Abstract
Alemtuzumab is a humanized anti-CD52 antibody licensed for refractory B-cell chronic lymphocytic leukemia (B-CLL), when given intravenously at 30 mg thrice weekly. However, the intravenous route is associated with infusion-related reactions and is inconvenient. We measured blood concentrations in 30 relapsed patients treated with intravenous alemtuzumab and in 20 patients from a previously untreated group who received similar doses subcutaneously. Highest trough samples in the intravenous group were less than 0.5 μg/mL to 18.3 μg/mL (mean 5.4 μg/mL). The cumulative dose required to reach 1.0 μg/mL was 13 mg to 316 mg (mean 90 mg). Higher blood concentrations correlated with the achievement of better clinical responses and minimal residual disease. The highest measured concentrations in the subcutaneous group were similar (0.6 μg/mL to 24.8 μg/mL, mean 5.4 μg/mL). However, the cumulative dose to reach 1.0 μg/mL was higher: 146 mg to 1106 mg (mean 551 mg). No antiglobulin responses were detected in 30 patients given intravenous alemtuzumab whereas 2 of 32 patients given subcutaneous alemtuzumab made substantial anti-idiotype responses. Thus, subcutaneous alemtuzumab achieved concentrations similar to those for intravenous alemtuzumab, although with slightly higher cumulative doses. Subcutaneous alemtuzumab is more convenient and better tolerated but may be associated with some patients forming anti–alemtuzumab antibodies, particularly those patients who were previously untreated.
Introduction
Alemtuzumab (CAMPATH-1H, Campath; ILEX Pharmaceuticals, San Antonio, TX) is a humanized immunoglobulin G1 (IgG1) antibody that recognizes the CD52 antigen, a lipid-anchored glycoprotein, on lymphocytes.1-3 Alemtuzumab is exceptionally lympholytic and has been tested as an immunosuppressive agent in transplantation and autoimmune diseases. It is active against a range of lymphoid malignancies and in 2001 was approved for the treatment of B-cell chronic lymphocytic leukemia (B-CLL) in patients who have been treated with alkylating agents and who failed fludarabine therapy. This was based largely on a trial in 93 patients, most of whom had advanced disease with an extremely poor prognosis.4 After a short dose escalation, alemtuzumab was given intravenously at 30 mg thrice weekly for 4 to 12 weeks. This gave an overall response rate (complete remission [CR] + partial remission [PR]) of 33%. Other trials in chemoresistant CLL reported comparable response rates of 42% and 29% respectively.5,6
The dose regimen was developed empirically without the benefit of detailed pharmacodynamic or pharmacokinetic studies. Alemtuzumab often causes substantial first-dose reactions, and although the reactions can now be minimized by appropriate premedication, they are the reason why an initially escalating dose was followed by frequent small doses. This regimen is not the most convenient for patients or physicians. Therefore, the subcutaneous route has been studied in an attempt to reduce side effects and make the treatment more manageable.7,8
The different routes of administration can be compared by analysis of the biodistribution of antibody, on the principle that treatments that give comparable blood concentrations should produce similar clinical effects. Some information is available about the pharmacokinetics of intravenous alemtuzumab in patients undergoing stem cell transplantation who have no substantial leukemic burden.9,10 The terminal half-life was estimated to be approximately 15 to 20 days. However, in patients with advanced CLL, tumor cells could absorb antibody from the circulation.
The humanization of alemtuzumab reduced the risk of antiglobulin responses.11 However, the antigen binding sites are still potentially immunogenic. The ability to provoke a response depends on the target antigen, the immune status of the patient, the use of immunosuppressive drugs, and other factors, perhaps including the route of administration. There is a suggestion that subcutaneous administration might be more immunogenic than intravenous administration in patients with rheumatoid arthritis,12,13 though it is well known that such patients are particularly able to make anti–Ig antibodies.
Here we measured blood concentrations of alemtuzumab and antiglobulin responses against it during 2 different clinical trials. In the first, alemtuzumab was administered intravenously to patients who had previously failed alkylating agents and fludarabine as in the licensed indication. In the second, alemtuzumab was administered subcutaneously to patients who required therapy but had received no prior treatment.8 We compared the blood concentrations and antiglobulin responses and investigated the possible relationship between alemtuzumab concentrations and clinical responses.
Patients, materials, and methods
Patients
Clinical studies were carried out with the approval of local and, in the United Kingdom, regional ethical committees. All patients gave written informed consent prior to enrolment.
Study design: intravenous study
An open-label phase 2 trial was carried out at 5 centers in the United Kingdom (Aberdeen, Birmingham, Bournemouth, Glasgow, Leeds). Primary objectives included the assessment of safety and efficacy as well as measurement of pharmacokinetics. Patients were eligible if they were at least 18 years old, had confirmed B-CLL requiring treatment, had failed previous treatment with purine analogs, had a World Health Organization (WHO) performance status of 2 or less, and had creatinine and bilirubin concentrations less than twice the upper limit of normal (unless due to disease). Exclusion criteria were active infection, HIV positivity, pregnancy or lactation, history of anaphylaxis to antibodies, less than 3 weeks since chemotherapy or less than 6 weeks since investigational therapy, central nervous system (CNS) involvement or persisting severe pancytopenia, other severe concurrent diseases, secondary malignancy, or mental disorders. Patients were premedicated with paracetamol and chlorpheniramine 30 minutes before the first infusion. Septra (sulfamethoxazole/trimethoprim 800 mg/160 mg twice a day, 3 times per week) and aciclovir (200 mg thrice daily) were given prior to therapy and continued until normal blood lymphocytes returned to the normal range. Allopurinol was given during the first 4 weeks of treatment. Alemtuzumab (M&I Partnership, Cambridge, MA) was diluted in 100 mL of 0.9% saline and administered intravenously over approximately 2 hours. A dose of 3 mg was given on day 1 and if it was well tolerated, a 10-mg dose was given on day 2 and a 30-mg dose on day 3. If severe reactions occurred, the same dose was repeated daily until it was well tolerated and then the dose was escalated. Subsequent 30-mg doses were given thrice weekly for up to 12 weeks, though treatment was occasionally extended if clinically indicated.
Before therapy, a full history, physical examination, and laboratory evaluation were performed, including assessment of lymph node, liver, and spleen size, computed tomographic (CT) scan, Rai staging, blood analysis, bone marrow aspirate, and trephine and immunophenotyping by flow cytometry. Disease parameters were reassessed and blood and bone marrow were reanalyzed by flow cytometry for residual CLL cells every 4 weeks. Therapy was stopped if there was no evidence of CLL or if sequential bone marrow samples showed no fall in the level of CLL. Blood samples were collected weekly to measure alemtuzumab concentrations and antiglobulins.After the last dose, samples were collected daily for 4 days and then weekly for 8 weeks. This was not always possible for logistic or clinical reasons.
During follow-up, patients were assessed monthly for 6 months. Responders were assessed every 6 months thereafter until disease progression. Disease response and toxicity were graded according to the criteria of the National Cancer Institute (NCI).
Study design: subcutaneous study
An open-label phase 2 trial was conducted at 4 clinics at the Karolinska Institute, Stockholm, Sweden.8 The primary objective was to assess the efficacy of alemtuzumab administered subcutaneously. Patients were eligible if they had a diagnosis of B-CLL, were age 18 to 75, had a WHO performance status of 1 or less, life expectancy at least 12 weeks, required treatment, and had not been treated previously. Other details were comparable to the intravenous study except the treatment regimen. Alemtuzumab was obtained from the Therapeutic Antibody Centre, Oxford, United Kingdom (patients 1-29) or from M&I Partner (patients 30-41). The 2 preparations were similar although the formulations were slightly different and antibody from the Therapeutic Antibody Centre was stored frozen.14 On day 1, 3 mg alemtuzumab was administered by subcutaneous injection in the thigh. If well tolerated, 10 mg was given on day 3 and 30 mg (in 2 injection sites) was given on day 5. In the event of erythema or edema, the same dose was repeated until well tolerated. After dose escalation, most patients self-administered the antibody. The 30-mg dose was given thrice weekly for up to 18 weeks. If treatment was interrupted for more than 7 days the dose was reinitiated at 3 mg or 10 mg. Therapy was stopped if patients achieved a complete remission (CR) or fulfilled NCI criteria for progressive disease (PD).
Measurement of alemtuzumab in patient serum
Serum samples were collected before doses and at various times afterward and stored at –70° C until analysis by immunofluorescence.15 Test samples were incubated at 56° C for 30 minutes to inactivate complement then incubated with HUT-78 cells at room temperature for 30 minutes. The cells were washed and resuspended in fluorescein isothiocyanate (FITC)–labeled polyclonal anti–human IgG Fc domain (F-9512; Sigma, Poole, United Kingdom), incubated for 30 minutes, washed and fixed with formaldehyde prior to flow cytometry. Alemtuzumab concentrations were calculated from the median fluorescence by interpolation on a standard curve. The lower limit of quantitation was 0.5 μg/mL (allowing for 2-fold sample dilution), overall precision was plus or minus 13%, and overall accuracy was 109%. There was no interference by normal or patient control sera and no reactivity with F(ab')2 fragments of alemtuzumab.15
Measurement of antiglobulin responses
Antiglobulin responses to alemtuzumab were measured in serum samples by sandwich enzyme-linked immunosorbent assay (ELISA) as previously described11,16 with some modifications. Microtiter plates were coated with alemtuzumab (M&I Partner) and blocked with diluent (phosphate-buffered saline [PBS] containing 2% bovine serum albumin [BSA]). Standards, quality-control samples, and test samples were added, incubated for 1 hour at room temperature, and rinsed 4 times with wash buffer (PBS containing 0.05% Tween 20). Biotin-labeled alemtuzumab was added to each well, incubated for 1 hour, and rinsed as before. The assay was developed with extravidin-peroxidase (E-2886; Sigma) and ortho-phenylenediamine. Color development was measured at 450 nm for 20 minutes by Genesis II software (Thermo Life Sciences, Basingstoke, United Kingdom). The standard was YID13, a monoclonal anti-idiotype specific for alemtuzumab.16 One U/mL of antiglobulin activity is the signal equivalent to 1 ng/mL of YID13 reference standard. The limit of detection (LOD) and lower limit of quantitation (LLOQ) was 488 U/mL serum (allowing for 2-fold sample dilution), overall precision was plus or minus 14%, and overall accuracy was 94%. There were no false positives and no interference by normal or patient control sera. Positive signals were obtained with serum containing rheumatoid factor or spiked with native CD52 antigen. The assay was reproducible despite variations in reagent concentrations, incubation and washing conditions, blood clotting conditions, and sample storage conditions within the specified ranges. Up to 3-fold enhancement of antiglobulin activity was seen in samples that had been heat-treated or stored for over a month at 4° C. This might be due to aggregation, since aggregates of the control anti-idiotype reagent also gave enhanced activity. Alemtuzumab and anti-alemtuzumab interact with each other to reduce the signal in either assay if both were present in the same sample. Thus, the antiglobulin assay measures only the concentration of free anti–alemtuzumab antibody, that is, the excess over the levels of alemtuzumab itself. Considering that alemtuzumab concentration was never more than 26 μg/mL, then it might interfere with measurement of antiglobulin responses less than about 26 000 U/mL. However, the antiglobulin levels in a patient who made a clear-cut response exceeded 106 U/mL. We report data for all samples measured, both during and, where available, after treatment. For complete assessment of antiglobulin responses, it is always preferable to analyze samples over an appropriate period of time after the end of treatment.
Measurement of minimal residual disease by flow cytometry
Residual CLL cells were quantified by sensitive 4-color flow cytometry; a method more sensitive than consensus-primer polymerase chain reaction (PCR) and more practicable than sequence-specific quantitative PCR.17 At least 5 × 106 bone marrow leukocytes were incubated with a mixture of fluorescent-labeled antibodies, washed twice, and analyzed using a FACSort (Becton Dickinson, Oxford, United Kingdom) with CELLQuest v3.1 software. The antibodies used were CD45/CD48 (Chemicon, Chandlers Ford, United Kingdom)/CD19/CD3, kappa/lambda/CD19/CD5, CD20 (Beckman Coulter, High Wycombe, United Kingdom)/CD79b (Beckman Coulter)/CD19/CD5, and CD20 (Beckman Coulter)/CD38 (BD Biosciences, Oxford, United Kingdom)/CD19/CD5 conjugated to FITC/phycoerythrin (PE)/PE-Cy5/allophycocyanin (APC), respectively. Reagents without named manufacturer were produced in-house. Between 50 000 and 500 000 cells were analyzed. B cells were identified by setting a region on CD19 versus side scatter (SSC), followed by a forward scatter region, and then ensuring that no CD3+ events fell within the combined gate. CLL cells were discriminated from mature B cells by their stronger expression of CD5 and weaker expression of CD20 and CD79b, and from B progenitors by their weaker expression of CD38. This assay can detect 1 CLL cell in approximately 104 to 105 leukocytes.
Results
Collection and analysis of samples
Serum samples were obtained from all 30 patients in the intravenous study and 20 of the 41 patients in the subcutaneous study, almost exclusively from a single clinic (Karolinska Hospital). Samples were tested in duplicate and the mean concentration was calculated. If the mean was less than 0.5 μg/mL (LLOQ), a level of less than 0.5 μg/mL was recorded. If either result was more than 20 μg/mL or a measurement was technically faulty, then the test was repeated, using a higher dilution if appropriate, and the repeat results were used.
Optimal time for collection of peak samples following intravenous administration
A preliminary experiment was carried out to identify the time following intravenous administration when the peak antibody concentration might be found. Samples were collected from 3 patients at 15 minutes, 30 minutes, and 60 minutes after the end of one dose each week for 4 to 7 weeks. The peak sample occurred at 15 minutes, significantly more often (21 times) than at 30 minutes (6 times) or 60 minutes (2 times) (P < .01; paired Wilcoxon signed rank test). Therefore, for subsequent patients a single sample was collected at 15 minutes to determine the peak concentration.
Serum concentrations of alemtuzumab during intravenous treatment
A total of 1561 patient samples were tested. Terminal samples (after the last dose) were not available for some patients, and in a few cases only a small number of terminal samples were available. All samples were tested in duplicate; the overall mean coefficient of variation (CV) was 10%. Many of the early samples and many trough samples were below the limit of quantitation (< 0.5 μg/mL). Five samples gave anomalous results, substantially diverging from other results for the same patients. These could be explained by accidental mix-ups at the clinical centers and were omitted from analysis. An example of the doses and blood concentrations throughout the course of treatment for one patient is shown in Figure 1.
Example of alemtuzumab concentrations in patient treated with intravenous antibody. After an initial dose escalation, 30 mg of alemtuzumab was administered 3 times a week for 8 weeks (•). Serum samples were taken before (▵) and after (▾) the dose once a week. This patient made a good clinical response to the therapy.
Example of alemtuzumab concentrations in patient treated with intravenous antibody. After an initial dose escalation, 30 mg of alemtuzumab was administered 3 times a week for 8 weeks (•). Serum samples were taken before (▵) and after (▾) the dose once a week. This patient made a good clinical response to the therapy.
In contrast to patients who had received transplants,9,10 there were wide variations in alemtuzumab concentrations between different patients with CLL. The highest peak ranged from 2.8 μg/mL to 26.4 μg/mL (mean 10.7 μg/mL). The highest trough ranged from less than 0.5 μg/mL to 18.3 μg/mL (mean 5.4 μg/mL). The cumulative dose before the trough concentration reached 1.0 μg/mL ranged from 13 mg to 316 mg (mean 90 mg; Table 1). In 9 of 21 evaluable patients, the highest trough concentration was measured just before the last dose, indicating that a steady state had not been reached. Total cumulative doses varied between patients, but did not correlate with the final antibody concentrations (Table 1).
Alemtuzumab dose and serum concentrations: intravenous study
Patient no. . | Cumulative dose, mg . | Number of samples . | Terminal samples . | Dose to reach 1 μg/mL . | Maximum peak, μg/mL . | Maximum trough, μg/mL . | Before last dose, μg/mL . | After last dose, μg/mL . |
|---|---|---|---|---|---|---|---|---|
| 001 | 1026 | 80 | 12 | 143 | 16.3 | 8.3 | 7.7 | 11.7 |
| 002 | 673 | 56 | 12 | 13 | 19.1 | 13.0 | 13.0 | 19.1 |
| 003 | 1106 | 91 | 23 | 43 | 6.7 | 1.1 | 0.9 | 5.9 |
| 004 | 836 | 54 | 0 | 103 | 26.4 | 18.3 | ND | ND |
| 005 | 1064 | 69 | 7 | 46 | 16.3 | 6.2 | 6.2 | 16.3 |
| 006 | 736 | 55 | 8 | 16 | 9.5 | 3.7 | 2.6 | 8.5 |
| 007 | 1093 | 75 | 11 | 133 | 19.8 | 11.9 | 11.9 | 16.4 |
| 008 | 291 | 19 | 3 | 111 | 5.8 | 0.7 | 0.7 | 0.7 |
| 009 | 1026 | 73 | 12 | 53 | 13.1 | 7.7 | 7.7 | 12.2 |
| 010 | 179 | 21 | 0 | 46 | 4.0 | 0.8 | ND | ND |
| 011 | 1076 | 71 | 11 | 133 | 8.3 | 1.9 | 1.9 | 8.3 |
| 012 | 473 | 28 | 0 | 53 | 4.4 | 0.2 | ND | ND |
| 013 | 946 | 60 | 13 | 183 | 4.0 | 0.5 | < 0.5 | 2.9 |
| 014 | 436 | 31 | 0 | 46 | 12.5 | 9.9 | ND | ND |
| 015 | 2053 | 89 | 0 | 43 | 6.4 | 2.8 | ND | ND |
| 016 | 1016 | 58 | 11 | 103 | 9.5 | 4.9 | 4.5 | 9.0 |
| 017 | 1093 | 55 | 8 | 133 | 6.6 | 1.7 | 1.2 | 6.0 |
| 018 | 733 | 40 | 1 | 43 | 8.2 | 2.6 | ND | ND |
| 019 | 493 | 27 | 1 | 73 | 19.9 | 14.8 | ND | ND |
| 020 | 1093 | 55 | 12 | 43 | 17.7 | 9.4 | 7.7 | 12.9 |
| 021 | 583 | 32 | 0 | 163 | 4.0 | 1.1 | ND | ND |
| 022 | 1003 | 45 | 9 | 43 | 7.1 | 1.0 | 0.6 | 6.3 |
| 023 | 1063 | 47 | 6 | 43 | 26.0 | 17.0 | ND | 24.1 |
| 024 | 1126 | 57 | 12 | 316 | 6.0 | 2.6 | 2.0 | 5.5 |
| 025 | 1093 | 54 | 11 | 73 | 6.9 | 5.2 | 3.1 | 6.7 |
| 026 | 969 | 57 | 13 | 133 | 5.4 | 1.5 | 1.4 | 4.7 |
| 027 | 1066 | 45 | 10 | 73 | 2.8 | 0.9 | < 0.5 | 2.5 |
| 028 | 673 | 42 | 13 | 43 | 8.5 | 4.0 | 4.0 | 6.9 |
| 029 | 416 | 40 | 10 | 133 | 7.6 | 3.3 | 3.3 | 7.6 |
| 030 | 763 | 35 | 5 | 133 | 12.2 | 3.7 | 3.7 | 12.2 |
| Minimum | 179 | 19 | 0 | 13 | 2.8 | < 0.5 | < 0.5 | 0.7 |
| Mean | 873 | 52 | 8 | 90.4 | 10.7 | 5.4 | 4.0 | 9.4 |
| Maximum | 2063 | 91 | 23 | 316 | 26.4 | 18.3 | 13.0 | 24.1 |
Patient no. . | Cumulative dose, mg . | Number of samples . | Terminal samples . | Dose to reach 1 μg/mL . | Maximum peak, μg/mL . | Maximum trough, μg/mL . | Before last dose, μg/mL . | After last dose, μg/mL . |
|---|---|---|---|---|---|---|---|---|
| 001 | 1026 | 80 | 12 | 143 | 16.3 | 8.3 | 7.7 | 11.7 |
| 002 | 673 | 56 | 12 | 13 | 19.1 | 13.0 | 13.0 | 19.1 |
| 003 | 1106 | 91 | 23 | 43 | 6.7 | 1.1 | 0.9 | 5.9 |
| 004 | 836 | 54 | 0 | 103 | 26.4 | 18.3 | ND | ND |
| 005 | 1064 | 69 | 7 | 46 | 16.3 | 6.2 | 6.2 | 16.3 |
| 006 | 736 | 55 | 8 | 16 | 9.5 | 3.7 | 2.6 | 8.5 |
| 007 | 1093 | 75 | 11 | 133 | 19.8 | 11.9 | 11.9 | 16.4 |
| 008 | 291 | 19 | 3 | 111 | 5.8 | 0.7 | 0.7 | 0.7 |
| 009 | 1026 | 73 | 12 | 53 | 13.1 | 7.7 | 7.7 | 12.2 |
| 010 | 179 | 21 | 0 | 46 | 4.0 | 0.8 | ND | ND |
| 011 | 1076 | 71 | 11 | 133 | 8.3 | 1.9 | 1.9 | 8.3 |
| 012 | 473 | 28 | 0 | 53 | 4.4 | 0.2 | ND | ND |
| 013 | 946 | 60 | 13 | 183 | 4.0 | 0.5 | < 0.5 | 2.9 |
| 014 | 436 | 31 | 0 | 46 | 12.5 | 9.9 | ND | ND |
| 015 | 2053 | 89 | 0 | 43 | 6.4 | 2.8 | ND | ND |
| 016 | 1016 | 58 | 11 | 103 | 9.5 | 4.9 | 4.5 | 9.0 |
| 017 | 1093 | 55 | 8 | 133 | 6.6 | 1.7 | 1.2 | 6.0 |
| 018 | 733 | 40 | 1 | 43 | 8.2 | 2.6 | ND | ND |
| 019 | 493 | 27 | 1 | 73 | 19.9 | 14.8 | ND | ND |
| 020 | 1093 | 55 | 12 | 43 | 17.7 | 9.4 | 7.7 | 12.9 |
| 021 | 583 | 32 | 0 | 163 | 4.0 | 1.1 | ND | ND |
| 022 | 1003 | 45 | 9 | 43 | 7.1 | 1.0 | 0.6 | 6.3 |
| 023 | 1063 | 47 | 6 | 43 | 26.0 | 17.0 | ND | 24.1 |
| 024 | 1126 | 57 | 12 | 316 | 6.0 | 2.6 | 2.0 | 5.5 |
| 025 | 1093 | 54 | 11 | 73 | 6.9 | 5.2 | 3.1 | 6.7 |
| 026 | 969 | 57 | 13 | 133 | 5.4 | 1.5 | 1.4 | 4.7 |
| 027 | 1066 | 45 | 10 | 73 | 2.8 | 0.9 | < 0.5 | 2.5 |
| 028 | 673 | 42 | 13 | 43 | 8.5 | 4.0 | 4.0 | 6.9 |
| 029 | 416 | 40 | 10 | 133 | 7.6 | 3.3 | 3.3 | 7.6 |
| 030 | 763 | 35 | 5 | 133 | 12.2 | 3.7 | 3.7 | 12.2 |
| Minimum | 179 | 19 | 0 | 13 | 2.8 | < 0.5 | < 0.5 | 0.7 |
| Mean | 873 | 52 | 8 | 90.4 | 10.7 | 5.4 | 4.0 | 9.4 |
| Maximum | 2063 | 91 | 23 | 316 | 26.4 | 18.3 | 13.0 | 24.1 |
ND indicates not determined.
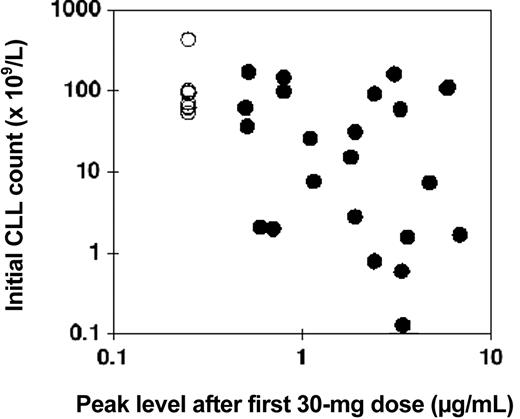
Comparison of the starting lymphocyte count with the peak concentration following the first 30-mg infusion showed a modest negative correlation, that is, the higher the initial lymphocyte count, the lower tended to be the peak concentration of alemtuzumab (Figure 2).
Relationship between initial CLL count and alemtuzumab blood concentration: intravenous study. The numbers of CLL cells in the blood directly before intravenous therapy are plotted against the peak concentration of alemtuzumab measured directly after the first 30-mg dose (•). In 8 patients the concentration was below the limit of quantitation, and these have all been plotted at an arbitrary level of 0.25 μg/mL (○). Pearson correlation coefficient for log (initial CLL count) versus log (alemtuzumab concentration) was –0.44, P = .01.
Relationship between initial CLL count and alemtuzumab blood concentration: intravenous study. The numbers of CLL cells in the blood directly before intravenous therapy are plotted against the peak concentration of alemtuzumab measured directly after the first 30-mg dose (•). In 8 patients the concentration was below the limit of quantitation, and these have all been plotted at an arbitrary level of 0.25 μg/mL (○). Pearson correlation coefficient for log (initial CLL count) versus log (alemtuzumab concentration) was –0.44, P = .01.
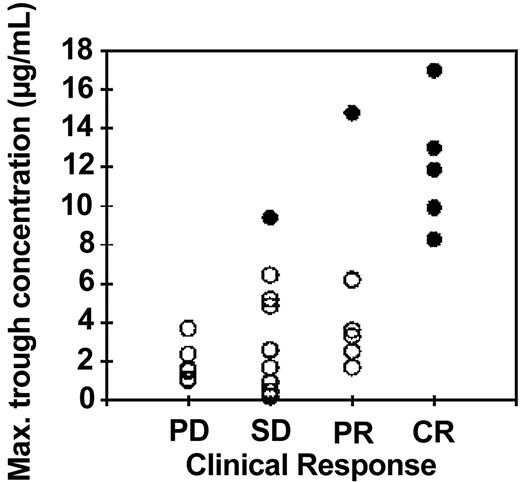
In Figure 3 the highest trough concentration for each patient is plotted against the response measured by NCI criteria for 27 patients where the clinical response was reported within 4 months of the end of treatment. There was a significant increase in trough concentrations with increasing clinical response (P = .006, Kruskal-Wallis test). There was an even more striking correlation between trough concentrations and the attainment of minimal residual disease (MRD) negativity in 7 patients (filled symbols) compared with the 17 patients who still had residual CLL cells in the bone marrow at the end of treatment as measured by 4-color flow cytometry (P < .0001, Kruskal-Wallis test)
Relationship between maximum trough concentrations of alemtuzumab and clinical response: intravenous study. The highest trough concentration of alemtuzumab (which generally occurred following the penultimate or last dose) is plotted against the clinical response at the end of intravenous treatment as determined by NCI criteria. PD indicates progressive disease; SD, stable disease; PR, partial remission; CR, complete remission. A better clinical outcome was significantly associated with higher alemtuzumab concentrations (P = .006, Kruskal-Wallis test). There was an even stronger correlation between high trough concentrations (> 8 μg/mL) and good responses measured by less than 0.1% CLL cells in the bone marrow (•; P < .0001). Three patients who died before the end of the planned treatment course are not included in this figure. Of those patients, 2 had low alemtuzumab concentrations (≤ 1.1 μg/mL) and progressive disease at the time of death. The third had a high antibody concentration (18.3 μg/mL) and low levels of residual disease (0.21%). Each symbol represents a single patient; • indicates a patient with no detectable MRD; ○, a patient with detectable MRD.
Relationship between maximum trough concentrations of alemtuzumab and clinical response: intravenous study. The highest trough concentration of alemtuzumab (which generally occurred following the penultimate or last dose) is plotted against the clinical response at the end of intravenous treatment as determined by NCI criteria. PD indicates progressive disease; SD, stable disease; PR, partial remission; CR, complete remission. A better clinical outcome was significantly associated with higher alemtuzumab concentrations (P = .006, Kruskal-Wallis test). There was an even stronger correlation between high trough concentrations (> 8 μg/mL) and good responses measured by less than 0.1% CLL cells in the bone marrow (•; P < .0001). Three patients who died before the end of the planned treatment course are not included in this figure. Of those patients, 2 had low alemtuzumab concentrations (≤ 1.1 μg/mL) and progressive disease at the time of death. The third had a high antibody concentration (18.3 μg/mL) and low levels of residual disease (0.21%). Each symbol represents a single patient; • indicates a patient with no detectable MRD; ○, a patient with detectable MRD.
In Figure 4 the mean trough concentration is plotted throughout the treatment for 2 groups of patients: (1) 8 responders who reached MRD less than 0.4%, and (2) 22 others. The mean trough concentration was significantly higher in the responders throughout the whole course of treatment from week 2 to week 11 (P < .02 at each time point, Kruskal-Wallis test).
Mean trough concentrations of alemtuzumab during intravenous therapy. The mean trough concentrations during treatment (measured 48 hours after a dose, once a week) are plotted, with standard deviations, for 8 patients who ultimately reached less than 0.4% CLL cells in the bone marrow (▵) and compared with 22 patients who still had residual CLL cells at the end of treatment (•).
Mean trough concentrations of alemtuzumab during intravenous therapy. The mean trough concentrations during treatment (measured 48 hours after a dose, once a week) are plotted, with standard deviations, for 8 patients who ultimately reached less than 0.4% CLL cells in the bone marrow (▵) and compared with 22 patients who still had residual CLL cells at the end of treatment (•).
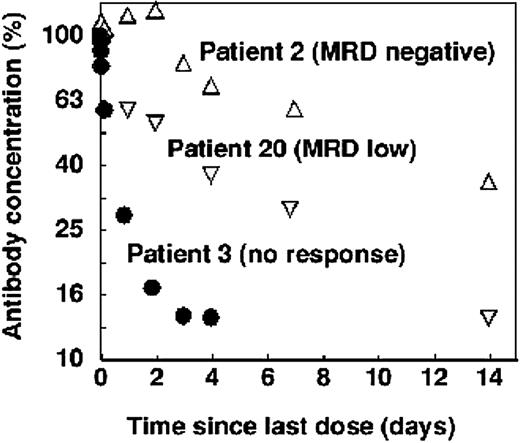
Clearance of alemtuzumab following the last dose showed a similar correlation with residual disease. In patients with undetectable CLL cells, there was a single clearance phase characterized by a relatively long half-life. In patients who still had a substantial tumor burden, most of the antibody was rapidly cleared from the blood. Patients with low levels of residual disease showed an intermediate pattern. An example of each is shown in Figure 5.
Clearance of alemtuzumab in selected patients treated intravenously. In patients with minimal residual disease (by flow cytometry) at the end of therapy, clearance was slow and single phase (▵). Patients who still had a small amount of CLL cells showed biphasic clearance (▿) and patients who still had bulky disease cleared the antibody rapidly (•). However, in each case the terminal half-life was roughly similar.
Clearance of alemtuzumab in selected patients treated intravenously. In patients with minimal residual disease (by flow cytometry) at the end of therapy, clearance was slow and single phase (▵). Patients who still had a small amount of CLL cells showed biphasic clearance (▿) and patients who still had bulky disease cleared the antibody rapidly (•). However, in each case the terminal half-life was roughly similar.
Sixteen patients contributed informative terminal samples after the last 30-mg dose. From these data the following mean pharmacokinetic parameters were calculated (ILEX Oncology, unpublished data, September 2001). The apparent steady-state volume of distribution (VSS) was 0.185 L/kg and the apparent volume of distribution during the terminal phase (VZ) was 0.252 L/kg. These are larger than for other monoclonal antibodies in humans but are consistent with the notion that alemtuzumab distributes through the plasma compartment and a substantial extravascular lymphocyte compartment. The mean terminal phase half-life (t1/2, Z) was 6.1 days, which is consistent with previous measurements for alemtuzumab and other chimeric antibodies in humans.
Serum concentrations of alemtuzumab during subcutaneous treatment
This study was started in 1998 using alemtuzumab manufactured at the Therapeutic Antibody Centre. During June 2000, commercially produced material became available and was subsequently used. No obvious differences were observed in the clinical responses. A total of 542 patient samples were received and tested. Two were mislabeled and are not included here. The overall mean CV was 8%. Assessment of pharmacokinetics in 7 patients was inadequate due to the limited number or inappropriate timing of samples.
Patient no. 8036 (who started with alemtuzumab from the Therapeutic Antibody Centre and was switched to commercial alemtuzumab) never achieved concentrations above the LLOQ. This patient had rapidly made a strong antiglobulin response to the alemtuzumab, which probably neutralized all of the administered antibody (“Antiglobulins following subcutaneous treatment”). The patient never showed any clearance of lymphocytes but showed continued reactions at the injection site. Because of this, the patient was switched to intravenous administration after 6 weeks.
There were some changes from the dose regimen due to clinical reactions. There were 2 patients who received lower doses throughout (10 mg and 20 mg) and 2 (including patient no. 8036, above) who were switched to intravenous dosing. The dose escalation rate was often slower than originally planned, typically taking 1 to 2 weeks to reach 30 mg. After this, the planned dose was usually maintained but only 1 of the 21 patients analyzed received the maximum planned dose of 1573 mg. The mean total dose was 1249 mg and the lowest dose was 712 mg.
The highest concentrations ranged from 0.6 μg/mL to 24.8 μg/mL and the mean (5.4 μg/mL) was the same as in the intravenous study (Table 2). However, the cumulative dose before the concentration reached 1.0 μg/mL ranged from 146 mg to 1106 mg (mean 551 mg), which was substantially more than in the intravenous study. An example of the time course in one patient illustrating this delay is shown in Figure 6.
Alemtuzumab dose and serum concentrations: subcutaneous study
Patient no. . | Cumulative dose, mg . | No. of samples . | Above LOQ . | Terminal samples . | Dose to reach 1 μg/mL . | Max concentration, μg/mL . | After last dose, μg/mL . |
|---|---|---|---|---|---|---|---|
| 8010 | 1358 | 24 | 2 | 0 | 728 | 1.3 | ND |
| 8015 | 1506 | 28 | 1 | 0 | > 156 | 0.6 | ND |
| 8016 | 1573 | 29 | 3 | 0 | 673 | 1.5 | ND |
| 8017 | 1213 | 20 | 2 | 0 | > 193 | 0.9 | ND |
| 8021 | 983 | 29 | 12 | 3 | 353 | 5.3 | 4.1 |
| 8026 | 1379 | 23 | 3 | 0 | 959 | 1.4 | ND |
| 8027 | 1269 | 43 | 9 | 1 | 639 | 9.0 | 6.8 |
| 8028 | 1363 | 37 | 13 | 3 | 403 | 5.8 | 5.8 |
| 8029 | 1133 | 34 | 9 | 1 | 503 | 7.3 | 7.3 |
| 8030 | 1553 | 45 | 16 | 1 | 473 | 4.3 | 4.2 |
| 8031 | 712 | 27 | 2 | 1 | 712 | 1.4 | 1.4 |
| 8032 | 723 | 17 | 2 | 0 | 393 | 2.5 | 2.5 |
| 8033 | 1302* | 28 | 10 | 0 | 542 | 3.4 | 3.0 |
| 8034 | 1412 | 24 | 16 | 4 | 269 | 6.3 | 5.8 |
| 8035 | 1036 | 24 | 15 | 6 | 406 | 17.1 | 17.1 |
| 8036 | 819* | 21 | 0 | 0 | > 819 | < 0.5 | < 0.5 |
| 8038 | 1486 | 19 | 9 | 3 | 1106 | 2.6 | 2.6 |
| 8039 | 1526 | 19 | 10 | 0 | 596 | 4.4 | ND |
| 8040 | 1496 | 26 | 19 | 7 | 146 | 24.8 | 24.8 |
| 8041 | 852 | 23 | 6 | 0 | 462 | 3.5 | 3.5 |
| Minimum | 712 | 17 | 0 | 0 | 146 | 0.6 | < 0.5 |
| Mean | 1249 | 27 | 8 | 1.5 | 551 | 5.4 | 6.4 |
| Maximum | 1573 | 45 | 19 | 7 | 1106 | 24.8 | 24.8 |
Patient no. . | Cumulative dose, mg . | No. of samples . | Above LOQ . | Terminal samples . | Dose to reach 1 μg/mL . | Max concentration, μg/mL . | After last dose, μg/mL . |
|---|---|---|---|---|---|---|---|
| 8010 | 1358 | 24 | 2 | 0 | 728 | 1.3 | ND |
| 8015 | 1506 | 28 | 1 | 0 | > 156 | 0.6 | ND |
| 8016 | 1573 | 29 | 3 | 0 | 673 | 1.5 | ND |
| 8017 | 1213 | 20 | 2 | 0 | > 193 | 0.9 | ND |
| 8021 | 983 | 29 | 12 | 3 | 353 | 5.3 | 4.1 |
| 8026 | 1379 | 23 | 3 | 0 | 959 | 1.4 | ND |
| 8027 | 1269 | 43 | 9 | 1 | 639 | 9.0 | 6.8 |
| 8028 | 1363 | 37 | 13 | 3 | 403 | 5.8 | 5.8 |
| 8029 | 1133 | 34 | 9 | 1 | 503 | 7.3 | 7.3 |
| 8030 | 1553 | 45 | 16 | 1 | 473 | 4.3 | 4.2 |
| 8031 | 712 | 27 | 2 | 1 | 712 | 1.4 | 1.4 |
| 8032 | 723 | 17 | 2 | 0 | 393 | 2.5 | 2.5 |
| 8033 | 1302* | 28 | 10 | 0 | 542 | 3.4 | 3.0 |
| 8034 | 1412 | 24 | 16 | 4 | 269 | 6.3 | 5.8 |
| 8035 | 1036 | 24 | 15 | 6 | 406 | 17.1 | 17.1 |
| 8036 | 819* | 21 | 0 | 0 | > 819 | < 0.5 | < 0.5 |
| 8038 | 1486 | 19 | 9 | 3 | 1106 | 2.6 | 2.6 |
| 8039 | 1526 | 19 | 10 | 0 | 596 | 4.4 | ND |
| 8040 | 1496 | 26 | 19 | 7 | 146 | 24.8 | 24.8 |
| 8041 | 852 | 23 | 6 | 0 | 462 | 3.5 | 3.5 |
| Minimum | 712 | 17 | 0 | 0 | 146 | 0.6 | < 0.5 |
| Mean | 1249 | 27 | 8 | 1.5 | 551 | 5.4 | 6.4 |
| Maximum | 1573 | 45 | 19 | 7 | 1106 | 24.8 | 24.8 |
Some doses for patients no. 8033 and no. 8036 were given intravenously due to infusion site reactions during the subcutaneous administration.
Example of alemtuzumab concentrations in a patient treated with subcutaneous antibody. After an initial dose escalation, 30 mg of alemtuzumab was administered 3 times a week for 12 weeks ( ). Serum samples were taken before the dose, once a week (▵). This patient made a good clinical response to the therapy and achieved relatively high concentrations of antibody.
). Serum samples were taken before the dose, once a week (▵). This patient made a good clinical response to the therapy and achieved relatively high concentrations of antibody.
Example of alemtuzumab concentrations in a patient treated with subcutaneous antibody. After an initial dose escalation, 30 mg of alemtuzumab was administered 3 times a week for 12 weeks ( ). Serum samples were taken before the dose, once a week (▵). This patient made a good clinical response to the therapy and achieved relatively high concentrations of antibody.
). Serum samples were taken before the dose, once a week (▵). This patient made a good clinical response to the therapy and achieved relatively high concentrations of antibody.
Inadequate samples were available for analysis of terminal phase pharmacokinetics. No correlation could be demonstrated between alemtuzumab concentrations and clinical responses because nearly all of the patients (18/20) were responders (CR or PR), and levels of MRD were not reported.
Antiglobulins following intravenous treatment
A total of 519 serum samples from 30 patients treated with intravenous alemtuzumab were tested for antiglobulins. These included all samples taken more than 24 hours after the last dose and representative pre-dose samples during treatment. All of them were below the limit of detection (488 U/mL). In 12 patients there were no or very few samples available after the end of treatment, and in the other 18 patients samples were only available between 2 and 6 weeks after the last dose. Therefore, we cannot completely exclude the possibility of delayed responses.
Antiglobulins following subcutaneous treatment
A total of 281 samples were tested from 21 patients (one patient had adequate late samples for antiglobulin measurement, but none suitable for pharmacokinetic analysis). These included all samples taken after the last dose and representative samples taken during treatment. There were 4 patients who had no or few samples after the end of treatment but 17 who had adequate posttreatment samples. All samples except those described below were below the LOD.
One patient (no. 8027) had a single sample taken at day 238 (114 days after the last dose) that gave a weak signal just above the LOD. All previous and subsequent samples were less than the LOD. The patient made a good clinical response to therapy, achieving a CR, and no relevant adverse effects were noted. It is possible that the patient made a very weak, transient response to the alemtuzumab, but we doubt that it could have been clinically significant.
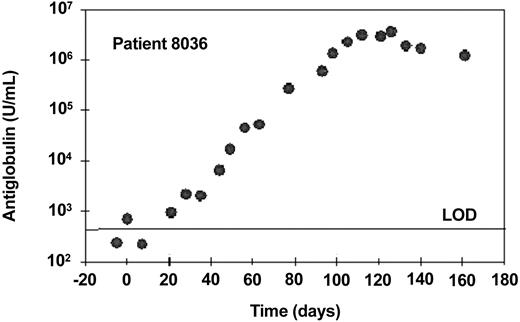
One patient (no. 8036) made a very high-titer response, reaching a peak of approximately 3.7 × 106 U/mL 24 days after the last dose of alemtuzumab (Figure 7). This antiglobulin bound strongly to CAMPATH-1G, the parental rat antibody from which alemtuzumab was humanized, but did not bind other human IgG1 monoclonal antibodies. Therefore, essentially all of the response was directed against the alemtuzumab idiotype. No free alemtuzumab could be detected throughout treatment and there was no significant depletion of tumor cells during therapy. This patient developed a pronounced injection site reaction including erythema, edema, and slight local pain but otherwise no marked general symptoms. As a result, the patient did not achieve the 30-mg dose level during 6 weeks of subcutaneous dosing and alemtuzumab was subsequently administered intravenously. Due to nonresponsiveness, the patient was subsequently treated with fludarabine and went into a long-lasting partial remission. He was retested 18 months after the end of alemtuzumab treatment (continuing in PR) and still had a high-titer antiglobulin (approx 1.8 × 105 U/mL). There was a very low anti-alemtuzumab signal in one of 2 samples taken before therapy. This patient might have had a natural anti-IgG (rheumatoid factor) prior to therapy and perhaps was predisposed to make an antiglobulin response.
Antiglobulin response to alemtuzumab following subcutaneous treatment. This patient received subcutaneous alemtuzumab at doses up to 10 mg until day 37 (cumulative dose, 89 mg) and subsequently received 30 mg 3 times a week intravenously until day 80, followed by 30 mg 3 times a week subcutaneously until day 102. Samples were taken once a week to measure antiglobulin responses. One U/mL is equivalent to 1 ng/mL of a standard monoclonal anti-idiotype. The horizontal line indicates the limit of detection (LOD).
Antiglobulin response to alemtuzumab following subcutaneous treatment. This patient received subcutaneous alemtuzumab at doses up to 10 mg until day 37 (cumulative dose, 89 mg) and subsequently received 30 mg 3 times a week intravenously until day 80, followed by 30 mg 3 times a week subcutaneously until day 102. Samples were taken once a week to measure antiglobulin responses. One U/mL is equivalent to 1 ng/mL of a standard monoclonal anti-idiotype. The horizontal line indicates the limit of detection (LOD).
A search was made for samples from other patients who had received subcutaneous alemtuzumab. Samples were collected between 4 to 50 months after the end of treatment from an additional 9 patients of the original subcutaneous study plus 2 others who received subcutaneous alemtuzumab as part of a compassionate program. A substantial response was detected in patient no. 8020 of approximately 4800 U/mL at 32 months after the end of treatment. Like the response in patient no. 8036, this response cross-reacted on the parental rat antibody but not on other human antibodies, showing it to be essentially anti-idiotype. The patient had not responded to the therapy, but was reported to have prolonged skin reactions to the infusions. A very weak response was detected in one other patient of 541 U/mL, barely above the LOD. Unlike the other 2 patients, this serum cross-reacted with other human IgG but not with CAMPATH-1G. We consider that it is a nonspecific rheumatoid-like factor and probably had no clinical significance with respect to alemtuzumab.
In total therefore, of 32 patients treated with subcutaneous alemtuzumab (30 who were previously untreated with any anti-CLL agent), 2 produced a significant antiglobulin response which persisted at potentially neutralizing concentrations for more than a year. Both of these patients had received alemtuzumab manufactured at the Therapeutic Antibody Centre, as did most of the patients in this study, although patient no. 8036 had been switched to the commercial product after 3 weeks.
Discussion
Alemtuzumab is extensively used for patients with B-CLL who have failed treatment with alkylating agents and purine analogues and investigators are exploring its use in other disease settings. To enable safe and effective use it is essential to understand alemtuzumab's pharmacokinetics and biodistribution as well as the potential for anti-idiotype responses, which all may differ according to disease, patient status, and route of administration. We have started to address these issues by comparing blood concentrations of alemtuzumab and antiglobulin responses in 2 different studies for treatment of CLL, namely intravenous treatment of chemotherapy-resistant disease and first-line subcutaneous treatment. The studies were initiated independently and had different objectives; nevertheless the pharmacologic results are instructive.
Alemtuzumab may cause substantial “first dose” reactions as a result of cytokine release when it is given intravenously.3,18,19 Therefore, the standard treatment regimen involves dose escalation and frequent, relatively small doses, though it has not been formally established whether the reactions are strictly dose related. A treatment course for chemotherapy-resistant CLL consists of approximately one gram of the antibody. However, a cumulative dose of 40 mg to 100 mg is highly immunosuppressive and clinically effective in patients without bulky tumors, for example those treated for prevention of transplant rejection,11,20 multiple sclerosis,21 and other autoimmune diseases,22-24 as well as to eradicate detectable minimal residual disease in CLL.25 The biodistribution of alemtuzumab is likely to depend on the bulk of tumor cells, possibly more so than other monoclonals because CD52 is such a highly expressed antigen.
There are approximately 5 × 105 binding sites for alemtuzumab on a normal lymphocyte.26,27 There are about 1012 lymphocytes in a healthy adult.28 At least 125 mg of alemtuzumab is therefore required to saturate all of the CD52 sites. CLL cells express a similar amount of CD52 antigen to normal lymphocytes27 but their total number may be 10 times greater. Therefore, more than 1 g of alemtuzumab could be required to saturate all the receptors in some patients, especially considering that the antibody has a relatively low affinity and that there will be a significant clearance during a 12-week course of treatment. Hence, it is not surprising that the biodistribution and clearance of antibody during the treatment of patients with CLL is dominated by the tumor burden and cannot be fitted to a simple pharmacokinetic model.
There was a strong correlation between the concentrations of antibody throughout intravenous treatment and the ultimate clinical response, or the achievement of minimal residual disease (MRD) negativity in the bone marrow by flow cytometry. It has recently been reported that survival correlates better with MRD than with the standard NCI criteria for clinical responses.29 Without a good measure for the initial total tumor burden it is difficult to distinguish cause and effect. One possibility is that patients with high tumor burden were treated with inadequate amounts of antibody to achieve sufficient receptor site saturation and see a clinical effect. In any event, it appears that measurements of alemtuzumab concentrations after 2 to 4 weeks of treatment might provide an indicator of the ultimate outcome and guide the physician whether to continue, stop, or adjust the dose of antibody. Most of the antibody was cleared within a few days of the final dose in the nonresponders. However, the half-life at the end of treatment in the responders was comparable with those previously reported for patients without bulky tumors.
Accumulation of alemtuzumab in the blood was significantly slower in the subcutaneous study and it took on average about 6 weeks longer to reach 1.0 μg/mL (an arbitrary threshold known to be potentially lympholytic). This could be a slight overestimate because samples were not always collected every week. Nevertheless, the eventual maximum trough concentrations were very similar in both studies. We cannot tell whether the slower accumulation of antibody in the blood was due to less favorable biodistribution, more effective binding to tumor cells, or the slower dose escalation used in the subcutaneous study compared with that used with intravenously treated patients. It should be possible to resolve this by studies of alemtuzumab given subcutaneously to patients not suffering from malignancies (ie, without excess CD52+ cells).
Because clinical responses were much more frequent (19% CR and 68% PR),8 we could not detect a significant correlation between blood concentrations and clinical responses in the subcutaneous study. With only 2 nonresponders in the group of patients we analyzed, the sample was too small to discern any relationship.
Clear-cut results were obtained from the measurements of antiglobulin responses (antihuman antibody [HAHA]). There were no detectable responses at all in the intravenous study (heavily pretreated patients) and only one patient in the subcutaneous study (previously untreated patients) made a strong response, reaching a peak of approximately 3.7 × 106 U/mL 3 weeks after the final dose. This was equivalent to 3.7 mg/mL of the monoclonal anti-idiotype standard and represented a substantial proportion of the total Ig in the patient. Similar titers were previously reported during treatment of rheumatoid arthritis patients with subcutaneous alemtuzumab13 when all of the patients (10/10) made a positive response. After screening 11 other patients we found one with a significant antiglobulin titer of 4800 U/mL. Considering that this sample was taken 32 months after the last treatment with alemtuzumab, it seems likely that this patient also had made a very strong response.
Previous studies on antiglobulin responses to alemtuzumab have given widely different results. In 12 patients treated for kidney transplant rejection no antiglobulins were detected, in contrast to 15 of 17 patients treated with the parental rat antibody CAMPATH-1G.11 In 167 patients treated for chemotherapy-resistant lymphoid malignancies there were only 3 weak or borderline responses. However, out of 115 patients treated for rheumatoid arthritis, there were 59 positive responses (ILEX Pharmaceuticals, unpublished data on file, July 2003), some of high titer similar to that reported here for patient no. 8036.13 Many factors could affect the immunogenicity of alemtuzumab, including the dose and length of treatment, the underlying disease, prior exposure to chemotherapy, concomitant immunosuppressive drugs, and possibly the route of administration or the formulation of the drug. Subcutaneous administration might allow more effective antigen presentation, for example, by Langerhans cells and with less “excess” antibody circulating to provide tolerogenic signals, the balance might be tipped toward an immune response. From our present study we cannot say whether subcutaneous administration in CLL is more immunogenic than intravenous administration since there were other differences between the 2 groups, most notably the subcutaneous group had received no prior chemotherapy. Even if the 2 groups had been comparable, the difference in responses (0/30 vs 2/32) did not reach statistical significance. This issue needs to be investigated in larger numbers of patients. Both patients who made an antiglobulin response had been treated with alemtuzumab manufactured at the Therapeutic Antibody Centre. We are aware that the manufacture, formulation, or storage of biologics might affect their immunogenicity, but so far we have no evidence to suggest that there is a major difference between the academic or commercial source since antiglobulin responses have been reported in other patients treated with alemtuzumab from either source. The 2 patients (no. 8020 and no. 8036) who produced anti–alemtuzumab antibody on subcutaneous treatment, unlike the other patients, did not show significant reductions in lymphocyte counts but had marked local skin reactions which did not diminish with continued therapy. An antiglobulin response should be considered when there is no drop in the blood lymphocyte count and/or there are persistent injection site reactions.
Alemtuzumab provides a useful therapeutic option for patients with CLL. It has excellent activity as first-line treatment and can give good responses in patients who are unresponsive to conventional chemotherapy. The optimal dose route and regimen are not yet known. However, the subcutaneous route can deliver concentrations of antibody comparable to the original intravenous route. The dominant factor influencing biodistribution and pharmacokinetics appears to be the tumor burden. With intravenous dosing, serum concentrations of alemtuzumab can provide an early prediction of the ultimate response and might be useful to guide dose adjustments to improve responses or reduce side effects. Strong anti-idiotype responses may occur in a small minority of patients. Although not associated with serious adverse effects, such responses neutralize the therapeutic benefit and therefore patients with a known antiglobulin response should not be further treated with this monoclonal antibody.
Prepublished online as Blood First Edition Paper, April 15, 2004; DOI 10.1182/blood-2004-02-0593.
Supported by The EP Abrahams Trust, Oxford, United Kingdom; Millennium Pharmaceuticals Inc, Cambridge, MA; Yorkshire Cancer Research, Yorkshire, United Kingdom; Schering AG, Berlin, Germany; and the Swedish Cancer Society and the Cancer Society in Stockholm, Sweden.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are very grateful to the staff of the Therapeutic Antibody Centre, Oxford, United Kingdom, for the manufacture and supply of some of the alemtuzumab used in this study. We thank Pete Bonate, Ian Clements, and the team at ILEX Oncology for constructive comments and for sharing unpublished data.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal