Abstract
We have carried out HLA-matched unrelated donor hematopoietic cell transplantation (HCT) after nonmyeloablative conditioning in patients with hematologic malignancies who were ineligible for conventional transplantations because of age, comorbidities, or both. The nonmyeloablative regimen consisted of 90 mg/m2 fludarabine and 2 Gy total body irradiation given before and mycophenolate mofetil and cyclosporine given after HCT. This report compares, retrospectively, morbidity and mortality among 60 consecutive patients given nonmyeloablative conditioning (nonablative patients) to those among 74 concurrent and consecutive patients given myeloablative conditioning (ablative patients) before unrelated HCT. The Charlson Comorbidity Index was used to assess pretransplantation comorbidities. Even though nonablative patients had significantly higher pretransplantation comorbidity scores, were older, and had more often failed preceding ablative transplantations and cytotoxic therapies, they experienced fewer grades III to IV toxicities than ablative patients. Further, the incidence of grades III to IV acute graft-versus-host disease (GVHD) was significantly lower in nonablative patients. Both patient groups had comparable 1-year probabilities of chronic GVHD. The 1-year nonrelapse mortality rate was 20% in nonablative patients compared to 32% in ablative patients (hazard ratio = 1.4). After adjustment for pretransplantation differences between the 2 patient groups, the hazard ratio was 3.0 (P = .04). Multivariate analyses showed higher pretransplantation comorbidity scores to result in increased toxicity and mortality.
Introduction
Based on preclinical studies in a canine model,1 we have developed a nonmyeloablative conditioning regimen for hematopoietic cell transplantation (HCT) in patients with hematologic malignancies. The regimen was first used in patients receiving transplants from HLA-matched related donors2 and, since January 2000, also for patients given transplants from HLA-matched unrelated donors.3,4 It included conditioning with 90 mg/m2 fludarabine (FLU) and 2 Gy total body irradiation (TBI) and postgrafting immunosuppression with mycophenolate mofetil (MMF) and cyclosporine (CSP).3,4 The regimen has been well tolerated without the typical side effects associated with conventional high-dose conditioning. It relied on graft-versus-tumor effects for eradicating malignant cells. The relatively low degree of toxicity has allowed extending unrelated HCT to include patients older than 50 years and those with comorbid conditions who would be excluded from conventional HCT. In this way, more patients benefited from unrelated HCT because median patient ages at diagnoses of most hematologic malignancies ranged between 65 and 70 years.5
To better understand the indications for nonmyeloablative transplantation, the current report compared, in a retrospective manner, morbidity and mortality among 134 patients given unrelated HCT between January 2000 and January 2002; 60 patients received nonmyeloablative conditioning and 74 received myeloablative conditioning. To describe transplant-related toxicities (TRTs), the bone marrow transplant–specific Common Toxicity Criteria (CTC) of the National Cancer Institute (NCI) (http://ctep.cancer.gov/reporting/CTC-3.html; then select link “Common Toxicity Criteria Document”) were used.
Additionally, we evaluated the impact of pretransplantation factors, including patient comorbidities at the time of HCT as assessed by the Charlson Comorbidity Index (CCI) on transplantation outcomes.6
Patients and methods
The analysis was approved by the institutional review board of the Fred Hutchinson Cancer Research Center (FHCRC; Seattle, WA).
Patients
Data from 60 consecutive patients with hematologic malignancies receiving unrelated hematopoietic cell transplants after nonmyeloablative conditioning (2Gy TBI + FLU) between January 2000 and January 2002 were analyzed retrospectively (nonablative patients). The patients were ineligible for conventional HCT because of age, pretransplantation comorbidities, and/or extensive pretransplantation treatment including high-dose ablative HCT. Results were compared to those among concurrent and consecutive patients (n = 74) given one of 2 standard myeloablative conditioning regimens, either busulphan (BU)/cyclophosphamide (CY) or CY/TBI (ablative patients). The relatively small number of patients in the 2 cohorts precluded matching for pretransplantation factors.
HLA typing and matching
Assessment of pretransplantation comorbidities
Information concerning comorbidities was obtained from medical notes, pathology reports, and laboratory data. The CCI (Table 1) provided a scoring system for multiple comorbidities.6 In the original study, the scores were weighted from 1 to 6, and the total scores were divided into 4 categories as prognostic factors for mortality.6 In a cohort of 559 medical patients, the 1-year mortality rates for the different scores were: 12%, 0; 26%, 1 to 2; 52%, 3 to 4; and 85%, 5 or higher.6 The index has been validated in predicting mortality risks over periods of weeks to 10 years for patients with different medical problems including breast cancer.6,8,9 Also, the CCI successfully predicted mortality among patients with other malignancies.10-14 Charlson et al added age to the CCI, and each decade of age, starting at 50 years, was counted as an extra point.15 The current study did not add age to the score because it was evaluated as a separate factor.
Adapted weighted CCI
Comorbid condition . | Assigned weights for diseases . |
|---|---|
| Hypertension | 0 |
| Angina | 0 |
| Arrhythmia | 0 |
| Gastrointestinal disease | 0 |
| Endocrine disease (other than diabetes) | 0 |
| Pulmonary disease (mild) | 0 |
| Renal disease (mild) | 0 |
| Other | 0 |
| Myocardial infarction | 1 |
| Congestive heart failure | 1 |
| Cerebrovascular disease | 1 |
| Ulcer | 1 |
| Hepatic disease (mild) | 1 |
| Diabetes (mild or moderate) | 1 |
| Pulmonary disease (moderate or severe) | 1 |
| Connective tissue disease | 1 |
| Diabetes (severe with end-organ damage) | 2 |
| Renal disease (moderate or severe) | 2 |
| Solid tumor (without metastases) | 2 |
| Hepatic disease (moderate or severe) | 3 |
| Solid tumor (with metastases) | 6 |
| Total score | Summation |
Comorbid condition . | Assigned weights for diseases . |
|---|---|
| Hypertension | 0 |
| Angina | 0 |
| Arrhythmia | 0 |
| Gastrointestinal disease | 0 |
| Endocrine disease (other than diabetes) | 0 |
| Pulmonary disease (mild) | 0 |
| Renal disease (mild) | 0 |
| Other | 0 |
| Myocardial infarction | 1 |
| Congestive heart failure | 1 |
| Cerebrovascular disease | 1 |
| Ulcer | 1 |
| Hepatic disease (mild) | 1 |
| Diabetes (mild or moderate) | 1 |
| Pulmonary disease (moderate or severe) | 1 |
| Connective tissue disease | 1 |
| Diabetes (severe with end-organ damage) | 2 |
| Renal disease (moderate or severe) | 2 |
| Solid tumor (without metastases) | 2 |
| Hepatic disease (moderate or severe) | 3 |
| Solid tumor (with metastases) | 6 |
| Total score | Summation |
Evaluation of TRTs
TRTs, using CTC version 2.0 of NCI (http://ctep.cancer.gov/reporting/CTC-3.html; click on “Common Toxicity Criteria Document”), were assessed by review of the patients' medical records. Additional information was contained in laboratory and pathology reports in the FHCRC database. The CTC contained toxicity categories, and each category contained several adverse events. Adverse events were graded as: 0 indicates none; I, mild; II,moderate; III, severe; IV, life-threatening or debilitating; and V, death from nonrelapse mortality (NRM).
To avoid multiple grading of individual toxicities, infections and their consequences were graded only under the infection category without grading sites of infections. Likewise, toxicities related to acute graft-versus-host disease (GVHD) were not graded under corresponding organ toxicities.
Definitions
Low-risk diseases included chronic myeloid leukemia (CML) in first chronic phase, acute leukemia in first complete remission, and myelodysplasia (MDS) with refractory anemia or refractory anemia with ringed sideroblasts. High-risk diseases included more advanced CML, acute leukemia, and MDS added to all other hematologic malignancies.4 Cytomegalovirus (CMV) risk groups were defined as previously described.16
Preparative regimens
Nonablative conditioning consisted of FLU, 30 mg/m2/d intravenously, on days –4to –2, and 2 Gy TBI delivered at 7 cGy/min on day 03,4 (Table 2). Ablative conditioning included CY, 60 mg/kg/d intravenously for 2 consecutive days, combined with either 12 Gy (n = 44) or 13.2 Gy (n = 10, all were < 18 years old) fractionated TBI (delivered at 7 cGy/min) administered over 3 or 4 days.17 A combination of BU, 4 mg/kg/d orally for 4 days with levels targeted to a mean concentration at steady state of 800 to 900 ng/mL, and CY, 60 mg/kg/d intravenously for 2 days, was given to 20 ablative patients.17
Patient and disease characteristics
Characteristics . | Nonablative patients (n = 60) . | Ablative patients (n = 74) . | P . |
|---|---|---|---|
| Conditioning regimens, % | |||
| FLU + 200 cGy TBI | 100 | 0 | |
| CY + 1200 cGy TBI | 0 | 58 | |
| CY + 1320 cGy TBI | 0 | 14 | |
| BU + CY | 0 | 28 | |
| Postgrafting immunosuppression, % | |||
| CSP + 30 mg/kg/d MMF | 80 | 9 | |
| CSP + 45 mg/kg/d MMF | 20 | 0 | |
| CSP + MTX | 0 | 90 | |
| CSP + MTX + sirolimus | 0 | 1 | |
| Disease, % | |||
| MDS | 22 | 19 | |
| Acute leukemia | 20 | 34 | |
| CML | 18 | 36 | |
| NHL/HD | 20 | 3 | .0002 |
| MM | 8 | 0 | |
| CLL | 7 | 3 | |
| Other* | 5 | 5 | |
| Disease risk group, % | |||
| High | 78 | 59 | .02 |
| Low | 22 | 41 | |
| Greater than 2nd CR, relapse, or refractory, % | 40 | 16 | .002 |
| Age at transplantation | |||
| Median, y (range) | 54 (5-69) | 41 (1-58) | < .0001 |
| Age older than 55 y, % | 43 | 3 | < .0001 |
| Preceding cytotoxic regimens | |||
| Median no. | 3 (0-7) | 1 (0-8) | .01 |
| 3 or more regimens, % | 58 | 34 | .004 |
| Preceding ablative HCT, % | 33 | 4 | < .0001 |
| Autologous/allogeneic, % | 30/3 | 1/3 | |
| Hematopoietic cell source, % | |||
| G-PBMCs | 82 | 43 | < .0001 |
| Marrow | 18 | 57 | |
| CMV risk group, %† | |||
| Low | 25 | 39 | |
| Intermediate | 18 | 16 | |
| High | 57 | 45 | .16 |
| Sex of patients, % | |||
| Male/female | 62/38 | 47/53 | 0.10 |
| Sex of donors, % | |||
| Male/female | 62/38 | 55/45 | 0.45 |
Characteristics . | Nonablative patients (n = 60) . | Ablative patients (n = 74) . | P . |
|---|---|---|---|
| Conditioning regimens, % | |||
| FLU + 200 cGy TBI | 100 | 0 | |
| CY + 1200 cGy TBI | 0 | 58 | |
| CY + 1320 cGy TBI | 0 | 14 | |
| BU + CY | 0 | 28 | |
| Postgrafting immunosuppression, % | |||
| CSP + 30 mg/kg/d MMF | 80 | 9 | |
| CSP + 45 mg/kg/d MMF | 20 | 0 | |
| CSP + MTX | 0 | 90 | |
| CSP + MTX + sirolimus | 0 | 1 | |
| Disease, % | |||
| MDS | 22 | 19 | |
| Acute leukemia | 20 | 34 | |
| CML | 18 | 36 | |
| NHL/HD | 20 | 3 | .0002 |
| MM | 8 | 0 | |
| CLL | 7 | 3 | |
| Other* | 5 | 5 | |
| Disease risk group, % | |||
| High | 78 | 59 | .02 |
| Low | 22 | 41 | |
| Greater than 2nd CR, relapse, or refractory, % | 40 | 16 | .002 |
| Age at transplantation | |||
| Median, y (range) | 54 (5-69) | 41 (1-58) | < .0001 |
| Age older than 55 y, % | 43 | 3 | < .0001 |
| Preceding cytotoxic regimens | |||
| Median no. | 3 (0-7) | 1 (0-8) | .01 |
| 3 or more regimens, % | 58 | 34 | .004 |
| Preceding ablative HCT, % | 33 | 4 | < .0001 |
| Autologous/allogeneic, % | 30/3 | 1/3 | |
| Hematopoietic cell source, % | |||
| G-PBMCs | 82 | 43 | < .0001 |
| Marrow | 18 | 57 | |
| CMV risk group, %† | |||
| Low | 25 | 39 | |
| Intermediate | 18 | 16 | |
| High | 57 | 45 | .16 |
| Sex of patients, % | |||
| Male/female | 62/38 | 47/53 | 0.10 |
| Sex of donors, % | |||
| Male/female | 62/38 | 55/45 | 0.45 |
NHL indicates non-Hodgkin lymphoma; HD, Hodgkin disease; MM, multiple myeloma; CLL, chronic lymphocytic leukemia; CR, complete remission.
Myelofibrosis; plasma cell leukemia; and Waldenström macroglobulinemia.
Low indicates both recipient and donor have negative CMV serostatus; intermediate, donor has positive whereas recipient has negative CMV serostatus; and high, recipient has positive CMV serostatus regardless of donor condition.
GVHD prophylaxis
Nonablative patients received MMF and CSP as posttransplantation immunosuppression.3,4 MMF was scheduled to be given orally at 15 mg/kg twice daily (n = 48) or at 15 mg/kg 3 times daily (n = 12) from days 0 to +40 with subsequent taper through day +96. CSP was scheduled to be given at 6.25 mg/kg twice daily orally from day –1 with trough levels targeted at 500 ng/mL during the first month and then at 300 to 400 ng/mL, until day +100 with subsequent taper through day +177.
Most ablative patients received methotrexate (MTX)/CSP immunosuppression.18 CSP was scheduled to be given twice daily at 1.5 mg/kg intravenously or 3.75 mg/kg orally, from days –1 to +50 and then tapered until day +180 (with trough levels targeted between 150 and 450 ng/mL). MTX was scheduled to be given intravenously at 15 mg/m2 on day +1, and 10 mg/m2/d on days +3, +6, and +11. Seven ablative patients received MMF orally, 15 mg/kg twice daily from day 0 until day +40, and then discontinued, along with CSP, 3 mg/kg/d intravenously initially and shifted, whenever tolerated, to oral tablets (using correction fraction of 2.5) from day –1 to +100 and then tapered to day +240. One ablative patient received MTX and CSP combined with sirolimus, 12 mg/d orally on day –1 and 4 mg/d from days 0 to 30.
Grading and treatment of GVHD
Diagnosis and clinical grading of acute and chronic GVHD were performed using standard criteria.19,20 Grading was done retrospectively by one independent clinician for both nonablative and ablative patients. Primary treatment of GVHD consisted of systemic corticosteroids, oral beclomethasone with or without systemic corticosteroids, or reinstitution of CSP.
Infection prophylaxis
Early detection of CMV antigenemia and preemptive ganciclovir therapy were used for all patients21 as were standard prophylaxis against candidal infections (fluconazole),22 bacterial infections when neutropenic (ceftazidime or ciprofloxacin), Pneumocystis carinii infection (trimethoprim-sulfamethoxazole or dapsone),23 and herpes simplex virus24 and varicella zoster virus25 reactivation in serologically positive patients (acyclovir).
Statistical methods
For comparison of findings, summary statistics, including frequency counts and percentages for categorical variables, as well as medians and ranges for ages at HCT, were calculated. Cumulative incidence curves were calculated26 for grades III to IV toxicities, NRM, and the impact of comorbidity scores on overall grade IV toxicity, NRM, and overall survival. Univariate comparisons of proportions were performed with the χ2 test. Multivariate analyses of proportions used logistic regression. Analyses of survival, NRM, and relapse used the Cox regression model. Relapse was treated as a competing risk for NRM, and vice versa. Multivariate P values for a given variable were based on adjustment for all other variables in the model. All P values were based on likelihood ratio statistics and were 2-sided. In the univariate analyses done for the nonablative patients, 8 pretransplantation factors were analyzed for their influences on outcome: recipient age, donor sex, CMV risk group, disease risk, hematopoietic cell source, prior ablative HCT, prior chemotherapy regimens, and CCI scores. Subsequent multivariate analyses, using these risk factors, with the addition of conditioning type, included both nonablative and ablative patients for greater statistical power.
Results
Pretransplantation characteristics
Major differences existed between nonablative and ablative patients at the time of HCT (Table 2). Whereas diagnoses among nonablative patients were evenly distributed between MDS, acute leukemias, CML, lymphomas, and to a lesser extent, multiple myeloma and chronic lymphocytic leukemia, diagnoses among ablative patients were mainly CML (24 of 27 in first chronic phase) and acute leukemia (8 of 25 were in first complete remission) followed by MDS (P = .0002). Nonablative patients had a higher percentage of high-risk diseases (P = .02), and more frequently had advanced (more than a second complete remission, relapsed, or refractory) diseases than ablative patients (P = .002). The median age of nonablative patients was 54 years compared to 41 years for ablative patients (P < .0001). Nonablative patients were more heavily pretreated than ablative patients with a median of 3 prior regimens compared to one (P = .01), and with 58% versus 34% receiving more than 2 chemotherapy regimens (P = .004), respectively. Additionally, more nonablative patients had failed prior ablative HCT (P < .0001). More nonablative patients received granulocyte colony-stimulating factor peripheral blood mononuclear cells (G-PBMCs) than ablative patients (P < .0001).
Pretransplantation comorbidities using CCI
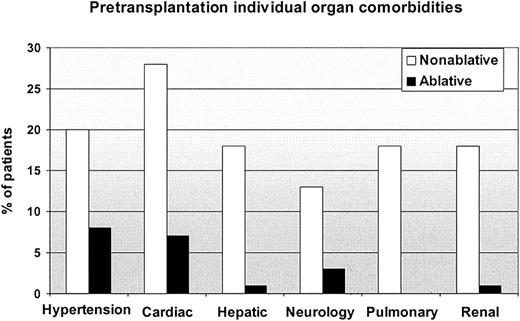
Comorbidity scores at the time of HCT were greater among nonablative patients (Table 3). Eleven nonablative patients had scores of 3 or higher compared to none of the ablative patients; conversely 88% of ablative patients had scores of 0 versus 47% of nonablative patients (P < .0001). Eight of the 11 nonablative patients with scores of 3 or higher had hepatic comorbidities; other common comorbidities were pulmonary (n = 4), cardiac (n = 3), diabetes (n = 3), and renal (n = 2). Of the 11 patients, 6 had active malignancies and another 3 were beyond first complete remission at HCT. Figure 1 shows individual organ comorbidities among nonablative and ablative patients.
Pretransplantation comorbidity scores in patients undergoing HCT
CCI . | Nonablative patients, % (n = 60) . | Ablative patients, % (n = 74) . | P . |
|---|---|---|---|
| Score | |||
| 0 | 47 | 88 | |
| 1-2 | 35 | 12 | |
| 3 or higher | 18 | 0 | < .0001 |
| Total score, comorbidities + age | |||
| 0 | 13 | 74 | |
| 1-2 | 58 | 23 | |
| 3 or higher | 28 | 3 | < .0001 |
CCI . | Nonablative patients, % (n = 60) . | Ablative patients, % (n = 74) . | P . |
|---|---|---|---|
| Score | |||
| 0 | 47 | 88 | |
| 1-2 | 35 | 12 | |
| 3 or higher | 18 | 0 | < .0001 |
| Total score, comorbidities + age | |||
| 0 | 13 | 74 | |
| 1-2 | 58 | 23 | |
| 3 or higher | 28 | 3 | < .0001 |
Comparison of pretransplantation individual organ comorbidities between nonablative and ablative patients.
Comparison of pretransplantation individual organ comorbidities between nonablative and ablative patients.
Posttransplantation events using CTC
Numbers of grades III to IV adverse events in each toxicity category. Lower mean numbers of hematologic (P < .0001), gastrointestinal (P < .0001), hepatic (P = .005), infection-related (P = .02), and hemorrhagic (P = .02) adverse events were seen in nonablative compared to ablative patients, whereas cardiovascular, metabolic, pulmonary, and renal toxicities were comparable (Table 4).
Grades III to IV toxicities by CTC
. | Mean nos. of adverse events per patient . | . | . | Cumulative incidences . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Category . | Nonablative (n = 60) . | Ablative (n = 74) . | P . | Nonablative patients, % (n = 60) . | Ablative patients, % (n = 74) . | P . | ||||
| Hematologic | 3.5 | 4.9 | < .0001 | 100 | 100 | .99 | ||||
| Cardiovascular | 0.9 | 1.1 | 0.4 | 68 | 80 | .1 | ||||
| Gastrointestinal | 0.6 | 1.7 | < .0001 | 27 | 80 | < .0001 | ||||
| Hemorrhage | 0.3 | 0.6 | .02 | 13 | 34 | .005 | ||||
| Hepatic | 0.7 | 1.2 | .005 | 48 | 68 | .02 | ||||
| Infection | 1.6 | 2.2 | .02 | 77 | 88 | .09 | ||||
| Metabolism | 1.6 | 1.8 | .3 | 72 | 87 | .03 | ||||
| Neurologic | 0.6 | 0.5 | .8 | 23 | 37 | .1 | ||||
| Pulmonary | 0.2 | 0.3 | .6 | 12 | 18 | .4 | ||||
| Renal | 0.1 | 0.2 | .1 | 7 | 15 | .1 | ||||
. | Mean nos. of adverse events per patient . | . | . | Cumulative incidences . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Category . | Nonablative (n = 60) . | Ablative (n = 74) . | P . | Nonablative patients, % (n = 60) . | Ablative patients, % (n = 74) . | P . | ||||
| Hematologic | 3.5 | 4.9 | < .0001 | 100 | 100 | .99 | ||||
| Cardiovascular | 0.9 | 1.1 | 0.4 | 68 | 80 | .1 | ||||
| Gastrointestinal | 0.6 | 1.7 | < .0001 | 27 | 80 | < .0001 | ||||
| Hemorrhage | 0.3 | 0.6 | .02 | 13 | 34 | .005 | ||||
| Hepatic | 0.7 | 1.2 | .005 | 48 | 68 | .02 | ||||
| Infection | 1.6 | 2.2 | .02 | 77 | 88 | .09 | ||||
| Metabolism | 1.6 | 1.8 | .3 | 72 | 87 | .03 | ||||
| Neurologic | 0.6 | 0.5 | .8 | 23 | 37 | .1 | ||||
| Pulmonary | 0.2 | 0.3 | .6 | 12 | 18 | .4 | ||||
| Renal | 0.1 | 0.2 | .1 | 7 | 15 | .1 | ||||
Cumulative incidences of grades III to IV toxicities. Given that grades III to IV toxicities seriously affected both quality of life and survival, we separately determined the cumulative probabilities of developing such toxicities, as overall maximum grades (Table 4).
Hematologic toxicity. Grade IV hematologic toxicity was found among 62% of nonablative and 100% of ablative patients (P < .0001). Median days to absolute neutrophil count engraftment were 15 for nonablative and 18 for ablative patients (P = .002), whereas platelets engrafted at medians of 10 and 16 days (P < .0001), respectively. Sixty-three percent of nonablative patients received a median of one platelet transfusion compared to all ablative patients given a median of 7 transfusions (P < .0001), respectively. Eighty-eight percent of nonablative patients received a median of 3 red blood cell transfusions compared to all ablative patients given a median of 5 transfusions (P = .005). Twelve nonablative patients, all given MMF twice daily, rejected their grafts compared to none of the ablative patients (P < .0001). Before HCT, 6 nonablative patients had poor marrow function compared to no ablative patient.
Cardiovascular toxicity. There was a trend for fewer grades III to IV toxicities among nonablative than ablative patients (68% versus 80%, P = .1). Most common were hypertension (55% versus 65%) and arrhythmias (12% versus 9%). Twelve nonablative and 6 ablative patients had pretransplantation hypertension, and all 18 had grades III to IV hypertension after HCT. Another 20 nonablative patients had pretransplantation angina, arrhythmias, ejection fraction less than 50%, cardiac ischemia, myocardial infarction, congestive heart failure, valvular heart disease, or atrial myxoma, and 14 of them (70%) had grades III to IV cardiac toxicities after transplantation. Three of 6 ablative patients with pretransplantation cardiac comorbidities had grades III to IV cardiac toxicities after transplantation.
Gastrointestinal toxicity. Grades III to IV gastrointestinal toxicities were less frequent in nonablative than ablative patients (27% versus 80%, P < .0001). Whereas 53 ablative patients had grades III to IV mucositis, no nonablative patient experienced this toxicity. Nausea and vomiting were common (17% versus 39%, respectively, P = .004). Two nonablative patients had grade IV gastrointestinal toxicity compared to 22 ablative patients (P < .0001).
Hepatic toxicity. Nonablative patients had fewer grades III to IV hepatic toxicities than ablative patients (48% versus 68%, P = .02). No nonablative patient developed veno-occlusive disease (VOD) compared to 13 ablative patients, 8 of whom had grades III to IV VOD (0% versus 11%, P = .002). Seven of 11 (64%) nonablative patients with mild to moderate pretransplantation hepatic comorbidities had grade III (n = 3) and grade IV (n = 4) hepatic toxicities after HCT. One ablative patient had mild pretransplantation hepatic comorbidity and developed grade II VOD after HCT.
Infection. Grades III to IV infections were slightly less frequent in nonablative than ablative patients (77% versus 88%, P = .09). There were no significant differences for fungal infections and CMV antigenemia/disease (12% versus 14% and 45% versus 35%). Bacterial infections were slightly lower among nonablative patients (48% versus 64%, respectively, P = .08). Five nonablative patients had pretransplantation infections requiring continued antibiotic therapy through the early posttransplantation period compared to no ablative patient.
Pulmonary toxicity. Although pulmonary events were rare, grades III to IV pulmonary toxicities were comparable between both patient groups. Interstitial pneumonitis/diffuse alveolar damage was seen among 4 nonablative versus 10 ablative patients. Eleven nonablative patients had significant lung comorbidities before HCT compared to no ablative patient. Three of the 11 (27%) had grades III to IV pulmonary toxicities after HCT and 8 had grade II toxicities.
Renal toxicity. There was a trend toward fewer grades III to IV renal toxicities among nonablative than ablative patients (7% versus 15%, P = .1). Three nonablative patients had grade IV renal toxicity, as did 6 ablative patients. Seven nonablative patients had mild and 4 had moderate to severe renal comorbidities before HCT, one of whom had grade IV renal toxicity after HCT. One ablative patient had moderate renal comorbidity before and grade IV renal failure after HCT.
Other toxicities. Nonablative patients had less grades III to IV hemorrhage than ablative patients (13% versus 34%, P = .005). Grades III to IV metabolic toxicities were less frequent in nonablative than ablative patients (72% versus 87%, P = .03). There was a trend toward fewer grades III to IV neuropsychiatric toxicities in nonablative than ablative patients (23% versus 37%, P = .1).
Time of onset of grades III to IV toxicities. The highest toxicity grades (III-IV) occurred during the first 30 to 40 days after HCT (Figure 2), confirming their close relationship to conditioning regimens. Some toxicities, especially among ablative patients, continued to develop after day 40, consistent with added effects of GVHD complications and treatment.
Cumulative incidences of grades III to IV toxicities versus time. (A) Cardiovascular toxicity; (B) gastrointestinal toxicity; (C) hemorrhage; (D) hepatic toxicity; (E) infection; (F) metabolic toxicity; (G), neurologic toxicity; (H) pulmonary toxicity; (I) renal toxicity; and (J) grades III to IV acute GVHD.
Cumulative incidences of grades III to IV toxicities versus time. (A) Cardiovascular toxicity; (B) gastrointestinal toxicity; (C) hemorrhage; (D) hepatic toxicity; (E) infection; (F) metabolic toxicity; (G), neurologic toxicity; (H) pulmonary toxicity; (I) renal toxicity; and (J) grades III to IV acute GVHD.
GVHD
The incidence of grades II to IV acute GVHD was significantly lower in nonablative than ablative patients (77% versus 91%, P = .03), in part because of less grades III to IV GVHD (17% versus 35%, P = .01). One nonablative and 5 ablative patients had grade IV acute GVHD (P = .1). The times of onset of grades III to IV acute GVHD were similar for both groups (Figure 2J). The incidences and times of onset of chronic extensive GVHD were also similar (53% versus 58%, respectively).
NRM
Although ablative patients had higher incidences of overall grade IV toxicities (odds ratio [OR] = 4.8, P < .0001), differences in NRM at day 100 and 1 year were not statistically significant (hazard ratio [HR] = 1.4 for both; Table 5). After adjusting for age, number of prior chemotherapy regimens, prior ablative HCT, disease risk, and CCI scores, ablative patients had an OR of 9.4 (P = .0001) for overall grade IV toxicities and HRs of 3.6 (P = .07) and 3.0 (P = .04) for day 100 and 1-year NRM, respectively. Table 6 shows causes of 1-year NRM.
Overall grade IV toxicity and NRM
. | Nonablative patients, % (n = 60) . | Ablative patients, % (n = 74) . | OR or HR*(95% CI) . | Adjusted†OR or HR (95% CI) . | P‡ . |
|---|---|---|---|---|---|
| Overall grade IV toxicity | 32 | 69 | 4.8 (2.3-10) | 9.4 (2.7-32) | .0001 |
| Day 100 NRM | 12 | 18 | 1.4 (0.5-3.4) | 3.6 (0.9-16) | .07 |
| 1-y NRM | 20 | 32 | 1.4 (0.7-2.8) | 3.0 (1.0-8.7) | .04 |
. | Nonablative patients, % (n = 60) . | Ablative patients, % (n = 74) . | OR or HR*(95% CI) . | Adjusted†OR or HR (95% CI) . | P‡ . |
|---|---|---|---|---|---|
| Overall grade IV toxicity | 32 | 69 | 4.8 (2.3-10) | 9.4 (2.7-32) | .0001 |
| Day 100 NRM | 12 | 18 | 1.4 (0.5-3.4) | 3.6 (0.9-16) | .07 |
| 1-y NRM | 20 | 32 | 1.4 (0.7-2.8) | 3.0 (1.0-8.7) | .04 |
OR for overall grade IV toxicity and HR for NRM.
Adjusted for age, number of prior regimens (3 versus ≥ 4), prior HCT, disease risk, and CCI scores (0 versus 1-2 versus ≥ 3).
P reflects adjusted comparisons.
Causes of 1-year NRM among nonablative and ablative patients
. | Percent of patients . | . | |
|---|---|---|---|
| Causes of death . | Nonablative (n = 60) . | Ablative (n = 74) . | |
| GVHD and infections | 5 | 9 | |
| GVHD complications | 5 | 7 | |
| Infections | 3 | 9 | |
| Other | 7* | 7† | |
. | Percent of patients . | . | |
|---|---|---|---|
| Causes of death . | Nonablative (n = 60) . | Ablative (n = 74) . | |
| GVHD and infections | 5 | 9 | |
| GVHD complications | 5 | 7 | |
| Infections | 3 | 9 | |
| Other | 7* | 7† | |
One patient died from hepatic failure, one from complications following induction chemotherapy for de novo acute myelogenous leukemia, one from congestive heart failure, and one from suicide.
One patient died from acute renal failure, 2 from interstitial pneumonitis, one from cardiac failure, and one from myocardial infarction with disseminated intravascular coagulopathy.
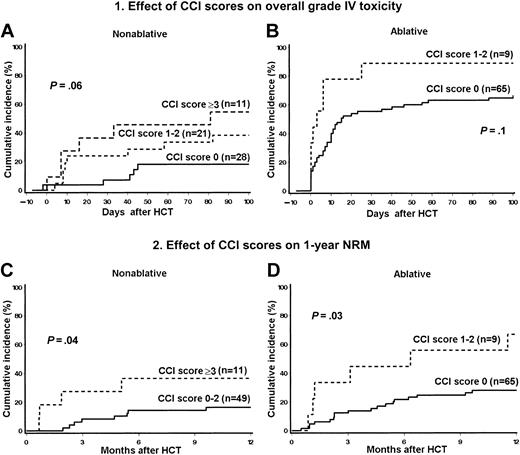
Pretransplantation CCI scores and HCT outcome
The risks for overall grade IV toxicity and NRM among nonablative and ablative patients increased in direct relationship to increasing CCI scores (Figure 3). Six of 28 nonablative patients (21%) with CCI scores of 0, 8 of 21 (38%) with scores of 1 to 2, and 6 of 11 (55%) with scores of 3 or higher experienced overall grade IV toxicity. By comparison, 66% of ablative patients with a score of 0 had overall grade IV toxicity as did 89% of those with scores of 1 to 2. Eight of 49 nonablative patients (16%) with CCI scores of 0 to 2 died within 1 year of HCT compared to 4 of 11 (36%) with scores of 3 or higher. The 1-year NRM rate among ablative patients with a CCI score of 0 was 28% compared to 67% among those with scores of 1 to 2. There were no ablative patients with scores of 3 or higher.
Stratification of CCI scores. Probability of overall grade IV (nonhematologic) toxicity among nonablative (A) and ablative patients (B), and NRM among nonablative (C) and ablative patients (D) stratified by CCI scores.
Stratification of CCI scores. Probability of overall grade IV (nonhematologic) toxicity among nonablative (A) and ablative patients (B), and NRM among nonablative (C) and ablative patients (D) stratified by CCI scores.
The findings were validated by univariate analyses. Higher CCI scores predicted both higher overall grade IV toxicity and NRM. Among nonablative patients, scores of 1 to 2 and 3 or higher had ORs of 2.8 (95% CI, 0.8-10) and 5.5 (95% CI, 1.2-26), respectively, for overall grade IV toxicity (P = .06). Nonablative patients with scores of 3 or higher had HRs of 5.5 (95% CI, 1.2-25) and 4.2 (95% CI, 1.3-14) for day 100 and 1-year NRM, respectively (P = .04 for both). Among ablative patients, CCI scores of 1 to 2 had an OR of 4.1 (95% CI, 0.5-35) for overall grade IV toxicity (P = .13) and a HR of 3.0 (95% CI, 1.2-7.6) for 1-year NRM (P = .03).
In subsequent multivariate analyses, which included both nonablative and ablative patients (Table 7), CCI scores of 3 or higher, present only among nonablative patients, were an independent risk factor for overall grade IV toxicity (P = .06), day 100 NRM (P = .04), and 1-year NRM (P = .05).
Multivariate analyses of risk factors for overall grade IV toxicity and NRM in both patient groups
. | Overall grade IV toxicity . | . | Day 100 NRM . | . | 1-y NRM . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| CCI score . | OR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |||
| 0 (n = 93) | Reference | Reference | Reference | ||||||
| 1-2 (n = 30) | 2.9 (0.9-9.2) | 2.4 (0.8-7.5) | 1.6 (0.7-3.8) | ||||||
| 3 or higher (n = 11) | 5.5 (1.1-29) | .06 | 10.5 (1.8-61) | .04 | 6.4 (1.6-25) | .05 | |||
. | Overall grade IV toxicity . | . | Day 100 NRM . | . | 1-y NRM . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| CCI score . | OR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |||
| 0 (n = 93) | Reference | Reference | Reference | ||||||
| 1-2 (n = 30) | 2.9 (0.9-9.2) | 2.4 (0.8-7.5) | 1.6 (0.7-3.8) | ||||||
| 3 or higher (n = 11) | 5.5 (1.1-29) | .06 | 10.5 (1.8-61) | .04 | 6.4 (1.6-25) | .05 | |||
Factors included in the analyses were recipient age, donor sex, CMV risk group, disease risk, hematopoietic cell source, prior transplantation and number of prior chemotherapy regimens, and CCI scores.
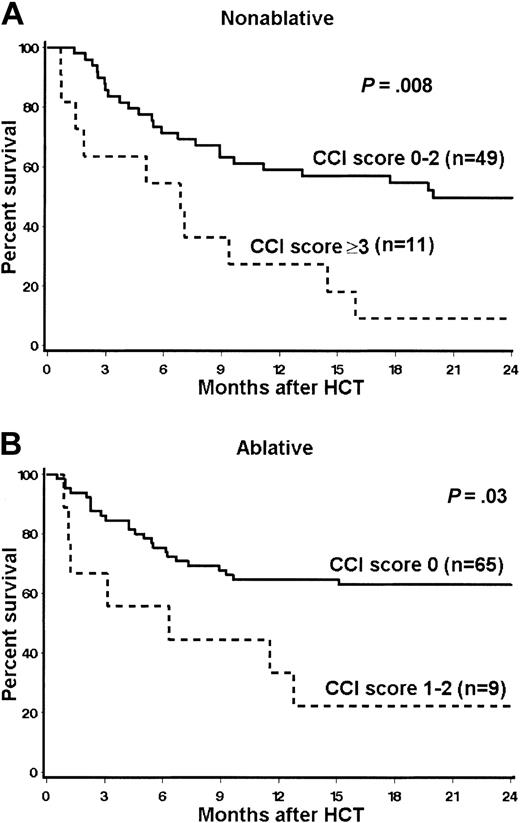
After stratification for CCI scores, 2-year estimates of overall survival for nonablative patients with scores of 0 to 2 versus 3 or higher were 50% versus 9% (HR = 3, P = .008). Six of the 11 (55%) nonablative patients with scores of 3 or higher died because of relapse-related mortality. The 2-year survival rates for ablative patients with scores of 0 versus 1 to 2 were 63% versus 22% (HR = 2.9; P = .03; Figure 4).
Kaplan-Meier estimates of 2-year overall survival stratified by CCI scores. (A) Nonablative patients; (B) ablative patients.
Kaplan-Meier estimates of 2-year overall survival stratified by CCI scores. (A) Nonablative patients; (B) ablative patients.
Discussion
The current study was aimed at evaluating severe toxicities and day 100 and 1-year NRM after unrelated HCT in concurrent and consecutive patients given either nonablative or ablative conditioning regimens. Additionally, the study quantified pretransplantation comorbidities, using the CCI, and assessed their effects on transplantation outcome. The CCI has been widely used in scoring comorbidities in patients with medical diseases including cancer.10-14 By virtue of the protocol eligibility criteria, nonablative patients were significantly older. Increasing age has been associated with higher morbidity and NRM after conventional HCT.27 Also, one third of nonablative patients had failed prior high-dose ablative HCT, a condition previously shown to be associated with high degrees of both morbidity and mortality.28 Further, nonablative patients had more advanced diseases at HCT which, in earlier studies,29,30 predicted higher risks for NRM. Finally, nonablative patients had significantly higher CCI scores, which, again, was a reflection of the protocol eligibility criteria. According to Charlson et al, scores of 1 to 2 and 3 or higher were associated with increasing risks of mortality.6 The increased CCI scores among nonablative patients resulted mainly from more cardiac, hepatic, diabetic, pulmonary, and renal comorbidities. Although more nonablative than ablative patients received G-PBMCs, previous studies have failed to show consistent differences in outcome between unrelated G-PBMCs and marrow grafts.31-35
Despite higher frequencies of adverse risk factors, nonablative patients experienced significantly less posttransplantation grade IV toxicity as assessed by NCI-CTC. Individual toxicities for which differences were seen included hematologic, hemorrhagic, infectious, gastrointestinal, hepatic, metabolic, and grades III to IV acute GVHD events. Directly or indirectly, differences in the severity of the toxicities were related to differences in the intensity of the conditioning regimens.
By definition, the nonablative regimen caused less severe myelosuppression, which, in turn, resulted in decreased transfusion requirements and fewer hemorrhagic events. Also, the less severe neutropenia likely contributed to the lower rate of infections among nonablative patients, as was observed in the comparison of nonablative versus ablative HLA-matched related recipients.36 Other factors reducing the infection rate in nonablative patients might include both the prolonged persistence of host immunocompetent cells37 and the lack of serious gastrointestinal mucosal damage, thereby reducing the risk of enteric organisms entering the bloodstream.
The reasons for the observed lessened incidence of grades III to IV acute GVHD among nonablative patients were likely multifactorial and could include lack of tissue injury and an associated “cytokine storm”38 following low-intensity conditioning, effective immunosuppression with MMF and CSP,39 and initial mixed donor-host T-cell chimerism. Incidences of chronic GVHD were similar in the 2 patient groups, suggesting additional mechanisms being operative in the development of this complication.
Although CSP was, in part, responsible for elevated serum bilirubin and hepatic enzyme levels in both patient groups,40 ablative conditioning41 appeared to cause more elevations in liver function tests, perhaps augmented by the frequent use of total parenteral nutrition.42 VOD of the liver43 was seen exclusively among ablative patients, probably both through direct cell kill and the release of proinflammatory cytokines from injured host tissues,44 and possibly intensified by postgrafting MTX.45
The day 100 and 1-year NRM rates were 12% and 20% for nonablative and 18% and 32% for ablative patients, respectively, and these differences were not statistically significant. However, after adjusting for independent risk factors, differences became either strongly suggestive (day 100 NRM) or significant (1-year NRM).
The adverse effect of one of the risk factors, pretransplantation CCI scores, on outcome was impressive in both patient groups. For example, 1-year NRM was 16% for nonablative patients with scores of 0 to 2 and 36% for those with scores of 3 or higher. This compared to 28% 1-year NRM for ablative patients with a score of 0 and 67% for those with scores of 1 to 2. CCI scores also correlated with 2-year overall survivals, even though those outcomes were also influenced by deaths from disease relapse and progression. The latter effect was particularly strong among nonablative patients with CCI scores of 3 or higher, owing to the advanced stages of their underlying malignancies at the time of HCT. Because survival was not a prespecified end point of the current study and the sample size was small, these results must be interpreted with caution. Nevertheless, the data suggest that patients who are younger than 50 to 55 years and have CCI scores of 0 should continue to receive ablative conditioning, whereas those with scores of 1 or higher should be considered for nonablative conditioning. In patients with both CCI scores of 3 or higher and advanced refractory malignancies, allogeneic HCT might not be a recommended option. The conclusions have to be considered tentative, and the importance of CCI scores on HCT outcome needs to be validated prospectively in larger numbers of patients.
Other investigators have described toxicities after reduced-intensity conditioning for unrelated HCT using toxicity criteria of Bearman et al,46,47 which were originally developed to assess toxicities after ablative HCT. One report suggested that the Bearman criteria were less sensitive than CTC in grading toxicities after nonablative or reduced-intensity regimens.48 Therefore, direct comparisons between the current and other studies were difficult. Giralt et al49 found that day 100 NRM following 3 reduced-intensity regimens consisting of different doses of melphalan and purine analogues was independently predicted by intensities of prior cytotoxic therapy and poor patient performance status. The latter likely reflected the influence of comorbidities similar to those in the current analysis. Patients in the study by Giralt et al had a median age of 52 years, had multiple comorbidities, and had advanced disease status; their day 100 NRM was 53%.49 Badros et al50 used melphalan, low-dose TBI, and FLU as conditioning and reported NRM in 2 of 6 patients and grades III to IV acute GVHD in 3 of 6 patients who underwent unrelated HCT. Others reported day 100 NRM rates comparable to the current one, at 12%,51 14.9%,52 and 19%,53 respectively, using various reduced-intensity regimens; the median patient ages in the 3 studies were 47, 44, and 34 years, respectively. Two of the regimens did not cause VOD or greater than grade II mucositis on the Bearman scale (roughly comparable to grade III mucositis per CTC).52,53 None of the 3 reports defined pretransplantation comorbidities.
In summary, recipients of nonablative conditioning, despite older age, more advanced diseases, greater number of pretransplantation chemotherapy regimens, more frequent prior high-dose HCT, and higher pretransplantation CCI scores had lower rates of 1-year morbidity and NRM compared to concurrent recipients of high-dose ablative hematopoietic cell transplants. CCI scores proved to be sensitive in predicting toxicities and NRM after unrelated HCT, and their usefulness should be validated in prospective studies.
Prepublished online as Blood First Edition Paper, April 27, 2004; DOI 10.1182/blood-2004-02-0545.
Supported in part by grants CA78902, K23 CA92058, CA18029, CA15704, and HL36444 from the National Institutes of Health, Department of Health and Human Services.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank the data coordinators, Chris Davis and Heather Hildebrant, and the study nurses, Steve Minor, Mary Hinds, and John Sedgwick, for their invaluable help in making the study possible. The authors also wish to thank Bonnie Larson and Helen Crawford for help with manuscript preparation.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal