Abstract
Although generalized T-cell activation is an important factor in chronic HIV disease pathogenesis, its role in primary infection remains poorly defined. To investigate the effect of immune activation on T-cell changes in subjects with early HIV infection, and to test the hypothesis that an immunologic activation “set point” is established early in the natural history of HIV disease, a prospective cohort of acutely infected adults was performed. The median density of CD38 molecules on CD4+ and CD8+ T cells was measured longitudinally in 68 antiretroviral-untreated individuals and 83 antiretroviral-treated individuals. At study entry, T-cell activation was positively associated with viremia, with CD8+ T-cell activation levels increasing exponentially at plasma HIV RNA levels more than 10 000 copies/mL. Among untreated patients, the level of CD8+ T-cell activation varied widely among individuals but often remained stable within a given individual. CD8+ T-cell activation and plasma HIV RNA levels over time were independently associated with the rate of CD4+ T-cell loss in untreated individuals. These data indicate that immunologic activation set point is established early in HIV infection, and that this set point determines the rate at which CD4+ T cells are lost over time.
Introduction
Untreated HIV-1 infection is associated with a gradual loss of peripheral CD4+ T cells. Although the direct cytopathic effect of HIV-1 on CD4+ T cells almost certainly contributes to this gradual depletion,1 most cells destined to die in vivo as a consequence of HIV infection are not productively infected with HIV.2 This observation has led to the hypothesis that progressive CD4+ T-cell depletion occurs due to indirect effects of viral replication.3-6 The mechanism for these indirect effects of HIV replication on CD4+ T-cell depletion is not understood.
One widely accepted model postulates that HIV causes accelerated proliferation, expansion, and death of T cells, and that this heightened T-cell turnover eventually results in depletion or exhaustion of the regenerative capacity of the immune system.4,5 Multiple studies have shown that HIV infection results in a state of high T-cell turnover (ie, the rates of T-cell proliferation and death are increased). For example, in vivo labeling of T cells indicates that HIV infection results in increased numbers of rapidly cycling CD4+ and CD8+ T cells.7,8 These cells are primarily of memory-effector phenotype, and are destined to proliferate and die rapidly.9 The rate at which HIV recruits cells into this rapid turnover state is directly proportional to the level of viremia,8 which in turn is directly related to the rate at which CD4+ T cells are lost.10 In the absence of antiretroviral treatment, markers of T-cell activation and T-cell turnover predict the rate of disease progression11-14 and the rate of CD4+ T-cell loss.15 When antiretroviral therapy is initiated, the rate of T-cell turnover and the degree of generalized T-cell activation both decrease, suggesting that viral replication directly contributes to heightened levels of T-cell activation.8,16-19 Collectively, these observations support the hypothesis that HIV causes disease progression as a consequence of generalized T-cell activation, and that continuous high turnover of T cells—coupled to a suppressed ability of the immune system to regenerate new progenitor T cells—eventually results in gradual loss of peripheral CD4+ T cells.4,5
Several immunophenotypic and serum markers have been used to quantify the level of T-cell activation in vivo, including CD38, HLA-DR, CD25, CD69, CD70, neopterin, tumor necrosis factor receptor type II, and β2-microglobulin.14,20-22 Of these, the best characterized marker of immune activation has been CD38 expression on T cells; at least one study has shown that CD38 expression on CD8+ T cells had stronger prognostic significance than other commonly used markers of activation.13 CD38 is a multifunctional transmembrane glycoprotein that is up-regulated during the earliest stages of T-cell activation.23,24 Physiologically, CD38 expression and/or ligation has been associated with increased cell-to-cell adhesion,25 increased levels of cytokine production,26 and more rapid CD4+ T-cell proliferation.27 In addition, CD38 expression on T cells is strongly correlated with other markers of cellular activation.22,28 The prognostic significance of CD38 appears to be greater when measured based on the mean density per cell rather than the proportion of cells expressing CD38,29 although this has not been fully addressed in prospective studies.18
Despite a large number of studies focusing on the relationship between T-cell activation and outcome, only a few have elucidated this relationship over time during primary and early infection. This is surprising since the immunologic and virologic events that occur during the earliest stages of infection can have a strong impact on subsequent disease progression.30-32 We therefore assessed the effects of T-cell activation and plasma HIV RNA levels on CD4+ T-cell changes over time in a prospective cohort of acutely and recently HIV-infected adults. Our primary objective was to determine the relative contributions of viremia and T-cell activation to the rate of CD4+ T-cell loss. Our secondary objective was to describe the natural history of activation during untreated HIV infection, focusing on the unresolved question of whether activation reaches a steady state or continually increases over time.
Patients, materials, and methods
This is a prospective study of HIV-infected patients enrolled in the University of California San Francisco (UCSF) Options project, part of the National Institutes of Health–funded Acute Infection Early Disease Research Program network. Subjects for this cohort were recruited through a variety of techniques, including community physician referral, outreach efforts to HIV testing and counseling sites, AIDS information hotlines, hospital emergency departments, and community-based organizations.33 Enrolled subjects must have had evidence of acute or recent HIV infection as defined by (1) negative or weakly reactive HIV antibody, enzyme immunoassay, and Western blot with detectable plasma HIV-1 RNA levels; (2) documentation of a negative HIV antibody test result within the previous 12 months and a positive antibody test at screen; or (3) a history compatible with recent HIV infection, with laboratory confirmation of recent antibody seroconversion using a less sensitive enzyme immunoassay (LS-EIA) testing strategy.34
All subjects were offered 3-drug combination therapy immediately upon entry. However, the decision to initiate antiretroviral therapy was voluntary, and those who chose not to start therapy were followed in a manner identical to those who chose to start therapy. Both treated and untreated patients underwent a baseline visit, at which time a variety of virologic and immunologic studies were performed. Beginning in January 1998, all subjects enrolled in the Options project had measurements of T-cell activation performed at study baseline and at weeks 4, 8, 12, 24, 48, 72, and 96.
Subjects were included in this analysis if they had baseline immunophenotyping data available and remained in the study for at least 12 weeks. This study received approval of the UCSF Committee on Human Research, and all subjects gave written informed consent.
Immunologic and virologic measurements
Immunophenotyping was performed using freshly collected heparin-treated peripheral whole blood. Activated T cells were identified using fluorescein isothiocyanate (FITC)–conjugated anti-CD8, phycoerythrin (PE)–conjugated anti-CD38, and peridinin chlorophyll protein (PerCP) anti-CD4 (Becton Dickinson, San Jose, CA). Negative control tubes were stained with isotype control antibodies immunoglobulin G (IgG)–FITC and IgG-PE (Dako, Carpenteria, CA) and IgG-PerCP isotype control (Becton Dickinson). Evaluation of fluorescent antibody staining was evaluated by a FACSCAN flow cytometer driven by CELLQUEST software (Becton Dickinson), with 10 000 ungated events recorded for each acquisition. T-cell analysis was performed on a gated population of lymphocytes as defined from forward versus side scatter 2-dimensional dot plots for each sample. FLOW acquisition parameters for voltage gain and percent compensation were determined such that more than 99% of lymphocytes stained with isotype control antibodies exhibited fluorescence values of less than 10; these parameter settings remained fixed at a standard setting for all samples. The CD38 data were reported as the median relative fluorescence intensity on CD4+ and CD8+ T cells. This approach is similar to the Quantibrite approach35 ; however, we did not convert the relative fluorescence units to number of CD38 molecules per cell.
Plasma HIV RNA levels were tested by the bDNA technology (Bayer Diagnostics, Emeryville, CA). Prior to March 1998, version 2.0 was used (lower limit of quantification 500). All plasma HIV RNA levels were transformed to 3.0 equivalents (3.0 equivalents = 2.0 results/0.57), as previously described.36 All plasma HIV RNA levels less than 500 with Bayer 2.0 were imputed to be 250 copies RNA/mL. All plasma HIV RNA levels less than 50 with Bayer 3.0 were imputed to be 25 copies/mL.
Clinical symptoms
Acute retroviral symptoms were assessed in all subjects at study entry using a standardized interviewer-administered questionnaire, as previously described.33 The following 21 symptoms were assessed: fever, rash, oral ulcers, arthralgias, pharyngitis, loss of appetite, weight loss, malaise, myalgias, fatigue, nausea, vomiting, diarrhea, headaches, photophobia, stiff neck, night sweats, confusion, infected gums, genital ulcers, and anal ulcers. Start and stop dates were assessed for each symptom, and only symptoms lasting for fewer than 4 months were considered.
Statistical analysis
Both a cross-sectional analysis and a longitudinal analysis were performed. For the cross-sectional analysis, we included all individuals who enrolled in the cohort, regardless of whether they began antiretroviral therapy at their baseline visit (most individuals who chose to start therapy did so at the earliest time point). Baseline characteristics were compared using Spearman rank correlations. We also compared characteristics in the untreated versus the treated cohorts using nonparametric techniques. The longitudinal analysis focused on those individuals who chose not to begin antiretroviral therapy immediately after study entry. However, these untreated individuals may have subsequently initiated therapy. Their data were included in the longitudinal analyses but censored at the time treatment was begun.
Kaplan-Meier techniques were used to determine the effect of baseline characteristics on immunologic progression during antiretroviral-untreated HIV infection. Since most clinical guidelines now recommend considering initiation of antiretroviral therapy at a CD4+ T-cell count of less than 350 cells/mm3, we used as an end point the time at which individuals reached this level.37,38 Quartiles were formed for baseline CD4+ T-cell count, CD4+ T-cell activation, CD8+ T-cell activation, and plasma HIV RNA levels. Differences among the groups were assessed by the log-rank statistic. Observations were censored when patients became lost to follow-up or when they initiated a treatment regimen. Cox proportional hazards regression was used to determine the association between the following factors and time to reaching a CD4+ T-cell count less than 350 cells/mm3: (1) plasma HIV RNA quartile at baseline, (2) CD4+ T-cell activation quartile at baseline, (3) CD8+ T-cell activation quartile at baseline, and (4) CD4+ T-cell count quartile at baseline.
We also used mixed-effects modeling to determine the association of T-cell activation and viremia on CD4+ T-cell count changes over time. This approach models the impact of one or more continuous predictor variables measured longitudinally on a continuous outcome variable also measured longitudinally. This technique allows each measurement to follow a path that differs randomly from the mean pattern and accounts for within-subject correlations.
The primary outcome variable for the mixed-effects analysis was CD4+ T-cell counts, and the primary predictors were plasma HIV RNA levels, CD4+ T-cell activation, and CD8+ T-cell activation. Parallel plots indicated a skewed distribution of CD4+ cell counts, CD8+ T-cell activation, CD4+ T-cell activation, and plasma HIV RNA levels. A log transformation was therefore used to normalize these values. All analyses were performed using SAS version 8 (SAS Institute, Cary, NC).
Results
Baseline characteristics
A total of 151 acutely or recently infected adults entered our cohort at the time longitudinal assessments of immune activation were being measured. Most subjects were enrolled within the first 6 months of the estimated time that HIV infection was acquired (Table 1). The median plasma HIV RNA level at baseline was 4.6 log10 copies RNA/mL (interquartile range, 3.9 to 5.2), and the median CD4+ T-cell count was 512 cells/mm3 (interquartile range, 405 to 651). Most subjects were white men who had acquired HIV through homosexual contacts.
Baseline characteristics
Characteristics . | Treated, n = 83 . | Untreated, n = 68 . | P . |
|---|---|---|---|
| Age, y (IQR) | 35 (30-40) | 34 (30-41) | .97 |
| Sex, % | 94 | 96 | .73 |
| Infection stage, % | .001 | ||
| Less than 30 d | 24.1 | 2.9 | |
| 1 to 6 mo | 71.1 | 88.2 | |
| 6 to 12 mo | 4.8 | 8.8 | |
| Presence of one or more drug-resistance associated mutations, % | 17.4 | 17.8 | 1.0 |
| HIV risk factors, % | |||
| MSM | 94.0 | 100 | .07 |
| Intravenous drug user | 4.4 | 2.4 | 1.0 |
| CD4+ T-cell count, cells/mm3 (IQR) | 484 (374-646) | 539 (451-717) | .03 |
| CD8+ T-cell count, cells/mm3 (IQR) | 798 (572-1071) | 817 (632-1086) | .68 |
| Plasma HIV RNA level, log10 copies RNA/mL | 5.10 (4.18-5.63) | 4.26 (3.36-4.70) | < .0001 |
| Baseline CD4+ CD38, relative fluorescence units | 33.2 (23.3-46.4) | 19.7 (8.2-41.4) | .004 |
| Baseline CD8+ CD38, relative fluorescence units | 99.6 (39.6-256) | 33.4 (9.8-67.6) | < .0001 |
Characteristics . | Treated, n = 83 . | Untreated, n = 68 . | P . |
|---|---|---|---|
| Age, y (IQR) | 35 (30-40) | 34 (30-41) | .97 |
| Sex, % | 94 | 96 | .73 |
| Infection stage, % | .001 | ||
| Less than 30 d | 24.1 | 2.9 | |
| 1 to 6 mo | 71.1 | 88.2 | |
| 6 to 12 mo | 4.8 | 8.8 | |
| Presence of one or more drug-resistance associated mutations, % | 17.4 | 17.8 | 1.0 |
| HIV risk factors, % | |||
| MSM | 94.0 | 100 | .07 |
| Intravenous drug user | 4.4 | 2.4 | 1.0 |
| CD4+ T-cell count, cells/mm3 (IQR) | 484 (374-646) | 539 (451-717) | .03 |
| CD8+ T-cell count, cells/mm3 (IQR) | 798 (572-1071) | 817 (632-1086) | .68 |
| Plasma HIV RNA level, log10 copies RNA/mL | 5.10 (4.18-5.63) | 4.26 (3.36-4.70) | < .0001 |
| Baseline CD4+ CD38, relative fluorescence units | 33.2 (23.3-46.4) | 19.7 (8.2-41.4) | .004 |
| Baseline CD8+ CD38, relative fluorescence units | 99.6 (39.6-256) | 33.4 (9.8-67.6) | < .0001 |
Median and interquartile ranges (IQRs) are shown. MSM indicates men who have sex with men.
Of the 151 enrolled subjects, 68 (45%) subjects chose not to receive antiretroviral therapy for their acute infection (the remaining subjects initiated therapy at or soon after their baseline visit). There were significant differences between the patients who chose to initiate therapy and the patients who chose to remain untreated (Table 1). Patients who chose to initiate therapy had higher levels of plasma HIV RNA (P < .0001), lower CD4+ T-cell counts (P = .006), and higher CD4+ and CD8+ T-cell activation levels (P = .005 and P < .0001, respectively). Patients who started therapy were more likely to have been diagnosed prior to seroconversion. There were no clear trends in sex or ethnicity between those who chose treatment and those who did not choose treatment.
Association between symptoms of acute HIV infection and T-cell activation
The median number of symptoms at study entry was 9 (interquartile range, 3 to 11), with fatigue (71%), fever (67%), malaise (59%), and night sweats (58%) being most common. Subjects who initiated therapy had a greater number of symptoms than those who chose not to initiate therapy (median, 10 vs 7; P < .01). In bivariate analyses, the total number of symptoms correlated with viral load, CD8+ T-cell count, CD4+ T-cell activation levels, and CD8+ T-cell activation levels, but not the CD4+ T-cell count. In a multiple linear regression using log10 transformation for all markers, only CD8+ T-cell activation was independently associated with the number of symptoms (P = .006).
Correlation between viremia, T-cell activation, and CD4+ T-cell counts at study baseline
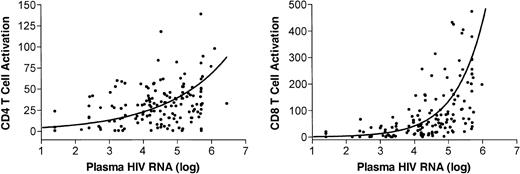
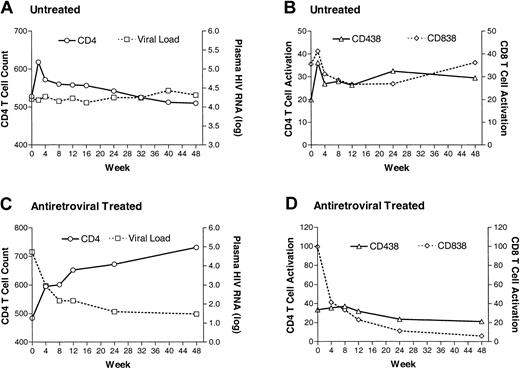
The relationship between viremia, T-cell activation, and CD4+ T-cell counts at study entry was examined using data from all 151 subjects. As expected, there was a negative correlation between plasma HIV RNA levels and CD4+ T-cell counts at study baseline (Spearman rho =–0.39, P < .0001, n = 151). There was also a positive correlation between plasma HIV RNA levels and both CD4+ T-cell activation and CD8+ T-cell activation levels (rho = 0.32, P < .0001 and rho = 0.69, P < .0001, respectively). Finally, there was a negative correlation between CD4+ T-cell counts and the levels of CD4+ and CD8+ T-cell activation (rho =–0.17, P = .001 and rho =–0.32, P < .0001, respectively). Notably, CD8+ T-cell activation levels appeared to remain low as long as the plasma HIV RNA level remained below 10 000 copies RNA/mL; at plasma HIV RNA levels above this threshold the level of CD8+ T-cell activation increased dramatically (Figure 1). This association between viremia and CD8+ T-cell activation persisted among untreated patients after resolution of primary infection symptoms (data not shown). T-cell activation declined rapidly after the introduction of highly active antiretroviral therapy (HAART), and remained low during subsequent observation (Figure 2).
The relationship between T-cell activation and plasma HIV RNA levels (log10transformed) in 153 individuals recently diagnosed with HIV infection. A smooth line was generated by linear regression with quadratic equations.
The relationship between T-cell activation and plasma HIV RNA levels (log10transformed) in 153 individuals recently diagnosed with HIV infection. A smooth line was generated by linear regression with quadratic equations.
The median change in plasma HIV RNA levels, CD4+T-cell count levels, and T-cell activation levels in 68 recently infected individuals who did not receive antiretroviral therapy and 83 recently infected individuals who did receive antiretroviral therapy.
The median change in plasma HIV RNA levels, CD4+T-cell count levels, and T-cell activation levels in 68 recently infected individuals who did not receive antiretroviral therapy and 83 recently infected individuals who did receive antiretroviral therapy.
Longitudinal changes in viremia and activation markers among untreated patients
The change in plasma HIV RNA levels, CD4+ T-cell counts, and activation markers during untreated HIV infection are shown in Figures 2 and 3. The level of viremia increased gradually over time (mean increase of +0.02 log10 copies RNA/mL per month; 95% CI = 0.01 to 0.03; P < .001), while the CD4+ T-cell count decreased (mean decrease of –8 CD4+ T cells/mm3 per month; 95% CI = –11 to –6; P < .001). CD4+ T-cell activation also increased over time (relative increase of 1.3% per month, P < .001). Although the level of CD8+ T-cell activation increased gradually, this change was not significantly different from zero (relative increase of 0.4% per month; P = .12).
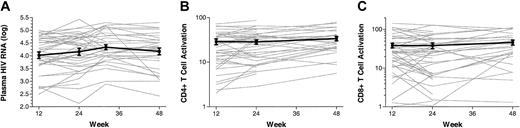
Longitudinal changes in plasma HIV RNA levels, CD4+T-cell activation levels, and CD8+T-cell activation in antiretroviral-untreated recently infected adults. Data are shown on all individuals who had a week-12 value and at least 24 weeks of observation (n = 41). The mean and standard error of the mean are shown in bold. Both plasma HIV RNA levels and T-cell activation levels tended to be variable during the first 12 weeks of observation (data not shown), followed by the emergence of a steady-state level.
Longitudinal changes in plasma HIV RNA levels, CD4+T-cell activation levels, and CD8+T-cell activation in antiretroviral-untreated recently infected adults. Data are shown on all individuals who had a week-12 value and at least 24 weeks of observation (n = 41). The mean and standard error of the mean are shown in bold. Both plasma HIV RNA levels and T-cell activation levels tended to be variable during the first 12 weeks of observation (data not shown), followed by the emergence of a steady-state level.
The individual trajectories demonstrated substantial variability in activation levels among a subset of patients during the first 8 to 12 weeks of observation (data not shown); however, after week 12 most subjects appeared to achieve a relatively steady-state level of viremia, CD4+ T-cell, and CD8+ T-cell activation (Figures 2-3). This level varied substantially between patients but remained relatively stable within patients.
Effect of viremia and T-cell activation on CD4+ T-cell decline
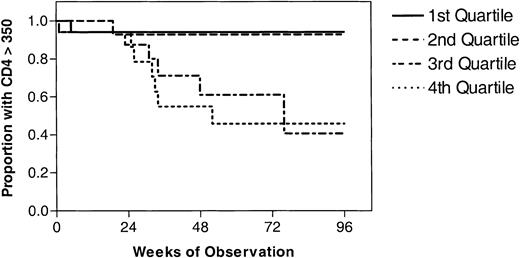
The effect of T-cell activation and viremia on time to immunologic progression was determined using Kaplan-Meier techniques. Since most clinical guidelines now recommend considering initiation of antiretroviral therapy at a CD4+ T-cell count of less than 350 cells/mm3, we used as an end point the time at which individuals reached this level.37,38 Of the 68 individuals who did not receive therapy, 15 eventually experienced a decrease in CD4+ T-cell counts to 350 cells/mm3 or less. Individuals with higher baseline quartiles of CD8+ T-cell activation were more likely to have this CD4+ T-cell count decrease (P = .002 by the log-rank test; Figure 4). Similar trends were observed for plasma HIV RNA levels (P = .004) but not CD4+ T-cell activation (P = .21; Figure 4). In univariate analyses, both baseline quartiles of CD8+ T-cell activation (hazard ratio, 2.12; P = .006) and baseline quartiles of log viral load (hazard ratio, 2.31; P = .003) were significant in predicting time to CD4+ T-cell count less than 350 (Table 2). In a multivariate Cox proportional hazards model, no factor was significant with P values less than .05.
The time to a CD4+T-cell count less than 350 cells/mm3is shown for 68 recently HIV-infected adults who did not receive antiretroviral therapy immediately after study entry. The cohort was stratified into quartiles based on the level of viremia, CD4+ T-cell activation, and CD8+ T-cell activation at baseline. Individuals with higher baseline quartiles of CD8+ T-cell activation (P = .002 by the log-rank test) and viremia (P = .004) but not CD4+ T-cell activation (P = .21) were more likely to experience subsequent CD4+ T-cell decline.
The time to a CD4+T-cell count less than 350 cells/mm3is shown for 68 recently HIV-infected adults who did not receive antiretroviral therapy immediately after study entry. The cohort was stratified into quartiles based on the level of viremia, CD4+ T-cell activation, and CD8+ T-cell activation at baseline. Individuals with higher baseline quartiles of CD8+ T-cell activation (P = .002 by the log-rank test) and viremia (P = .004) but not CD4+ T-cell activation (P = .21) were more likely to experience subsequent CD4+ T-cell decline.
Factors associated with immunologic progression in untreated HIV infection
Parameter . | Hazard ratio . | 95% confidence interval . | P . |
|---|---|---|---|
| Baseline CD8+ T-cell activation quartile | 2.12 | 1.25-3.73 | .006 |
| Baseline CD4+ T-cell activation quartile | 1.47 | 0.91-2.38 | .115 |
| Baseline log10 plasma HIV RNA quartile | 2.31 | 1.34-4.00 | .003 |
| Baseline CD4+ T-cell count quartile | 0.43 | 0.22-0.84 | .02 |
Parameter . | Hazard ratio . | 95% confidence interval . | P . |
|---|---|---|---|
| Baseline CD8+ T-cell activation quartile | 2.12 | 1.25-3.73 | .006 |
| Baseline CD4+ T-cell activation quartile | 1.47 | 0.91-2.38 | .115 |
| Baseline log10 plasma HIV RNA quartile | 2.31 | 1.34-4.00 | .003 |
| Baseline CD4+ T-cell count quartile | 0.43 | 0.22-0.84 | .02 |
Univariate proportional hazards regression analysis of time to reaching a CD4+ T-cell count of 350 cells/mm3 during untreated HIV infection.
To assess the role of activation and viral load in CD4 decline over time, a mixed-effects analysis was performed with the log CD4+ T-cell changes over time as the primary outcome variable. All available viral load and T-cell activation measurements were considered in the final model. We allowed the predictor variables—viral load and activation markers—to be time-varying covariates. As expected, plasma HIV RNA levels, CD8 T-cell activation, and, to a lesser degree, CD4+ T-cell activation were all predictive of CD4+ T-cell counts in univariate analyses (Table 3). In a multivariate mixed-effects analysis including all 3 of these variables, both plasma HIV RNA levels and CD8+ T-cell activation remained predictive (Table 3).
Mixed effects regression assessing the predictors of CD4+ T-cell count over time
Parameter . | Estimate . | Standard error . | P . |
|---|---|---|---|
| Univariate model | |||
| Plasma HIV RNA level, log10 | -0.032 | 0.007 | < .001 |
| CD8+ T-cell activation, log10 | -0.049 | 0.014 | < .001 |
| CD4+ T-cell activation, log10 | -0.039 | 0.017 | .021 |
| Multivariate model | |||
| Intercept | 2.921 | 0.042 | < .001 |
| Plasma HIV RNA level, log10 | -0.026 | 0.009 | .005 |
| CD8+ T-cell activation, log10 | -0.033 | 0.015 | .027 |
| CD4+ T-cell activation, log10 | -0.013 | 0.019 | .474 |
Parameter . | Estimate . | Standard error . | P . |
|---|---|---|---|
| Univariate model | |||
| Plasma HIV RNA level, log10 | -0.032 | 0.007 | < .001 |
| CD8+ T-cell activation, log10 | -0.049 | 0.014 | < .001 |
| CD4+ T-cell activation, log10 | -0.039 | 0.017 | .021 |
| Multivariate model | |||
| Intercept | 2.921 | 0.042 | < .001 |
| Plasma HIV RNA level, log10 | -0.026 | 0.009 | .005 |
| CD8+ T-cell activation, log10 | -0.033 | 0.015 | .027 |
| CD4+ T-cell activation, log10 | -0.013 | 0.019 | .474 |
Discussion
Although the role of immune activation in HIV disease pathogenesis has been extensively evaluated in chronically infected patients, its role in primary infection remains poorly defined. Using the density of CD38 expression on T cells as a measure of T-cell activation, we assessed the role of immune activation on disease outcome in acutely and recently infected adults, and have made a number of observations. First, there is a strong and consistent relationship between the level of viremia and the level of both CD4+ and CD8+ T-cell activation during acute HIV infection. Second, most untreated patients reach a steady-state level of T-cell activation in early HIV infection. This immunologic activation “set point” varies widely between individuals but is generally stable within a given individual. Third, the CD8+ T-cell activation set point during untreated HIV infection is a strong independent predictor of the rate of CD4+ T-cell decline. Fourth, initiation of antiretroviral therapy during early HIV infection dramatically reduces the level of CD8+ T-cell activation (and to a lesser degree CD4+ T-cell activation). Collectively, these data support the concept that the pathogenic potential of HIV in a given individual is determined both by the level of viral replication and by the ability of a given virus in a given host to cause sustained increases in CD8+ T-cell activation. This dynamic relationship between the virus and the infected individual appears to occur early in the natural course of HIV infection.
Most studies suggest that early infection is marked by the establishment of a relative steady-state level of viremia (the viral load set point).10,32,39 Our data support this concept as untreated patients exhibited only a small increase in viremia during longitudinal observation. Importantly, our data also suggest that an immunologic activation set point is established during early infection. This immunologic activation set point was negatively correlated with the viral load set point, suggesting that either the level of viremia in part determined the T-cell activation set point or that the level of T-cell activation determined the viral load set point (the former mechanism is supported by the observation that antiretroviral treatment resulted in rapid decreases in the level of CD8+ T-cell activation and proliferation/turnover).7,8,16,18,40
The strong association between T-cell activation and CD4+ T-cell decline among patients with antiretroviral-untreated HIV infection suggests that the in vivo pathogenicity of HIV (as defined by immunologic progression) in an individual host is determined in part by the ability of that virus to cause heightened immune activation. Similar conclusions can be drawn from a number of other studies. For example, simian immunodeficiency virus (SIV) infection of the sooty-mangabey and the macaque are associated with high-level viral replication and rapid killing of infected CD4+ T cells. However, whereas SIV-infected macaques—like HIV-infected humans—exhibit high-level immune activation and progressive immunodeficiency, SIV-infected sooty mangabeys exhibit no consistent increase in immune activation and rarely exhibit evidence of progressive immunodeficiency.41-43 Also, constitutive expression of CD70 (which binds the T-cell costimulatory molecule CD27) in transgenic mice results in chronic T-cell activation, progressive loss of memory and naive T cells, and the eventual development of an AIDS-like syndrome (even in the absence of a cytopathic virus). Finally, our group has recently assessed the role of monocyte/macrophage activation in recently infected individuals initiating highly active antiretroviral therapy. Heightened peripheral blood monocyte activation was strongly associated with low CD4+ T-cell counts before and during antiretroviral treatment.44 These observations, plus other observations made in patients infected with either HIV-2 or drug-resistant HIV-1,36,45-47 clearly point to immune activation as a critical step in the HIV-mediated immunopathogenesis.
There is growing uncertainty regarding the management of acute infection.37,38 Arguments for early and aggressive initiation of antiretroviral therapy include preservation of HIV-specific CD4+ T cells, prevention of viral evolution, progressive seeding of the latent reservoir, and, from a public health perspective, reduction of HIV transmission.48-50 Arguments against early initiation of antiretroviral therapy include costs, the potential for pill fatigue, and the potential for both short-term and long-term toxicity. Although our data do not bear directly on whether acute infection should be treated, they do indicate that measures of both the level of viremia and the level of T-cell activation provide important prognostic information that could guide therapy. For example, since patients with high levels of T-cell activation at presentation are more at risk for rapid CD4+ T-cell loss in the absence of therapy, such patients may be more likely to benefit from early treatment.
Prepublished online as Blood First Edition Paper, April 29, 2004; DOI 10.1182/blood-2003-09-3333.
Supported by the National Institute of Allergy and Infectious Diseases (AI41531 and AI052745), the Centers for Disease Control and Prevention (UC64/CCU91394), and the University of California San Francisco Center for AIDS Research (NIH P30 MH59037).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Plasma HIV RNA levels were performed by Bayer Diagnostics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal