, 30, or 60 days after gene delivery. Individual spleens (A-C, n = 4-8) or pooled inguinal nodes (D-F) from GFP- or ova-transduced mice were cultured in the presence of 100 μg ova/mL media for 3 days. Error bars represent SD. *P less than .05 for ova-versus GFP-transduced mice. • indicates cells from ova-transduced mice stimulated with ova antigen; ▪, cells from GFP-transduced mice stimulated with ova; ○ and □, mock-stimulated controls. (G) Proliferation of cultured splenocytes as determined by measurement of 3H-tymidine incorporation (day 60 after vector administration, n = 4/group). Splenocyte proliferation was measured in the presence or absence (mock) of ova in conditioned media. For cells from AAV-ova–transduced mice, cells were also cultured in the presence of murine IL-2 (50 U/mL) in a parallel experiment. Error bars represent SD. *P ≤ .05.
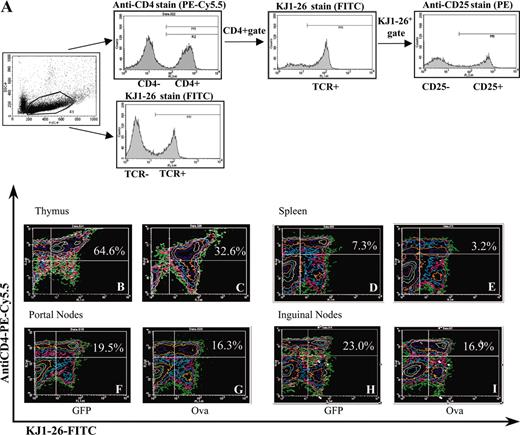
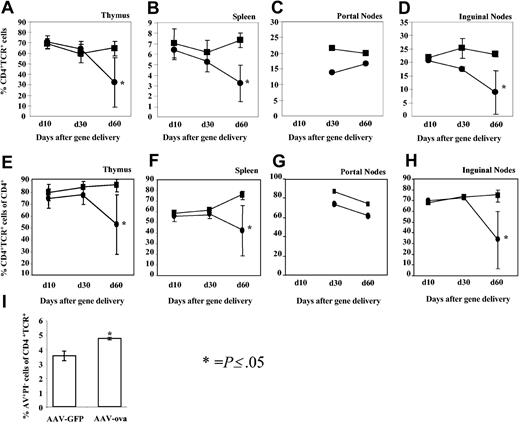
The strategy for analysis of cell populations in lymphoid organs by flow cytometry is outlined in Figure 3A. Data from day 10 revealed no difference between the percentage of TCR+CD4+ cells among total lymphocytes in thymuses, spleens, or ILNs isolated from ova-transduced or control animals (Figure 4A-B,D). Percent TCR+CD4+ cells in lymphoid organs from GFP-transduced control mice were identical to those measured for DO11.10 transgenic mice that had not received AAV vector (data not shown). However, the proportion of TCR+CD4+ cells was reduced for ILNs, portal LNs, and spleens at day 30 (Figure 3B-D) compared with controls. By day 60, percent TCR+CD4+ cells were substantially reduced in spleens, LNs, and thymuses (Figures 3B-I and 4A-D). Further analysis of splenocytes (1.5 months after gene transfer) showed an increased number of apoptotic cells among TCR+ cells for mice that had received AAV-ova vector (Figure 4I). While 60% to 95% of CD4+ cells in different lymphoid organs also expressed the DO11.10 TCR in our analyses of DO11.10 mice, this percentage was reduced in the spleen (to average of ∼45%), ILN (to ∼35%), and in the thymus (to ∼55%) of AAV-EF1α-ova–transduced DO11.10 mice by day 60 (Figure 4E-H). Deletion in portal nodes (in particular at day 30) was less obvious than in other lymphoid organs. This may reflect technical difficulties in processing this small node yielding limited numbers of cells for analysis by flow cytometry. Alternatively, antigen presentation and signals in the draining LNs of the target organ of gene transfer may be different from systemically delivered antigen.
Analyses of cell populations in lymphoid organs by flow cytometry. (A) Strategy of antibody stain and analyses by flow cytometry is outlined by examples of histograms obtained from splenocytes to determine TCR+CD4+ cells and TCR+CD4+CD25+ (see also Figure 6) in DO11.10 mice. (B-I) Quantitation of TCR+CD4+ cells 60 days after gene delivery by flow cytometry. Representative examples of FACS contour plots are shown for individual samples of viable lymphocytes from lymphoid organs of DO11.10 mice. The mice received either AAV-EF1α-GFP (B,D,F,H) or AAV-EF1α-ova (C,E,G,I). Percent dual-positive cells (top right quadrant) are indicated. Note that AAV-EF1α-ova–transduced mice showed significant reduction in TCR+CD4+ cells compared with control animals. Antibody stain was PE-Cy5.5–conjugated anti-CD4 and FITC-conjugated KJ1-26. Portal nodes had been pooled from 4 mice prior to flow cytometry.
Analyses of cell populations in lymphoid organs by flow cytometry. (A) Strategy of antibody stain and analyses by flow cytometry is outlined by examples of histograms obtained from splenocytes to determine TCR+CD4+ cells and TCR+CD4+CD25+ (see also Figure 6) in DO11.10 mice. (B-I) Quantitation of TCR+CD4+ cells 60 days after gene delivery by flow cytometry. Representative examples of FACS contour plots are shown for individual samples of viable lymphocytes from lymphoid organs of DO11.10 mice. The mice received either AAV-EF1α-GFP (B,D,F,H) or AAV-EF1α-ova (C,E,G,I). Percent dual-positive cells (top right quadrant) are indicated. Note that AAV-EF1α-ova–transduced mice showed significant reduction in TCR+CD4+ cells compared with control animals. Antibody stain was PE-Cy5.5–conjugated anti-CD4 and FITC-conjugated KJ1-26. Portal nodes had been pooled from 4 mice prior to flow cytometry.
Summary of percent TCR+CD4+ cells of total viable lymphocytes (as determined by flow cytometry) as a function of time after vector administration. Examined tissues were thymus (A), spleen (B), portal nodes (C), and inguinal nodes (D). Also graphed are percent TCR+CD4+ cells of total CD4+ cells as a function of time after vector administration (E-H). Examined tissues were thymus (E), spleen (F), portal nodes (G), and inguinal nodes (H). Mice had been transduced with AAV-EF1α-GFP (▪) or AAV-EF1α-ova (•). Antibody staining was carried out using PE-Cy5.5–conjugated anti-CD4 and FITC-conjugated KJ1-26. Results are average ± SD (error bars) for n = 4 mice (day 10) or n = 8 mice (days 30 and 60). Results from portal nodes were average from 2 FACS analyses for nodes pooled from 4 animals (only done for day 30 and 60 time points). (I) Percent apoptotic (annexin V+ and propidium iodide–, AV+PI–TCR+) cells of total TCR+ cells in the spleens of AAV-EF1α-GFP– or AAV-EF1α-ova–transduced DO11.10 mice (1.5 months after vector administration). Error bars represent SD. * indicates P ≤ .05.
Summary of percent TCR+CD4+ cells of total viable lymphocytes (as determined by flow cytometry) as a function of time after vector administration. Examined tissues were thymus (A), spleen (B), portal nodes (C), and inguinal nodes (D). Also graphed are percent TCR+CD4+ cells of total CD4+ cells as a function of time after vector administration (E-H). Examined tissues were thymus (E), spleen (F), portal nodes (G), and inguinal nodes (H). Mice had been transduced with AAV-EF1α-GFP (▪) or AAV-EF1α-ova (•). Antibody staining was carried out using PE-Cy5.5–conjugated anti-CD4 and FITC-conjugated KJ1-26. Results are average ± SD (error bars) for n = 4 mice (day 10) or n = 8 mice (days 30 and 60). Results from portal nodes were average from 2 FACS analyses for nodes pooled from 4 animals (only done for day 30 and 60 time points). (I) Percent apoptotic (annexin V+ and propidium iodide–, AV+PI–TCR+) cells of total TCR+ cells in the spleens of AAV-EF1α-GFP– or AAV-EF1α-ova–transduced DO11.10 mice (1.5 months after vector administration). Error bars represent SD. * indicates P ≤ .05.
Secretion of IL-2 cytokine and ova-specific lymphocyte proliferation as determined by 3H-thymidine incorporation was substantially reduced for splenocytes and ILN cells isolated from mice 60 days after AAV-EF1α-ova gene transfer and stimulated in vitro with ova antigen (Figure 2C,F,G). Ova-specific lymphocyte proliferation could largely be restored by addition of exogenous IL-2 to conditioned media indicating T-cell anergy among the remaining TCR+CD4+ population in AAV-EF1α-ova–transduced DO11.10 mice.
To evaluate whether T-cell tolerance can be correlated with absence of B-cell responses, we challenged DO11.10 mice 2 months after gene transfer by subcutaneous injection of 25 μg ova in complete Freund adjuvant (cFA). As shown in Figure 1C, none of 5 mice that had been transduced with AAV-EF1α-ova vector (resulting in expression of 50-300 ng/mL) formed antibody to ova (assayed 2 and 3 weeks after immunization), while 4 of 7 control mice formed IgG1 antiova within 2 weeks.
In order to test for evidence of T-cell tolerance in a more physiologic setting, we adoptively transferred CD4+ T cells from DO11.10 transgenic BALB/c mice to wild-type BALB/c mice (5 × 106 cells/recipient).29 After 24 hours, half of these mice were injected with 3 × 1012 vg's AAV-EF1α-ova (n = 4 per group). CD4+TCR+ cells were detected in the spleens and LNs (0.6% and 0.5%-1.2% of lymphocytes, respectively) when recipient mice were killed 11 days after adoptive T-cell transfer. There was no significant difference in these numbers for vector-transduced and control mice (data not shown). Vector-transduced mice expressed 50 to 100 ng ova/mL plasma at this time point (day 10 after vector administration).
After in vitro stimulation of splenocytes with ova peptide (stimulation of ova protein was not efficient enough for a response; not shown), we observed a reduction in levels of IL-2 cytokine secretion by cells from AAV-EF1α-ova–transduced mice that was similar to data from DO11.10 transgenic mice (compare Figure 5A and Figure 2A). TUNEL assay (Figure 5B) revealed increase in apoptosis in cultured CD4+TCR+ cells from vector-transduced mice compared with controls, which was observed independent of restimulation with antigen, suggesting that this phenotype had been induced by antigen stimulation in vivo (Figure 5C). Increased numbers of apoptotic CD4+TCR+ cells (cells that had incorporated Cy5-labeled dUTP catalyzed by terminal transferase) were found in Fas (CD95)+ and Fas– populations, indicating that increased apoptosis cannot be entirely explained by Fas-FasL–induced cell death.
Hepatic gene transfer (3 × 1012 vg's AAV-EF1α-ova/animal, n = 4) in BALB/c mice 24 hours after adoptive transfer of 5 × 106 CD4+ T cells from DO11.10 transgenic mice. Mice were killed at day 10, and pooled splenocytes were cultured. (A) IL-2 cytokine release upon in vitro stimulation with ova peptide ISQAVHAAHAEINEAGR as a function of time. Control cells were from mice (n = 4) that received vehicle control (5% sorbitol in HBS) instead of vector 24 hours after adoptive T-cell transfer. (B) TUNEL assay to measure apoptosis of DO11.10 TCR+ cells after 36 hours of in vitro culture. Shown are cells from AAV-EF1α-ova–transduced mice. Gated lymphocytes were analyzed for TCR+ and Fas+ (CD95+) cells. TCR+ were subsequently analyzed for incorporation of Cy5-labeled nucleotides (terminal transferase reaction to detect DNA fragmentation) to determine percent apoptotic cells. (C) Summary of flow cytometry results. Percent apoptotic cells of TCR+ cells after in vitro culture in mock, ova, ova peptide (Peptide), or dexamethasone (Dexa, positive control for induction of apoptosis) containing media are shown.
Hepatic gene transfer (3 × 1012 vg's AAV-EF1α-ova/animal, n = 4) in BALB/c mice 24 hours after adoptive transfer of 5 × 106 CD4+ T cells from DO11.10 transgenic mice. Mice were killed at day 10, and pooled splenocytes were cultured. (A) IL-2 cytokine release upon in vitro stimulation with ova peptide ISQAVHAAHAEINEAGR as a function of time. Control cells were from mice (n = 4) that received vehicle control (5% sorbitol in HBS) instead of vector 24 hours after adoptive T-cell transfer. (B) TUNEL assay to measure apoptosis of DO11.10 TCR+ cells after 36 hours of in vitro culture. Shown are cells from AAV-EF1α-ova–transduced mice. Gated lymphocytes were analyzed for TCR+ and Fas+ (CD95+) cells. TCR+ were subsequently analyzed for incorporation of Cy5-labeled nucleotides (terminal transferase reaction to detect DNA fragmentation) to determine percent apoptotic cells. (C) Summary of flow cytometry results. Percent apoptotic cells of TCR+ cells after in vitro culture in mock, ova, ova peptide (Peptide), or dexamethasone (Dexa, positive control for induction of apoptosis) containing media are shown.
When mice were killed 21 days after adoptive T-cell transfer, numbers of CD4+TCR+ cells had declined in experimental and control groups and in vitro responses to ova peptide were nearly undetectable. Inability of long-term follow-up in the absence of boost injections represents a known limitation of the adoptive transfer model.31 Nonetheless, data from day 10 are consistent with evidence for T-cell anergy and deletion of ova-specific CD4+ T cells obtained in DO11.10 transgenic mice.
Enrichment for CD4+CD25+ T cells
No differences in proportions of TCR+CD4+NK1.1+ cells (which had been identified to play a role in oral tolerance induction) were detected between experimental groups of mice (data not shown).32 Interestingly, percentage of CD25+ cells among TCR+CD4+ cells was increased by day 60 for ova-transduced mice (compared with control mice) in spleens, LNs, and thymuses (1.3-to 3-fold increase compared with controls, depending on the organ; Figure 6A-D).
Summary of analyses for percentage of CD25+ cells among TCR+CD4+ cells by flow cytometry. Thymocytes (A), splenocytes (B), portal (C), or inguinal lymph node cells (D) from DO11.10 mice were analyzed 30 or 60 days after ova (•) or GFP (▪) gene transfer (n = 4 per time point and group). Anti-CD4 (conjugated to PE-Cy5.5), anti-CD25 (PE), and KJ1-26 (FITC) antibodies were used for staining. Initial gating of viable lymphocytes was performed to identify CD4+ cells. CD4+ cell population was subsequently used to determine percent TCR+CD25+ cells. Error bars represent SD except for portal nodes, which had been pooled prior to flow cytometry. Examples for flow cytometry contour plots (inguinal lymph nodes, day 60) are shown in panels E (AAV-EF1α-GFP) and F (AAV-EF1α-ova). Percent TCR+CD4+CD25+ of TCR+CD4+ is indicated in top right quadrant.
Summary of analyses for percentage of CD25+ cells among TCR+CD4+ cells by flow cytometry. Thymocytes (A), splenocytes (B), portal (C), or inguinal lymph node cells (D) from DO11.10 mice were analyzed 30 or 60 days after ova (•) or GFP (▪) gene transfer (n = 4 per time point and group). Anti-CD4 (conjugated to PE-Cy5.5), anti-CD25 (PE), and KJ1-26 (FITC) antibodies were used for staining. Initial gating of viable lymphocytes was performed to identify CD4+ cells. CD4+ cell population was subsequently used to determine percent TCR+CD25+ cells. Error bars represent SD except for portal nodes, which had been pooled prior to flow cytometry. Examples for flow cytometry contour plots (inguinal lymph nodes, day 60) are shown in panels E (AAV-EF1α-GFP) and F (AAV-EF1α-ova). Percent TCR+CD4+CD25+ of TCR+CD4+ is indicated in top right quadrant.
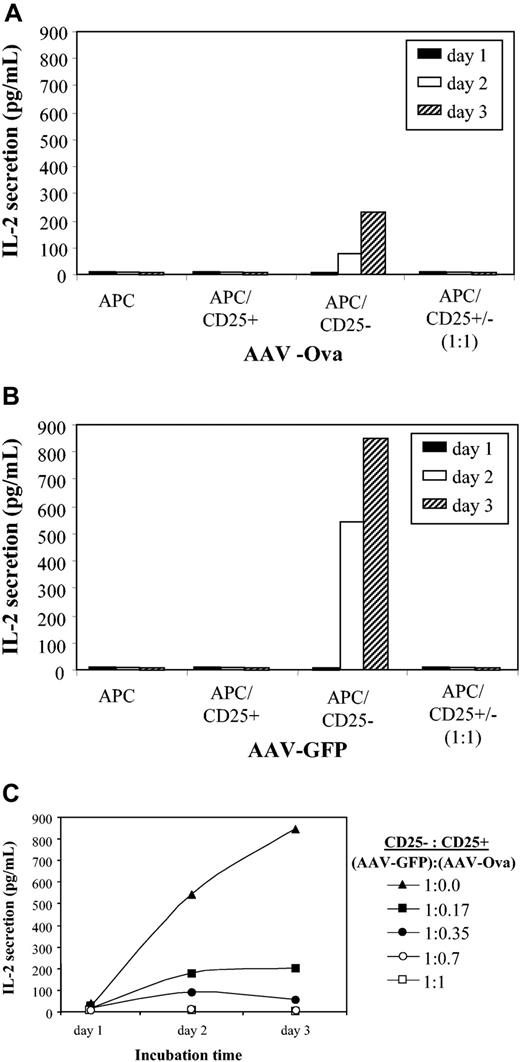
Regulatory CD4+CD25+ T cells have been described to be naturally occurring at a frequency of up to 10% of antigen-specific CD4+ T-cell populations and to be potent suppressors of CD4+CD25– T cells.33,34 Furthermore, activation of these cells has been documented in the context of oral and intravenous tolerance.30 In order to investigate whether CD4+CD25+ cells from ova-transduced mice behaved as regulatory cells, CD4+CD25+ and CD4+CD25– cells were isolated by magnetic cell sorting. CD4+CD25+ cells from either AAV-EF1α-ova–transduced (day 60 after gene transfer) or control DO11.10 mice failed to secrete IL-2 in response to coculture with antigen-presenting cells in the presence of ova antigen (Figure 7A-B), which is consistent with the anergic nature of these cells described in the literature.33
IL-2 release as a function of time after in vitro culture of CD4+CD25+ and CD4+CD25– cells. Cells were isolated by magnetic cell sorting from DO11.10 mice 60 days after gene transfer with AAV-EF1α-ova (A) or AAV-EF1α-GFP (B). All cultured cells were stimulated in vitro with ova (100 μg/mL). APC indicates antigen-presenting cells. Note that neither APCs alone nor CD25– cells nor CD25+ and CD25– cells cocultured at 1:1 ratio yielded a response to ova antigen for both experimental groups. Panel C shows CD4+CD25+ (from AAV-EF1α-ova–transduced DO11.10 mice) dose-dependent suppression of IL-2 release from CD4+CD25– cells (isolated from AAV-EF1α-GFP DO11.10 mice). Data represent average from n = 4 cultures based on cells pooled from several mice.
IL-2 release as a function of time after in vitro culture of CD4+CD25+ and CD4+CD25– cells. Cells were isolated by magnetic cell sorting from DO11.10 mice 60 days after gene transfer with AAV-EF1α-ova (A) or AAV-EF1α-GFP (B). All cultured cells were stimulated in vitro with ova (100 μg/mL). APC indicates antigen-presenting cells. Note that neither APCs alone nor CD25– cells nor CD25+ and CD25– cells cocultured at 1:1 ratio yielded a response to ova antigen for both experimental groups. Panel C shows CD4+CD25+ (from AAV-EF1α-ova–transduced DO11.10 mice) dose-dependent suppression of IL-2 release from CD4+CD25– cells (isolated from AAV-EF1α-GFP DO11.10 mice). Data represent average from n = 4 cultures based on cells pooled from several mice.
CD4+CD25– cell population from ova-transduced animals showed only a low level of IL-2 secretion in response to stimulation with ova compared with cells isolated from control mice (Figure 7A-B). Coculture of CD4+CD25+ cells from ova-transduced mice reduced IL-2 secretion by CD4+CD25– cells from control mice in a dose-dependent manner (Figure 7C), thereby illustrating the suppressive properties of this cell population.
Discussion
It has been the hope in the gene therapy field that sustained transgene expression would lead to induction of immune tolerance. While sustained expression of systemic proteins in immune competent animals has been described, data presented here are the first direct evidence for induction of T-cell tolerance by viral in vivo gene transfer. Moreover, the TCR transgenic model provides insights into underlying mechanisms of T-cell tolerance.
Induction of anergy and deletion among transgene product–specific CD4+ T cells
Results shown above demonstrate that tolerance induction after hepatic gene transfer involves a combination of mechanisms. Antigen-specific CD4+ T-cell anergy is achieved early after gene transfer and likely becomes more robust by an increase in the proportion of regulatory cells over time. Reduction in the size of the transgene product–specific CD4+ T-cell population in lymphoid organs is most likely due to antigen-specific T-cell deletion. This interpretation is supported by emergence of CD4+ cells in the thymus (and secondary lymphoid organs) that do not express the DO11.10 TCR, thus indicating a central tolerance mechanism based on negative selection against ova-specific CD4+ T cells. An increase in apoptotic TCR+CD4+ cells in the spleens of ova-transduced mice suggests deletion of ova-specific CD4+ T cells also in peripheral lymphoid organs. In an earlier study, we observed inability to induce tolerance to a FIX transgene product in Fas-deficient mice following AAV hepatic gene transfer.19 Taken together, these findings point toward a role of apoptotic cell death (at least in part mediated by Fas-FasL interaction) in tolerance induction during systemic antigen delivery. Interestingly, studies on oral tolerance in the DO11.10 mouse model showed that high-dose feeding of ova antigen caused deletion of TCR+CD4+ not only in gut-associated lymphoid tissues but also in spleen, thymus, and other lymphoid organs, presumably because of antigen presentation outside the gut.23,25 Furthermore, Alpan et al35 have shown that feeding of high-antigen doses (in this case FIX) results in systemic delivery of the antigen. We therefore hypothesize that systemic delivery of high protein levels by different means can lead to deletion of antigen-specific T cells. Persistence of anergic antigen-specific T cells is reminiscent of an earlier observation that functionally defective T cells may persist in the context of intravenous tolerance induction.36 Similar to data on FIX gene transfer, induction of T-cell tolerance to ova correlated with absence of antibody formation after challenge with adjuvant.19 Lack of antiova formation in some of the control mice may be due to some level of ignorance to ova in the TCR transgenic mice as noticed by others or may be caused by preferential stimulation of cellular rather than antibody-mediated response to ova (L.W. and R.W.H., unpublished observations, February 2004).37
Potential role of regulatory T cells
Increase in the proportion of regulatory CD25+ cells among antigen-specific CD4+ T cells should contribute to maintenance of T-cell tolerance by promoting T-cell anergy to the transgene product and suppressing activation of CD4+CD25– T cells. Antigen specificity of regulatory of CD4+CD25+ T cells in vivo is supported by a recent study that used T-cell transfer to suppress immune responses to the transgene product in AAV gene transfer to skeletal muscle.38 However, additional studies will be required to address the question of whether hepatic gene transfer specifically generates or activates regulatory CD4+CD25+ T cells or whether our finding reflects resistance of existing CD4+CD25+ T cells (which may have been generated by thymic presentation of self-antigens rather than ova) to deletion. Regulatory T cells have been shown to be more resistant to deletion, presumably because of their anergic properties (in particular lack of IL-2 expression, presence of which is required for activation-induced cell death).25,30 Future studies may identify other subsets of regulatory CD4+ cells that contribute to tolerance induction in hepatic gene transfer. With respect to treatment of hemophilia B, studies will be necessary to determine whether those CD4+ T cells with regulatory properties, which were activated during hepatic AAV-FIX gene transfer, are part of the CD25+ or CD25– subpopulation. Depending on the underlying FIX mutation, regulatory CD4+CD25+ T cells to FIX may be present or absent prior to gene transfer, and potential of gene transfer to generate, activate, or recruit these cells is unclear at this point.
In contrast to oral tolerance studies, we have not been able to demonstrate induction of T-helper 3 (Th3) regulatory cells, since lymphocyte cultures from mice tolerized by AAV gene transfer did not show evidence for secretion of TGF-β after in vitro restimulation with ova (data not shown). Th3 cells may be preferentially generated during antigen presentation on mucosal surfaces.39
Requirements for tolerance induction
We documented previously that tolerance induction to FIX after hepatic gene transfer is favored by higher levels of transgene expression.19 Minimal levels of systemic FIX expression required for tolerance were 0.5 to 2 nM, with the therapeutic range of expression starting at approximately 0.9 nM (50 ng/mL or 1% of normal levels in humans). Levels of ova expression in DO11.10 mice were 2 to 4 nM (days 30-60), providing a tolerogenic amount of antigen. It is possible that these numbers represent a general requirement for tolerance induction that may also apply to other antigens. It may therefore be harder to induce tolerance to antigens that are more difficult to express at high levels such as factor VIII hepatocyte-derived expression of which was described to be tolerogenic at superphysiologic levels.40 Tolerance induction may fail if antigen presentation combined with activation signals present early after viral gene transfer (such as interaction between viral vector particles and antigen-presenting cells or other cell types, tissue damage associated with the procedure, etc) leads to efficient T-helper cell activation, thereby initiating an adaptive immune response. In contrast to data presented here on hepatic gene transfer, we observed only transient expression of ova (∼2 weeks) after muscle-directed gene transfer to BALB/c or DO11.10 TCR transgenic BALB/c mice. In this case, a CD4+/Th1 and CD8+ T-cell driven inflammatory immune response to ova antigen eliminated ova-expressing muscle fibers (L.W. and R.W.H., unpublished results, September 2003). These data illustrate that the choice of target tissue for gene transfer substantially influences the probability of tolerance induction. The tolerogenic nature of the hepatic environment, perhaps the result of a unique combination of antigen-presenting cells (such as sinusoidal endothelial cells) and local cytokine expression (eg, IL-10 and TGF-β), has been widely recognized in transplant biology and in studies on oral tolerance and is now also increasingly being explored in the gene therapy field.8,19,41-45 Use of a gene transfer vector such as AAV with reduced potential for innate immunity, inflammation, and transduction of antigen-presenting cells is likely required in order not to change the hepatic microenvironment (eg, the cytokine milieu), since an inflammatory environment could skew antigen presentation and T-helper cell differentiation toward induction of an immune response.46-48 However, other vectors may also be adapted as tools for tolerance induction.11,18,49
Alternative strategies for tolerance induction
A number of strategies are actively pursued to attempt tolerance induction to protein antigens by gene transfer in animal models, including in utero and neonatal gene transfer, combination of gene transfer and immune modulation, injection of B cells that received ex vivo retroviral gene transfer of an IgG fusion protein, and infusion of ex vivo–transduced hematopoietic stem cells.6,50-55 It is encouraging for the field that transgene expression in the context of in vivo gene transfer to an animal with a fully developed immune system can induce immune tolerance by promoting T-cell tolerance. Examples of the potential of hepatic gene transfer to confer sustained systemic expression of a therapeutic protein neoantigen include FIX expression in hemophilia B dogs with an early stop codon in the FIX gene and in mice with a FIX gene deletion, as well as expression of human α1-antitrypsin, apo A-I, factor VIII, and β-glucuronidase after hepatic gene transfer with viral vectors.8,14,16,18,19,56-59 Tolerance induction has now been demonstrated for hepatic AAV gene transfer for FIX, ova, and α-galactosidase transgene products.19,20 However, one also has to realize that this strategy may not work equally well for all antigens, and success is also influenced by genetic factors of the recipient of gene transfer.19,60 In particular, requirements for tolerance induction to FVIII have not yet been exhaustively studied. Some successes with hepatocyte-restricted expression from viral vectors have been reported in adult animal models of hemophilia A, while in other studies immune-deficient animals or immune suppression were used to avoid immune responses.12,58,61-64 It is also noteworthy that induction of T-cell tolerance to the transgene product is not linked to tolerance to AAV vector particles, which elicit a neutralizing antibody (NAB) response to capsid antigens after hepatic gene transfer that blocks readministration.65 Furthermore, it is conceivable that preexisting immunity to AAV vector in humans may interfere with tolerance induction through NAB- or memory T-cell–mediated mechanisms. Another limitation to this approach may be presence of inflammation or other pathologic changes to the liver or the immune system, which can influence T-cell activation as was observed in a model of canine hemophilia B.16
In summary, we have documented antigen-specific CD4+ T-cell tolerance induced by transgene expression following hepatic in vivo gene transfer with a viral vector. Expression of a secreted transgene promoted deletion and anergy among CD4+ T cells in lymphoid organs resulting in a smaller and hypo-responsive transgene product–specific CD4+ T-cell population with increased proportion of regulatory cells. These results illustrate the potential of liver-directed gene therapy for tolerance induction to therapeutic proteins.
Prepublished online as Blood First Edition Paper, April 22, 2004; DOI 10.1182/blood-2004-03-0847.
Supported by National Institutes of Health (NIH) grant R01 AI/HL51390-01 (R.W.H.) and NIH training grant T32HL07439 (E.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Ovalbumin cDNA was a kind gift from Dr N. Shastri. The authors thank Drs M. K. Jenkins, Y. Chen, and H. C. J. Ertl for valuable suggestions for these studies and the Flow Cytometry Core Facility of the University of Pennsylvania Cancer Center for assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal