Abstract
Fanconi anemia (FA) is characterized by bone marrow (BM) failure and cancer susceptibility. Identification of the cDNAs of many FA complementation types allows the potential of using gene transfer technology to introduce functional cDNAs as transgenes into autologous stem cells and provide a cure for the BM failure in FA patients. Previous studies in FA murine models and in a phase 1 clinical trial suggest that myelopreparation is required for significant engraftment of exogenous, genetically corrected stem cells. Since myeloid progenitors from Fancc-/- mice and human Fanconi anemia group C protein (FANCC) patients have increased apoptosis in response to interferon γ (IFN-γ) in vitro, we hypothesized that IFN-γ may be useful as a nongenotoxic, myelopreparative conditioning agent. To test this hypothesis, IFN-γ was administered as a continuous infusion to Fancc-/- and wild-type (WT) mice for 1 week. Primitive and mature myeloid lineages were preferentially reduced in IFN-γ-treated Fancc-/- mice. Further, IFN-γ conditioning of Fancc-/- recipients was sufficient as a myelopreparative regimen to allow consistent engraftment of isogenic WT repopulating stem cells. Collectively, these data demonstrate that Fancc-/- hematopoietic cell populations have increased hypersensitivity to IFN-γ in vivo and that IFN-γ conditioning may be useful as a nongenotoxic strategy for myelopreparation in this disorder. (Blood. 2004;104:1204-1209)
Introduction
Fanconi anemia (FA) is a complex heterogeneous genetic disorder characterized by bone marrow (BM) failure and a 700- to 800-fold increased risk for the acquisition of myeloid malignancies and solid tumors.1,2 The progressive BM failure is a hallmark of FA and a leading cause (80%) of patient death.3-7 Currently, the only cure for the hematopoietic manifestations of FA is an HLA-compatible hematopoietic stem cell (HSC) transplantation,8 a therapy available to only one third of patients.9 Eleven complementation types, inferring the existence of 11 genes, have been identified. Eight of the 11 FA cDNAs (A, C, D2, E, F, G, L, and Brca2)10-12 have been sequenced, raising the potential of using gene transfer technology to express the functional cDNA in autologous stem cells. The BM hypoplasia commonly observed in FA patients and the reduced repopulating ability of murine FA stem cells13 would be predicted to allow an engraftment advantage of autologous genetically corrected stem cells. However, no long-term proliferation of autologous genetically corrected cells was observed in the peripheral blood of Fanconi anemia group C protein (FANCC) patients in a phase 1 clinical trial that did not use myelopreparative conditioning.9,14,15 Furthermore, in experimental Fancc knockout (Fancc-/-) murine models, neither wild-type (WT) nor genetically corrected Fancc-/- stem cells engraft in the absence of myelopreparation.16,17
FA cells exhibit increased chromosomal breaks in vitro in response to DNA-damaging treatments such as alkylating agents and ionizing radiation.6,18 Consequently, there is at least a theoretical potential of FA patients having an increase in secondary malignancies following myelopreparation with genotoxic agents. Although this risk is difficult to study formally in a clinical setting, especially in a rare disease with a high intrinsic risk of malignancy, some,19,20 but not all, clinical studies1 support the hypothesis that genotoxins may contribute to an increased risk for the acquisition of carcinomas following transplantation in FA patients. For these reasons, a nongenotoxic myelopreparative conditioning regimen would be desirable in selecting genetically corrected HSCs in vivo during gene therapy.
Interferon-gamma (IFN-γ) is an immunoregulatory lymphokine that is known to exert antiproliferative effects on neoplastic cells.21-25 IFN-γ has been used clinically to treat a number of malignancies (reviewed in Liles23 and Borden24 ) and has a high therapeutic index.25 Previously, in vitro studies in 2 murine models of Fancc,26,27 as well as cells from FA type C patients,28 established that cells lacking functional Fancc were hypersensitive to IFN-γ. Treatment with IFN-γ resulted in an increased apoptosis in Fancc-/- cells mediated by the hyperactivation of the double-stranded RNA protein kinase (PKR).18,29 Based on the hypersensitive phenotype of Fancc-/- cells to IFN-γ, we hypothesized that IFN-γ might be used as a nongenotoxic myelopreparative conditioning agent in Fancc-/- mice. Kurre et al30 recently reported that in vivo administration of IFN-γ by intraperitoneal or serial bolus infusion did not result in marrow aplasia in a similar line of Fancc-/- mice. However, since the half-life of IFN-γ in another rodent model (rat) is only 1.1 minutes with 90% drug elimination by 8 minutes after injection,31 we hypothesized that a continuous infusion of IFN-γ treatment may have a therapeutic impact. Therefore, we used micro-osmotic pumps as an alternative strategy to deliver a continuous administration of IFN-γ to WT and Fancc-/- mice. Here we show that continuous infusion of IFN-γ resulted in a preferential, dose-dependent, and reversible reduction in peripheral white blood cells as well as mature and primitive hematopoietic progenitor populations in Fancc-/- mice. Furthermore, treatment of Fancc-/- recipients with IFN-γ alone was sufficient to allow high-level engraftment of exogenous isogeneic WT HSCs.
Materials and methods
Mice
Fancc+/- mice (C57Bl/6 × SV129) were backcrossed into a C57Bl/6J strain (CD45.2+) for 10 generations and then bred to generate Fancc-/- and WT mice.32 Congenic C57Bl/6J (CD45.2+) and B6.SJL-PtrcaPep3b/BoyJ (B6.BoyJ) mice (CD45.1+) were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained in our animal facility.13,33
Treatment and monitoring
All experimental mice were housed in the Indiana University School of Medicine Animal Care Facility and examined regularly by one of the investigators. Murine IFN-γ was purchased from Peprotech (Rocky Hill, NJ), reconstituted as recommended by the manufacturer, and placed within micro-osmotic pumps to administer a defined volume (0.5 μL/h for 7 days; DURECT, Cupertino, CA). Micropumps containing either IFN-γ or phosphate-buffered saline (PBS) were implanted subcutaneously into the backs of WT and Fancc-/- mice and removed following a 7-day infusion. IFN-γ was administered at a dosage of 0, 120, or 400 μg/kg/day. These studies were reviewed and approved by the Indiana University School of Medicine Animal Advisory Committee.
Complete peripheral blood counts were obtained using an automated cell counter (Sysmex, Kobe, Japan) 7 days after treatment. The accuracy of abnormal blood counts was verified by direct examination of blood smears. Renal and hepatic functions of the mice were also examined. Mice were killed 0, 45, 90, or 180 days subsequent to completion of treatment. Spleen weight and BM cellularity were determined. The hematopoietic tissues were collected for clonogenic assays and histologic analysis.
Harvesting bone marrow
Bone marrow cells were flushed from the tibiae and femurs using Iscoves Modified Dulbecco Media (IMDM; Gibco-BRL, Gaithersburg, MD) containing 5% fetal calf serum (FCS; Hyclone Laboratories, Logan, UT). Low-density mononuclear cells (LDMNCs) were prepared by centrifugation on ficoll-hypaque (density, 1.119; Sigma, St Louis, MO).26
Hematopoietic progenitor growth
Recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 3 (IL-3), and stem cell factor (SCF) were obtained from Peprotech; recombinant murine IL-1 (mIL-1) and macrophage (M)-CSF were purchased from R&D Research Laboratories (Minneapolis, MN). LDMNCs were placed into culture in triplicate 35-mm plates (Becton Dickinson, Franklin Lakes, NJ) at a final concentration of 2 × 104 BM cells or 5 × 105 spleen cells per plate. To culture low proliferating potential colony-forming cells (LPP-CFCs) and high proliferating potential colony forming cells (HPP-CFCs), LDMNCs and recombinant growth factors were added to (0.66%-1.0%) agar and the solution was thoroughly mixed before plating as previously described.34 Growth factors used for culture of HPP-CFCs and LPP-CFCs included SCF, IL-1, M-CSF, GM-CSF, and IL-3.34 To evaluate growth inhibitory effects of IFN-γ on progenitor cells, LDMNCs were plated in the absence or presence of IFN-γ (0.3-1 ng/mL) in clonogenic assays as described elsewhere.26
Evaluation of IFN-γ as a myelopreparative conditioning agent
Fancc-/- and WT mice (CD45.2+) were treated with 400 μg/kg/day of IFN-γ or PBS for 7 days as described above and then received transplants of 1 × 107 BM nucleated B6.BoyJ cells (CD45.1+). The percentage of CD45.1+ cells in the peripheral blood was analyzed at 1, 4, and 6 months after transplantation as described previously.13 To evaluate multilineage reconstitution of donor CD45.1+ cells, peripheral blood of IFN-γ-treated or untreated mice was costained with phycoerythrin (PE)-conjugated lineage marker antibodies (Gr1, Mac1, B220, and CD3; BD PharMingen, San Diego, CA) and fluorescein isothiocyanate (FITC)-conjugated CD45.1 antibody (BD PharMingen) and analyzed by using fluorescence cytometry 6 months following transplantation. Six months following transplantation, secondary transplant studies were conducted using BM cells from IFN-γ-treated or untreated recipients as donor cells.
IFN-γ treatment of mice chimeric for WT and Fancc-/- cells
Lethally irradiated WT mice were reconstituted with 2 × 106 BM LDMNCs at a ratio of 3 Fancc-/- CD45.2+ cells to 1 WT CD45.1+ cell to generate chimeric mice whose CD45.2+ peripheral blood chimerism was around 40%. Following stable reconstitution (4 months), recipients were treated with 400 μg/kg/day of IFN-γ or PBS control for 7 days and the peripheral blood chimerism was evaluated up to 4 months after treatment. Sections were scored on a Zeiss Axioskop (Oberkochen, Germany) using Spot Advanced Software (Spot Software, Amsterdam, the Netherlands) and using a × 40 objective and × 10 subjective lens.
Apoptosis in situ (TUNEL assay)
Bone marrow was fixed in 1% paraformaldehyde. The TUNEL (terminal deoxynucleotidyl transferase [TdT]-mediated deoxyuridine triphosphate [dUTP] nick end labeling) assay was performed on BM sections using the ApopTag Plus Peroxidase in Situ Apoptosis Detection Kit (Intergen, Purchase, NY) as suggested by the manufacturer.
Statistical analyses
Computations were carried out using the Graph Pad Prism software package (San Diego, CA). Student t test was used to compare the various groups. A P value less than .05 was considered statistically significant.
Results
In vivo administration of IFN-γ results in preferential but reversible reduction of primitive and mature hematopoietic progenitor cell populations in Fancc-/- mice
To determine whether hematopoietic cell populations of Fancc-/- mice were hypersensitive to in vivo administration of IFN-γ, micro-osomotic pumps containing 0, 120, or 400 μg/kg/day of IFN-γ were implanted subcutaneously into the backs of Fancc-/- and WT mice for 7 days. Peripheral blood indices were determined at the conclusion of the treatment. Simple placement of the micro-osmotic pumps caused a modest though not significant rise in peripheral white blood cell (WBC) counts compared with WBC counts obtained prior to implantation of the pumps, especially in the Fancc-/- mice (13.5 ± 2.9 × 109/L [13 500 ± 2900/μL] baseline vs 18.2 ± 2.2 × 109/L [18 200 ± 2200/μL] after implantation). A modest though not significant reduction in the peripheral WBC count of WT mice was observed following a 7-day infusion of 400 μg/kg/day of IFN-γ (Figure 1A). However, Fancc-/- mice had an IFN-γ dose-dependent reduction in peripheral WBC counts (Figure 1A) and BM cellularity following treatment (Figure 1B). The profound decline in BM cellularity was associated with a demonstrable increase in TUNEL-positive cells in the marrow cavity of Fancc-/- mice (Figure 1C). No significant change in hematocrit or platelet counts in IFN-γ-treated mice of either genotype compared with mice treated with vehicle control (data not shown).
Effect of in vivo IFN-γ treatment on WBC count and BM cellularity. (A) Complete blood counts were obtained from experimental mice following completion of IFN-γ treatment. Data points represent the WBC count of individual mice. Bars represent the mean WBC count. *P < .01; **P < .001 comparing IFN-γ-treated versus vehicle-treated genotypic controls. (B) After 7 days of IFN-γ treatment, BM cellularity was counted. Data points represent BM cellularity of individual mice. Bars represent the mean BM cellularity. **P < .001 comparing IFN-γ-treated versus vehicle-treated genotypic controls. (C) TUNEL assays were conducted on BM sections from IFN-γ- or vehicle-treated Fancc-/- and WT mice (original magnification × 400). Arrows indicate the apoptotic cells. Data are representative of 5 experimental mice in each group.
Effect of in vivo IFN-γ treatment on WBC count and BM cellularity. (A) Complete blood counts were obtained from experimental mice following completion of IFN-γ treatment. Data points represent the WBC count of individual mice. Bars represent the mean WBC count. *P < .01; **P < .001 comparing IFN-γ-treated versus vehicle-treated genotypic controls. (B) After 7 days of IFN-γ treatment, BM cellularity was counted. Data points represent BM cellularity of individual mice. Bars represent the mean BM cellularity. **P < .001 comparing IFN-γ-treated versus vehicle-treated genotypic controls. (C) TUNEL assays were conducted on BM sections from IFN-γ- or vehicle-treated Fancc-/- and WT mice (original magnification × 400). Arrows indicate the apoptotic cells. Data are representative of 5 experimental mice in each group.
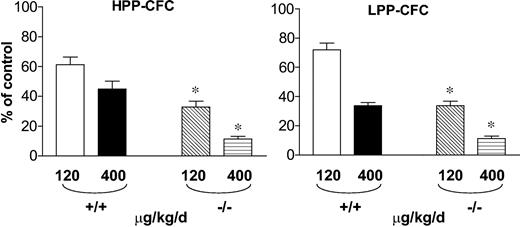
In order to determine the effect of IFN-γ treatment on primitive (HPP-CFC) and mature (LPP-CFC) hematopoietic progenitors, clonogenic assays were established. Even in the absence of IFN-γ treatment, Fancc-/- mice had a reduction in the number of HPP-CFCs per femur compared with WT mice (Table 1; P < .01). In addition, following administration of IFN-γ, Fancc-/- mice had a dramatic reduction in the proportion of HPP-CFCs in the BM (Figure 2; Table 1) when compared with equivalently treated WT mice. A similar pattern was observed in more mature progenitors, LPP-CFCs, from the bone marrow (Figure 2; Table 1). Further, a similar reduction in primitive and mature myeloid progenitor populations was observed in the spleen (Table 1). These data indicate that in vivo IFN-γ treatment preferentially reduces the number of primitive and mature myeloid progenitors in Fancc-/- mice.
Preferential but reversible reduction of BM and spleen primitive and mature progenitors in Fancc−/− mice following IFN-γ treatment
Time following completion of IFN-γ treatment and progenitor . | No. colonies per organ with progenitors in a femur (spleen progenitors), WT . | . | . | No. colonies per organ with progenitors in a femur (spleen progenitors), Fancc−/− . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | IFN-γ, μg/kg/d . | . | . | IFN-γ, μg/kg/d . | . | . | ||||
| . | 0 . | 120 . | 400 . | 0 . | 120 . | 400 . | ||||
| 0 d | ||||||||||
| HPP-CFCs | 4 580 ± 1 583 | 2 807 ± 886* | 2 061 ± 830* | 2 717 ± 1 115† | 893 ± 417* | 303 ± 199* | ||||
| (6 720 ± 1 287) | (3 278 ± 964*) | (2 274 ± 832*) | (1 667 ± 119†) | (176 ± 80*) | (51 ± 24*) | |||||
| LPP-CFCs | 31 537 ± 8 948 | 22 775 ± 5 812* | 10 639 ± 2 523* | 32 232 ± 5 471 | 10 894 ± 3 860* | 3 614 ± 2 340* | ||||
| (20 589 ± 4 022) | (13 879 ± 2 404*) | (8 067 ± 605*) | (26 570 ± 1 193) | (2 033 ± 573*) | (422 ± 27*) | |||||
| 45 d | ||||||||||
| HPP-CFCs | 3 753 ± 305 | 3 406 ± 408 | 3 237 ± 194 | 1 691 ± 415† | 1 651 ± 192† | 1 441 ± 145† | ||||
| (6 436 ± 1 403) | (5 820 ± 1 069) | (6 300 ± 1 208) | (1 724 ± 420†) | (1 486 ± 323†) | (1 571 ± 503†) | |||||
| LPP-CFCs | 36 331 ± 3 051 | 32 641 ± 5 902 | 35 802 ± 4 524 | 38 780 ± 1 800 | 28 553 ± 6 897 | 36 063 ± 2 873 | ||||
| (32 034 ± 2 009) | (36 467 ± 3 246) | (30 680 ± 5 107) | (34 684 ± 3 567) | (39 857 ± 6 098) | (36 004 ± 3 946) | |||||
| 90 d | ||||||||||
| HPP-CFCs | 4 278 ± 772 | 4 575 ± 1 038 | 4 861 ± 1 460 | 2 376 ± 405† | 2 130 ± 256† | 2 064 ± 445† | ||||
| (6 338 ± 479) | (6 747 ± 689) | (6 114 ± 2 017) | (2 372 ± 485†) | (2 544 ± 737†) | (2 237 ± 515†) | |||||
| LPP-CFCs | 30 338 ± 8 479 | 26 767 ± 6 897 | 30 114 ± 2 017 | 34 372 ± 1 885 | 32 004 ± 7 367 | 32 371 ± 5 005 | ||||
| (40 312 ± 4 068) | (39 906 ± 2 940) | (36 785 ± 3 416) | (31 116 ± 4 957) | (29 709 ± 4 210) | (32 110 ± 5 416) | |||||
Time following completion of IFN-γ treatment and progenitor . | No. colonies per organ with progenitors in a femur (spleen progenitors), WT . | . | . | No. colonies per organ with progenitors in a femur (spleen progenitors), Fancc−/− . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | IFN-γ, μg/kg/d . | . | . | IFN-γ, μg/kg/d . | . | . | ||||
| . | 0 . | 120 . | 400 . | 0 . | 120 . | 400 . | ||||
| 0 d | ||||||||||
| HPP-CFCs | 4 580 ± 1 583 | 2 807 ± 886* | 2 061 ± 830* | 2 717 ± 1 115† | 893 ± 417* | 303 ± 199* | ||||
| (6 720 ± 1 287) | (3 278 ± 964*) | (2 274 ± 832*) | (1 667 ± 119†) | (176 ± 80*) | (51 ± 24*) | |||||
| LPP-CFCs | 31 537 ± 8 948 | 22 775 ± 5 812* | 10 639 ± 2 523* | 32 232 ± 5 471 | 10 894 ± 3 860* | 3 614 ± 2 340* | ||||
| (20 589 ± 4 022) | (13 879 ± 2 404*) | (8 067 ± 605*) | (26 570 ± 1 193) | (2 033 ± 573*) | (422 ± 27*) | |||||
| 45 d | ||||||||||
| HPP-CFCs | 3 753 ± 305 | 3 406 ± 408 | 3 237 ± 194 | 1 691 ± 415† | 1 651 ± 192† | 1 441 ± 145† | ||||
| (6 436 ± 1 403) | (5 820 ± 1 069) | (6 300 ± 1 208) | (1 724 ± 420†) | (1 486 ± 323†) | (1 571 ± 503†) | |||||
| LPP-CFCs | 36 331 ± 3 051 | 32 641 ± 5 902 | 35 802 ± 4 524 | 38 780 ± 1 800 | 28 553 ± 6 897 | 36 063 ± 2 873 | ||||
| (32 034 ± 2 009) | (36 467 ± 3 246) | (30 680 ± 5 107) | (34 684 ± 3 567) | (39 857 ± 6 098) | (36 004 ± 3 946) | |||||
| 90 d | ||||||||||
| HPP-CFCs | 4 278 ± 772 | 4 575 ± 1 038 | 4 861 ± 1 460 | 2 376 ± 405† | 2 130 ± 256† | 2 064 ± 445† | ||||
| (6 338 ± 479) | (6 747 ± 689) | (6 114 ± 2 017) | (2 372 ± 485†) | (2 544 ± 737†) | (2 237 ± 515†) | |||||
| LPP-CFCs | 30 338 ± 8 479 | 26 767 ± 6 897 | 30 114 ± 2 017 | 34 372 ± 1 885 | 32 004 ± 7 367 | 32 371 ± 5 005 | ||||
| (40 312 ± 4 068) | (39 906 ± 2 940) | (36 785 ± 3 416) | (31 116 ± 4 957) | (29 709 ± 4 210) | (32 110 ± 5 416) | |||||
n = 5.
P < .01 comparing treated and untreated mice in the same genotype.
P < .01 comparing Fancc−/− and WT.
Effect of in vivo IFN-γ treatment on primitive and mature progenitors in the bone marrow. Cells from WT and Fancc-/- mice that were treated with IFN-γ or vehicle control were cultured at 2 × 104 LDMNCs/mL for the growth of high proliferative potential colony forming cells (HPP-CFCs) and low proliferative potential colony forming cells (LPP-CFCs). Each condition was plated in triplicate and scored on day 7 for LPP-CFCs and day 14 for HPP-CFCs. The dosage of IFN-γ that was administrated and the mouse genotypes are indicated. Error bars represent the standard error of the mean (SEM). *P < .001 comparing the genotypic differences in the reduction of clonogenic formation (n = 5).
Effect of in vivo IFN-γ treatment on primitive and mature progenitors in the bone marrow. Cells from WT and Fancc-/- mice that were treated with IFN-γ or vehicle control were cultured at 2 × 104 LDMNCs/mL for the growth of high proliferative potential colony forming cells (HPP-CFCs) and low proliferative potential colony forming cells (LPP-CFCs). Each condition was plated in triplicate and scored on day 7 for LPP-CFCs and day 14 for HPP-CFCs. The dosage of IFN-γ that was administrated and the mouse genotypes are indicated. Error bars represent the standard error of the mean (SEM). *P < .001 comparing the genotypic differences in the reduction of clonogenic formation (n = 5).
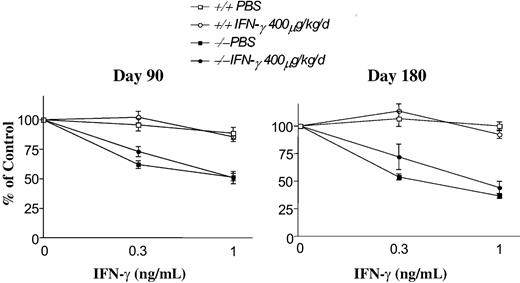
To determine whether pharmacologic administration of IFN-γ causes long-term consequences to hematopoietic populations, Fancc-/- and WT mice were followed for up to 6 months subsequent to treatment with IFN-γ. BM cellularity as well as myeloid progenitor populations from the marrow and spleen were examined. Primitive and mature myeloid progenitor populations from marrow and spleen of mice in both genotypes were comparable to mice treated with PBS vehicle 45 or 90 days following cessation of IFN-γ treatment (Table 1). Additionally, histologic examination of the bone marrow and spleen of these mice revealed normal BM and splenic histology and cellularity (data not shown). To examine whether short-term treatment with IFN-γ results in the genesis of IFN-γ-resistant populations of progenitors, Fancc-/- mice were killed at 90 and 180 days following a 1-week treatment with 400 μg/kg/day IFN-γ. As expected, WT cells from IFN-γ-treated or PBS-treated controls were resistant to IFN-γ at concentrations of 0.3 to 1 ng/mL (Figure 3). In contrast, progenitors cultured from the BM of Fancc-/- mice treated with either IFN-γ or PBS were hypersensitive to IFN-γ and comparable to previously published studies.26 Collectively, IFN-γ resulted in a significant though transient reduction in hematopoietic cell populations and did not result in the selection of a population of IFN-γ-resistant myeloid progenitors in Fancc-/- mice.
Myeloid progenitors isolated from Fancc-/- mice retain IFN-γ hypersensitivity following IFN-γ treatment in vivo. IFN-γ sensitivity of myeloid progenitors from WT and Fancc-/- mice was assessed 90 and 180 days following in vivo treatment with IFN-γ or vehicle control. From each day of evaluation, cells from 4 IFN-γ-treated or vehicle control-treated WT and Fancc-/- mice were cultured for the growth of granulocyte-macrophage progenitors in methylcellulose at the indicated concentrations of IFN-γ. Each condition was plated in triplicate and scored on day 7 of culture. The mean data at days 90 and 180 following treatment are shown. Error bars represent SEM.
Myeloid progenitors isolated from Fancc-/- mice retain IFN-γ hypersensitivity following IFN-γ treatment in vivo. IFN-γ sensitivity of myeloid progenitors from WT and Fancc-/- mice was assessed 90 and 180 days following in vivo treatment with IFN-γ or vehicle control. From each day of evaluation, cells from 4 IFN-γ-treated or vehicle control-treated WT and Fancc-/- mice were cultured for the growth of granulocyte-macrophage progenitors in methylcellulose at the indicated concentrations of IFN-γ. Each condition was plated in triplicate and scored on day 7 of culture. The mean data at days 90 and 180 following treatment are shown. Error bars represent SEM.
In vivo administration of IFN-γ enhanced engraftment of WT hematopoietic cells in Fancc-/- mice
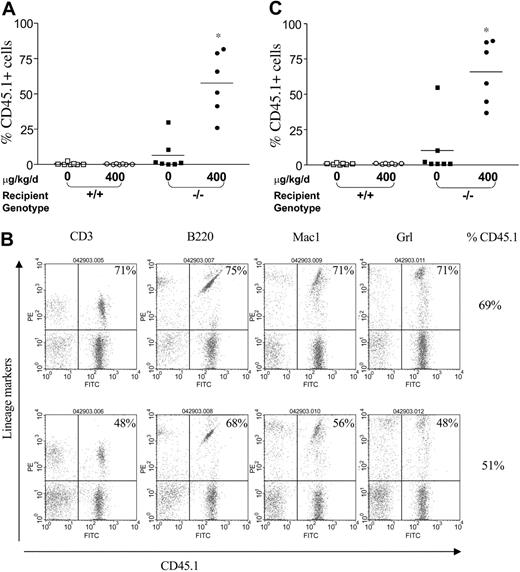
To evaluate whether myelopreparation with IFN-γ is sufficient to allow engraftment and proliferation of isogenic WT repopulating cells, 107 WT CD45.1+ BM nucleated cells were injected via tail vein into Fancc-/- and WT animals pretreated with IFN-γ or PBS. The percent CD45.1+ cells in peripheral blood was examined by fluorescence cytometry 6 months following transplantation. Treatment of WT mice with IFN-γ did not influence the engraftment of exogenous BM cells compared with mice treated with the PBS control (Figure 4A). In the absence of IFN-γ myelopreparation, 6 of 7 Fancc-/- mice failed to engraft significant numbers of isogenic WT cells. However, Fancc-/- recipients treated with IFN-γ prior to transplantation had a mean CD45.1+ chimerism of 58% (range, 25%-82%). Multilineage engraftment of CD45.1+ WT cells was confirmed using 2-color fluorescence cytometry, where expression of myeloid (Gr1 and Mac1) and lymphoid (CD3 and B220) antigens was observed in the peripheral blood of IFN-γ-treated Fancc-/- recipients (Figure 4B).
IFN-γ treatment of Fancc-/- recipients is sufficient to allow engraftment of syngeneic WT bone marrow cells. (A) CD45.1+ WT BM nucleated cells (107) were injected into the tail vein of WT and Fancc-/- C57Bl/6 recipients that express the CD45.2 antigen. Recipients were pretreated with IFN-γ or vehicle control. The percentage of CD45.1+ cells in the peripheral blood was determined by fluorescence cytometry 6 months following treatment. Data points represent CD45.1+ chimerism of individual mice. Bars represent the mean CD45.1+ chimerism. *P < .001 comparing chimerism of Fancc-/- recipients treated with IFN-γ-versus vehicle-treated Fancc-/- recipients and WT recipients. (B) Myeloid and lymphoid differentiation of isogenic WT cells in Fancc-/- mice was determined using fluorescence cytometry 6 months subsequent to IFN-γ treatment. Multilineage analyses of 2 representative mice with 69% and 51% CD45.1+ chimerism are shown. The percentage of WT CD45.1+ lymphoid (CD3 and B220) and myeloid (Gr1 and Mac1) cells is shown in the top right corner of each fluorescence-activated cell sorter (FACS) profile. (C) To evaluate long-term repopulating ability of syngeneic WT BM cells from primary recipients pretreated with IFN-γ, secondary recipients were reconstituted using bone marrow cells from primary recipients 6 months following IFN-γ treatment. CD45.1+ chimerism of secondary recipients was measured 4 months following transplantation. Bars represent the mean CD45.1+ chimerism. *P < .001 comparing chimerism of Fancc-/- recipients treated with IFN-γ-versus vehicle-treated Fancc-/- recipients and WT recipients.
IFN-γ treatment of Fancc-/- recipients is sufficient to allow engraftment of syngeneic WT bone marrow cells. (A) CD45.1+ WT BM nucleated cells (107) were injected into the tail vein of WT and Fancc-/- C57Bl/6 recipients that express the CD45.2 antigen. Recipients were pretreated with IFN-γ or vehicle control. The percentage of CD45.1+ cells in the peripheral blood was determined by fluorescence cytometry 6 months following treatment. Data points represent CD45.1+ chimerism of individual mice. Bars represent the mean CD45.1+ chimerism. *P < .001 comparing chimerism of Fancc-/- recipients treated with IFN-γ-versus vehicle-treated Fancc-/- recipients and WT recipients. (B) Myeloid and lymphoid differentiation of isogenic WT cells in Fancc-/- mice was determined using fluorescence cytometry 6 months subsequent to IFN-γ treatment. Multilineage analyses of 2 representative mice with 69% and 51% CD45.1+ chimerism are shown. The percentage of WT CD45.1+ lymphoid (CD3 and B220) and myeloid (Gr1 and Mac1) cells is shown in the top right corner of each fluorescence-activated cell sorter (FACS) profile. (C) To evaluate long-term repopulating ability of syngeneic WT BM cells from primary recipients pretreated with IFN-γ, secondary recipients were reconstituted using bone marrow cells from primary recipients 6 months following IFN-γ treatment. CD45.1+ chimerism of secondary recipients was measured 4 months following transplantation. Bars represent the mean CD45.1+ chimerism. *P < .001 comparing chimerism of Fancc-/- recipients treated with IFN-γ-versus vehicle-treated Fancc-/- recipients and WT recipients.
To test whether IFN-γ myelopreparation was sufficient to allow engraftment of donor long-term repopulating stem cells, 2 million BM cells from each primary recipient were transplanted into a secondary recipient. As expected, the CD45.1+ chimerism of secondary recipient mice that received cells from IFN-γ-treated and untreated WT primary recipients was low (< 1%; Figure 4C). Similarly, secondary recipients that received transplants with BM cells from Fancc-/- mice treated with PBS demonstrated chimerism similar to the respective primary recipients. However, secondary recipients reconstituted with BM cells from IFN-γ-treated Fancc-/- mice had a mean CD45.1+ chimerism of 66% comparable to that of the chimerism in primary recipients (Figure 4A). Collectively, these data indicate that in vivo administration of IFN-γ in Fancc-/- mice is sufficient as a myelopreparative agent to enhance engraftment of syngeneic WT cells in Fancc-/- mice.
IFN-γ treatment of chimeric mice results in selection of WT cells
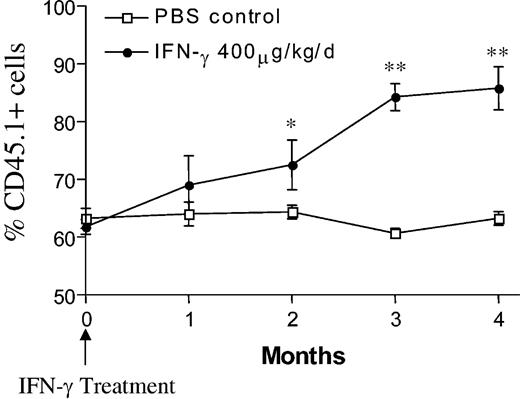
Previous clinical studies have determined that FA patients have relatively low populations of mobilizable stem/progenitor cells available for gene transduction.35 Given these observations and the relatively low efficiency of transducing stem cells, in vivo selection of genetically corrected stem cells could significantly enhance the efficacy of gene therapy in FA patients or preclinical animal models. As a proof of concept, to test whether IFN-γ can provide a selective advantage to WT cells in the setting of a stable hematopoietic microenvironment, lethally irradiated WT mice were reconstituted with CD45.2+Fancc-/- and CD45.1+ WT BM cells. Four months following transplantation, the chimeric mice were treated with either 400 μg/kg/day of IFN-γ or PBS for 1 week and chimerism was monitored for 4 months. As expected, the WT CD45.1 chimerism in the PBS group remained unchanged (63% ± 3.4% before treatment vs 63% ± 2.4% after treatment; Figure 5). However, treatment of chimeric mice with IFN-γ resulted in a significant increase of WT CD45.1+ cells (62% ± 2.5% before treatment vs 86% ± 7.5% 4 months after treatment; P < .0001, n = 5). Collectively, these data suggest that in vivo administration of IFN-γ preferentially reduces Fancc-/- HSC/progenitor cell populations in chimeric mice, which allow the outgrowth of syngeneic WT cells.
Treatment with IFN-γ results in an in vivo selection of WT cells in chimeric mice.Fancc-/- CD45.2+ and WT CD45.1+ BM cells were transplanted at a ratio of 3:1 into lethally irradiated WT mice to obtain similar chimerism. Four months after transplantation, the recipients were treated with 400 μg/kg/day of IFN-γ or vehicle control via micro-osmotic pumps. WT CD45.1+ cells were measured by fluorescence cytometry up to 4 months following IFN-γ treatment. Individual symbols represent the mean of WT CD45.1+ cells (n = 5). Error bars represent SEM. *P < .05; **P < .0001 comparing chimerism from vehicle-treated versus IFN-γ-treated mice.
Treatment with IFN-γ results in an in vivo selection of WT cells in chimeric mice.Fancc-/- CD45.2+ and WT CD45.1+ BM cells were transplanted at a ratio of 3:1 into lethally irradiated WT mice to obtain similar chimerism. Four months after transplantation, the recipients were treated with 400 μg/kg/day of IFN-γ or vehicle control via micro-osmotic pumps. WT CD45.1+ cells were measured by fluorescence cytometry up to 4 months following IFN-γ treatment. Individual symbols represent the mean of WT CD45.1+ cells (n = 5). Error bars represent SEM. *P < .05; **P < .0001 comparing chimerism from vehicle-treated versus IFN-γ-treated mice.
Discussion
Because of the reduction in progenitor/stem cell function in FANCC patients and in genetically engineered Fancc mutant mice, it was anticipated initially that FANCC-deficient (or Fancc- deficient) cells expressing the correct transgene would have a sufficient gain in function compared with uncorrected cells such that myelopreparation would not be required. However, data from numerous laboratories have demonstrated that Fancc-/- mice require some myelopreparation in order to consistently engraft exogenous cells.36,37 Further, in a phase 1 clinical trial, FANCC patients who received a transplant in the absence of any myelopreparation failed to have significant long-term proliferation of transduced cells.9 The failure to engraft genetically corrected cells may in part be related to observations that in vitro-cultured cells frequently do not engraft as well as freshly isolated cells38,39 or alternatively because of the relatively low number of stem cells that are transduced and transplanted compared with endogenous stem cells.
Given these observations, we were interested in exploring nongenotoxic myelopreparative strategies in a Fancc-/- mouse model. The rationale for pursuing IFN-γ in this preclinical model was based on previous in vitro data from our laboratory26 and others27 showing that Fancc-/- hematopoietic progenitors are hypersensitive to IFN-γ-induced apoptosis. In addition, IFN-γ has been used clinically in other disease states.24-26 The present study indicates that IFN-γ treatment results in a profound reduction in mature and primitive hematopoietic populations in Fancc-/- mice compared with WT mice. Importantly, this reduction in hematopoietic populations was sufficient to allow the engraftment and multilineage proliferation of exogenous repopulating stem cells. Further, IFN-γ treatment allows an in vivo selection of WT cells in mice that are stably engrafted with both WT and Fancc-/- cells. Both strategies (myelopreparation and in vivo selection) may ultimately be required to enhance the chimerism of genetically corrected FANCC stem cells since it is anticipated that FA patients will have comparatively low populations of mobilizable FA stem/progenitor cells35 available for transduction.
IFN-γ was overall well tolerated by both WT and Fancc-/- mice. Hematopoietic populations examined returned to normal by 45 days following treatment with IFN-γ, and evaluation of the hematopoietic organs for as long as 6 months following transplantation failed to reveal evidence of unexpected hematologic sequelae. Renal and hepatic toxicities are the known transient sequelae of IFN-γ treatment in humans.40,41 Mice of both genotypes had a less than 2-fold transient rise in alanine aminotransferase (ALT) and asparate aminotransferase (AST), although these returned to baseline levels by 14 days after cessation of treatment. No abnormalities in renal function were observed in any experimental animals (unpublished results). Further, histologic evaluation of the liver and kidney of 3 to 5 mice examined at days 45, 90, and 180 after IFN-γ treatment failed to show evidence of pathology (X.L., D.W.C., unpublished results, October 2003). Thus, the results are encouraging that no major sequelae occur following treatment with IFN-γ. However, future studies to evaluate subtle changes in behavior, appetite, and activity should be systematically examined prior to consideration in preclinical protocols.
Collectively, these studies provide a proof of concept that IFN-γ treatment is sufficient to allow for engraftment of WT cells in Fancc-/- mice and to in vivo select WT cells in hematopoietic chimeric mice. Future studies will focus on the potential of genetically corrected Fancc-/- stem cells to engraft into Fancc-/- mice following this treatment protocol.
Prepublished online as Blood First Edition Paper, April 27, 2004; DOI 10.1182/blood-2004-03-1094.
Supported by US Public Health Services grants P01 HL53586, P30DK49218, R01 HL63219, K08 HLDK 040071-01, and T32 HL-07910 and the Riley Children's Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors gratefully acknowledge Dr Manuel Buchwald (Hospital for Sick Children, University of Toronto) for providing us with the Fancc-/- mice. We thank Samantha Ciccone and Drs Mervin Yoder and Mary Dinauer (Indiana University) for numerous helpful discussions and for reading the manuscript. We also thank Lee Ann Baldrige for excellent technical expertise in conducting all histologic and apoptosis in situ analyses and Janice Walls for preparing the manuscript. Finally, we acknowledge Dr Grover Bagby (Oregon Health Sciences Medical Center) for originally suggesting that IFN-γ may be a useful approach for myelopreparation of FA patients.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal