Abstract
We analyzed herein whether members of the tetraspanin superfamily are involved in human immature dendritic cell (DC) functions such as foreign antigen internalization, phagocytosis, and cell migration. We show that CD63, CD9, CD81, CD82, and CD151 are present in immature DCs. Whereas CD9 and CD81 are mostly expressed at the cell surface, CD63 and CD82 are also located in intracellular organelles. Complexes of monoclonal antibody (Mab) FC-5.01-CD63 or Fab-5.01-CD63 were rapidly translocated “outside-in” and followed the endocytic pathway through early endosomes and lysosomes, reaching major histocompatibility complex (MHC) class II-enriched compartments (MIICs) in less than one hour. Internalization of CD63 was also observed during Saccharomyces cerevisiae phagocytosis. Moreover, an association of CD63 with the β-glycan receptor dectin-1 was observed. Mabs against CD9, CD63, CD81, and CD82 enhanced by 50% the migration induced by the chemokines macrophage inflammatory protein-5 (MIP-5) and MIP-1α. Concomitantly, Mabs against CD63 and CD82 diminished the surface expression of CD29, CD11b, CD18, and α5 integrins. By immunoprecipitation experiments we found that CD63 associated with integrins CD11b and CD18. These results suggest that CD9, CD63, CD81, and CD82 could play a role in modulating the interactions between immature DCs and their environment, slowing their migratory ability. However, only CD63 would intervene in the internalization of complex antigens. (Blood. 2004; 104:1183-1190)
Introduction
Dendritic cells (DCs) comprise a family of professional antigen-presenting cells (APCs) that are sentinels of the immune system.1 In this regard, DCs have been shown to efficiently stimulate both naive B and T cells and to elicit primary immune responses.2,3 Their remarkable effectiveness is due to their ability to capture, process and present antigens along with costimulatory signals, and to migrate to secondary lymphoid tissues.4 Different stages of maturation are responsible for the different functions of DCs. Immature DCs, widely present in peripheral tissues, efficiently uptake antigens but express moderate levels of major histocompatibility complex (MHC) class II and costimulatory molecules. In contrast, mature DCs poorly acquire antigens but express higher levels of MHC class II and costimulatory molecules, are able to migrate into lymph nodes, and become potent activators of resting T cells.2-5 Since antigen internalization and processing, as well as cell migration, are essential properties of DCs, the study of the possible involvement of tetraspanins in these processes is a challenging question, as it could bring new insight into the physiologic role of these molecules in DCs and other cells. In recent years, considerable interest has arisen in the expanding tetraspanins family, which are integral membrane proteins with 2 extracellular domains (EC1 and EC2) that are variably glycosylated.6 The most conspicuous members of this family are CD9,7 CD63/lamp-3,8 CD81/TAPA-1,9 CD82/KAI1,10 and CD151.11 Tetraspanins form several specific complexes implicated in a variety of cellular processes such as migration, adhesion, proliferation, and signal transduction, initially leading to the idea that these proteins could play a “molecular facilitator” role.6
There is growing evidence that points to a possible role of tetraspanins in antigen processing and presentation. In fact, it has been recently found that CD63 is modified after translation during maturation of DCs, and this event is accompanied by morphologic changes in MHC class II-enriched compartments (MIICs), the compartments where peptide loading takes place.12 However, there are conflicting reports about tetraspanins subcellular localization in APCs. On one hand, CD82 has been found in the MIICs of B cells13 and large complexes of MHC class II molecules, integrins, and the tetraspanins CD9, CD63, CD81, and CD82 have been observed in the plasma membrane of lymphoblastoid B-cell lines.14 In addition, CD63 has been detected at the surface of human immature DCs by immunofluorescence15 and CD9, CD63, and CD82 have also been found in B-cell- or DC-derived exosomes.16,17 On the other hand, using cell fractionation and immunoprecipitation techniques, other authors18 detected CD63 in the MIICs but not at the cell surface of immature DCs, and CD82 expression was not found.
DCs have developed various mechanisms for antigen internalization such as phagocytosis, macropinocytosis, and internalization of immune complexes via Fc receptors. We have previously demonstrated that the binding of FC-5.01, a CD63-specific monoclonal antibody (Mab),19 induced the translocation of surface CD63 to intracellular acidic vesicles in the breast cancer cell line IIB-BR-G.20 Thus, we examined if CD63 and other tetraspanins were present at the cell surface in immature DCs and if they translocated toward specific intracellular compartments following Mab FC-5.01 binding. Moreover, since CD63 has been found to be involved in the release of cytoplasmic granules in hematopoietic cells,21,22 we explored whether CD63 and other tetraspanins are also involved in the internalization of foreign antigens along the endosomal-lysosomal route. In consequence, we analyzed tetraspanin surface expression after yeast phagocytosis and fluorescein isothiocyanate (FITC)-dextran endocytosis and we investigated the possible association between CD63 and the phagocytic receptors macrophage mannose receptor (MMR) and dectin-1. Finally, since DCs are capable of tissue migration, a process that appears to be slowed down by tetraspanins in tumor cells,23,24 we investigated the effect of Mabs against different tetraspanins on DC migration and integrin expression.
We demonstrate herein the presence of CD63, CD9, CD81, and CD82 at the cell surface of immature DCs. We have also studied in detail the fate of the FC-5.01-CD63 complex, and have found that it is rapidly internalized and follows the endosomal-lysosomal-MIICs route. We also demonstrate that CD63 is involved in physiologic DC functions, such as yeast phagocytosis and cell migration. In addition, the comparative study of various tetraspanins revealed important differences in their respective behavior.
Materials and methods
Preparation of DCs
DCs were generated from adherent peripheral blood monocytes. After obtaining informed consent, leukocytes from healthy blood donors were collected by cytapheresis or from buffy coats. After Ficoll-Hypaque density centrifugation, mononuclear cells were resuspended in serum-free lymphocyte medium AIM-V (Gibco BRL, Grand Island, NY) and allowed to adhere to plastic dishes for 2 hours at 37°C. Cells in suspension were then removed and adherent cells were cultured for 5 to 7 days in AIM-V fresh medium containing 800 U/mL recombinant human granulocyte-monocyte colony-stimulating factor (GM-CSF; Molgramostim; Schering-Plough, Bray, Ireland) and 50 ng/mL recombinant human interleukin 4 (IL-4; Peprotech, Rocky Hill, NJ). In control experiments, after 5 days of culture, 1 μg/mL lipopolysaccharide (LPS) from Pseudomonas aeruginosa (Sigma, St Louis, MO) was added for 48 hours to induce DC maturation.
Antibodies
The antibodies (Abs) used were as follows: mouse Mab anti-human CD63 FC-5.01 (Zymed, South San Francisco, CA), rabbit polyclonal anti-human early endosome antigen-1 (EEA1; BD Pharmingen, San Diego, CA; a gift from Dr J. Salamero, Institute Curie, Paris, France), goat polyclonal anti-human lysosome associated membrane protein-2 (LAMP-2) (Santa Cruz Biotechnology, Santa Cruz, CA); mouse Mabs anti-human HLA-DR, -DP, -DQ FITC-conjugated (Tü39, IgG2a/κ), anti-human CD29 (IgG1/κ), anti-human CD40 (5C3), anti-human CD80 (BB1, IgM/κ), anti-human CD9 (IgG1/κ), anti-human CD81 (IgG1/κ), anti-human CD82 (IgG1/κ), anti-human CD83 FITC-conjugated (IgG1/κ), anti-human CD151 (IgG1/κ), and anti-human HLA class I (A, B, C, IgG1/κ) were from BD Pharmingen; polyclonal rabbit anti-human dectin-1 Abs were generated as described25 ; mouse anti-human CD11b and mouse anti-human CD18 were gifts from Dr V. Horejsi (Institute of Molecular Genetics, Academy of Sciences of the Czech Republic, Czech Republic); mouse anti-human α5 (IgG1; Chemicon International, Temecula, CA); mouse anti-human β3 (IgG1; Cymbus Biotechnology, Chandlers Ford, United Kingdom), alkaline phosphatase-conjugated goat antimouse immunoglobulins (AP-GAMs), horseradish peroxidase-conjugated goat antirabbit immunoglobulins (HRPGARs), cyanin 5 (Cy5)-conjugated goat antimouse immunoglobulins (Cy5-GAMs), Cy3-conjugated rabbit antimouse immunoglobulins (Cy3-RAMs), Cy5-conjugated rabbit antigoat immunoglobulins (Cy5-RAG), and Cy3-conjugated goat antirabbit immunoglobulins (Cy3-GARs) were from Jackson ImmunoResearch Laboratories (West Grove, PA). Fluorescein isothiocyanate-conjugated polyclonal goat antimouse immunoglobulins (GAM-FITCs) and tetramethylrhodamine isothiocyanate-conjugated polyclonal goat antimouse immunoglobulins (GAM-TRITCs) were from Sigma. Phycoerythrin-conjugated goat antimouse immunoglobulins (GAM-PEs) were from Dako (Dako, Carpintería, CA). Isotype-matched control immunoglobulins were as follows: mouse IgG2a, IgG1, IgM, IgG1 FITC-conjugated, and IgG2a FITC-conjugated (Sigma).
Fab-5.01 fragments were prepared by overnight papain digestion and purified through a protein A-Sepharose column (Sigma). Purity of Fab fragments was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie Brilliant Blue staining.
Indirect immunofluorescence
Indirect immunofluorescence assays were performed on suspended DCs removed from culture flasks by pipetting with phosphate-buffered saline (PBS). After blocking with 10% normal goat serum for 30 minutes, the cells were incubated with primary Abs for 60 minutes, washed, incubated with secondary Abs (GAM-FITC or GAM-PE for FACscan analysis [FACS Vantage SE; Becton Dickinson, San Jose, CA] or GAM-TRITC for microscope observations), and fixed with 3% paraformaldehyde for 10 minutes. All incubations were performed on ice in order to minimize Ab internalization. For intracellular staining, the cells were first fixed with 3% paraformaldehyde and then permeabilized with PBS plus 0.1% saponin, which was present in all subsequent incubations. Samples were mounted in Permafluor (Immunon, Pittsburgh, PA) and observed under a Zeiss LSM 510 confocal microscope (Carl Zeiss, Heidelberg, Germany). Images were acquired and processed using Zeiss LSM Image Browser Version 3.1.0.99 (Carl Zeiss).
In order to analyze integrin surface expression after CD63 and CD82 internalization, the following assay was performed: DCs were incubated for 1 hour at 4°C with FC-5.01 or anti-CD82 Mabs (10 μg/mL). After binding, the medium was removed and incubation was continued in PBS Ca++/Mg++ at 37°C for 30 minutes to allow internalization of surface-bound Abs. Cells were then incubated with Abs against CD29, CD11b, C18, α5, or β3 integrins at 4°C, followed by incubation with secondary antibodies (GAM-FITC). Cells were fixed with 3% paraformaldehyde and analyzed by FACS.
Tetraspanin internalization assay by confocal microscopy
At day 5, immature DCs were collected by pipetting and assayed for anti-CD63 Mab internalization. Alternatively, the assay was performed with immature DCs grown on 12-mm diameter coverslips (Marienfeld Laboratory Glassware, Lauda-Koenigshofen, Germany) treated with 0.1% poly-l-lysine (Sigma). The cells were washed 3 times with PBS plus Ca++/Mg++ and nonspecific sites were blocked with PBS-1% bovine serum albumin (PBS-BSA) for 30 minutes at 4°C. DCs were then incubated for 1 hour at 4°C with FC-5.01 (10 μg/mL) or Fab-5.01 (20 μg/mL) diluted in PBS-BSA. After binding, the medium was removed and incubation was continued in PBS Ca++/Mg++ at 37°C to allow internalization of surface-bound Abs. The cells were fixed with 3% paraformaldehyde for 10 minutes, permeabilized with PBS-0.05% saponin (15 minutes) at different times of incubation, and blocked with goat or rabbit serum (Laboratorio Alfredo C. Gutiérrez, Buenos Aires, Argentina). All further Ab dilutions were prepared in PBS-0.05% saponin. To visualize colocalization of internalized anti-CD63 Mab with intracellular markers, a double indirect immunofluorescence scheme was set up. The cells were sequentially incubated with the corresponding anti-mouse fluorescent secondary Ab to reveal anti-CD63 Mab, then with Abs against EEA1, LAMP-2, or HLA-DR, -DP, -DQ markers followed by incubation with the corresponding fluorescent secondary Ab, as indicated in the figures. After washing, the cells were mounted with Mowiol (Calbiochem, La Jolla, CA) and examined under a Zeiss LSM 510 confocal microscope (Carl Zeiss).
Controls for Cy5-GAM, Cy3-RAM, Cy3-GAR, and Cy5-RAG staining were performed with control immunoglobulins. Controls for HLA-II staining was performed with IgG2a FITC-conjugated isotype-matched control. No staining was observed in any case. Controls for double-labeling with FC-5.01 or Fab-5.01 were also performed. FC-5.01 and Fab-5.01 were incubated with Cy3-GAR or Cy5-RAG alone or sequentially incubated with Cy5-GAM and Cy3-GAR or with Cy3-RAM and Cy5-RAG. No cross-reaction was observed (Figure S1; see the Supplemental Figures link at the top of the online article on the Blood website).
Internalization of other tetraspanins was performed as described for CD63.
Cell lysis and immunoprecipitation
DCs were lysed in immunoprecipitation buffer (1% CHAPS [3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate] in PBS, 2 mM MgCl2, 2 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL aprotinin, 10 μg/mL leupeptine) for 1 hour at 4°C. Insoluble materials were pelleted at 11.600g for 10 minutes, and the cell lysates were first precleared by incubation with protein G Sepharose 4B (Amersham Pharmacia, Uppsala, Sweden). Then, cell lysates were incubated with FC-5.01 or anti-CD11b Mabs or with IgG2a isotype control immunoglobulins overnight at 4°C. The immune complexes were collected onto protein G Sepharose 4B, followed by extensive washing with the immunoprecipitation buffer. Immune complexes were eluted from beads with Laemmli sample buffer and resolved by 10% SDS-PAGE under nonreducing conditions. Proteins were transferred to nitrocellulose membranes (Sigma) and visualized with HRP-GAR using Western Blotting Luminol Reagent for enhanced chemiluminescence (Santa Cruz Biotechnology) or with AP-GAM using BCIP (5-bromo-4-chloro-3-indolyl-phosphate)/NBT (nitroblue tetrazolium) (Promega, Madison, WI) as indicated in “Results.”
Chemotaxis assay
The chemotaxis assay was performed in a 48-well chemotaxis chamber (Nucleopore, Pleasanton, CA). Macrophage inflammatory protein-1 α (MIP-1α)26 or MIP-527 (Peprotech), diluted to 100 ng/mL in RPMI-0.1% BSA, as well as RPMI-0.1% BSA alone were placed in the lower compartment. A 5-μm pore polycarbonate membrane (Neuro Probe, Gaithersburg, MD) was placed between the upper and lower compartments. DCs were incubated with the corresponding Abs for 30 minutes at 37°C and then seeded in the upper compartment (7.5 × 104 DCs/well). After 90 minutes at 37°C, the membrane was stained with Giemsa and the cells in the lower face were counted. Five medium-power (× 40) fields per well and 3 wells per condition were analyzed. Statistical analysis was performed using a 2-tailed Student t test.
Yeast phagocytosis assay
DCs were cultured as described above in 100-mm culture dishes (Nunc, Roskilde, Denmark) for 5 days. Autoclaved Saccharomyces cerevisiae yeasts were added at a DC-to-yeast ratio of 1:30 and incubated for 4 hours at 37°C in a 5% CO2 humidified atmosphere. Some plates were washed, stained with May-Grünwald-Giemsa, and phagocytosis was evaluated under light microscopy using a Zeiss Axioplan photomicroscope (Carl Zeiss) equipped with an MC 80 camera (Carl Zeiss) by counting the percentage of effector cells that had ingested more than 5 yeasts per cell. In other plates, the cells were resuspended by pipetting, centrifuged at 800g, washed, and resuspended in PBS. CD63 and MHC class II expression was assessed by indirect immunofluorescence performed at 4°C in order to prevent FC-5.01 internalization, using GAM-PE as revealing antibody, and analyzed by FACS. In some experiments, DCs were incubated with 400 μg/mL α-mannan (Sigma) or with 400 μg/mL laminarin (Calbiochem) for 20 minutes at 37°C before yeasts were added.
FITC-dextran uptake
Endocytosis was studied by incubating immature DCs with one mg/mL FITC-dextran (FITC-Dx; Sigma) for 30 minutes at 37°C or at 4°C. In some plates, preincubation with α-mannan was performed as indicated in “Yeast phagocytosis assay.” The cells were then resuspended, washed, and incubated with FC-5.01 Mab as indicated in “Yeast phagocytosis assay.” Flow cytometric cell analysis for double staining was subsequently performed.
Results
CD63 is expressed at the cell surface of monocyte-derived DCs
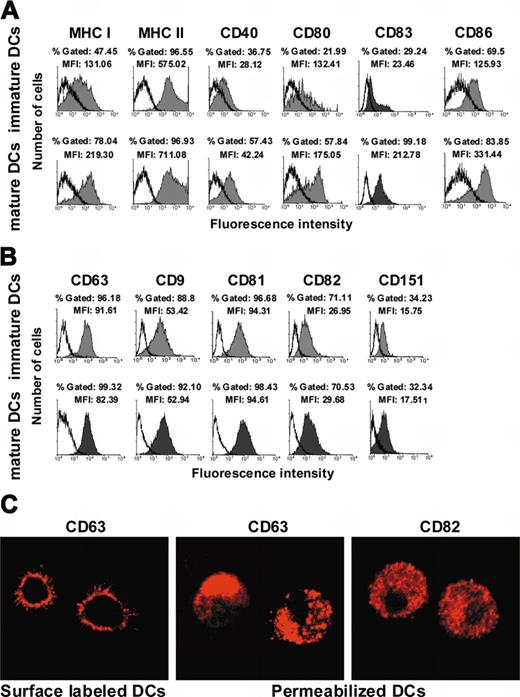
Immature and mature DCs, obtained by adding one μg/mL LPS at day 5 of the culture (see “Materials and methods”), were first characterized by indirect immunofluorescence, to validate our system. A marked increase on the expression of MHC class I and II, CD83, as well as of CD40, CD80, and CD86 costimulatory molecules on mature DCs was observed, as expected (Figure 1A).
Tetraspanin expression at the cell surface of monocyte-derived immature DCs. (A) Expression of MHC class I, MHC class II, CD40, CD80, CD83, and CD86 on immature and mature DCs (shaded histograms). Isotype-matched control immunoglobulin labeling is shown in open histograms. (B) Expression of CD63, CD9, CD81, CD82, and CD151 on immature and mature DCs (shaded histograms). Isotype-matched control immunoglobulins labeling is shown in open histograms. Obtention and FACS analysis of human immature and mature DCs were performed as described in “Materials and methods.” A total of 10 000 events were analyzed in each case. The histograms shown are representative of at least 4 experiments. (C) Microphotographs illustrating the pattern of CD63 expression before cell permeabilization (left) and of CD63 (middle) and CD82 (right) expression after cell permeabilization, observed by confocal microscopy in immature DCs. Objective lenses: C-Apochromat 63 ×/1.2 numerical aperture (NA), water.
Tetraspanin expression at the cell surface of monocyte-derived immature DCs. (A) Expression of MHC class I, MHC class II, CD40, CD80, CD83, and CD86 on immature and mature DCs (shaded histograms). Isotype-matched control immunoglobulin labeling is shown in open histograms. (B) Expression of CD63, CD9, CD81, CD82, and CD151 on immature and mature DCs (shaded histograms). Isotype-matched control immunoglobulins labeling is shown in open histograms. Obtention and FACS analysis of human immature and mature DCs were performed as described in “Materials and methods.” A total of 10 000 events were analyzed in each case. The histograms shown are representative of at least 4 experiments. (C) Microphotographs illustrating the pattern of CD63 expression before cell permeabilization (left) and of CD63 (middle) and CD82 (right) expression after cell permeabilization, observed by confocal microscopy in immature DCs. Objective lenses: C-Apochromat 63 ×/1.2 numerical aperture (NA), water.
We then investigated the expression of tetraspanins on immature DCs. Whereas CD63 was poorly detected at the surface of freshly isolated monocytes (data not shown), a significant expression was observed on immature DCs from 30 donors. CD9, CD81, and CD82 were also found to be expressed in immature DCs, whereas CD151 expression was much lower (Figure 1B). Tetraspanin expression on mature DCs did not significantly differ from their correspondent expression on inmmature DCs (Figure 1B)
Since CD63 expression at the surface of DCs is still controversial,15,16 confocal microscopy analysis of DCs labeled with anti-CD63 Mab at 4°C were then performed (Figures 1C, left panel, and Figure 2). These experiments confirmed the expression of CD63 at the cell membrane, which was generally uniform (Figure 1C, left panel, and Figure 2, upper panels), although in some cells CD63 molecules appeared to be polarized (Figure 2, upper left panel). Analysis of fixed and permeabilized cells (Figure 1C, center) demonstrated that CD63 is abundantly expressed in vesicles, which probably correspond to lysosomes and endosomes, as previously reported.18 Thus, CD63 is clearly expressed at the cell surface of immature DCs as well as in intracellular compartments. Analysis of permeabilized immature DCs demonstrated that CD82 is also expressed uniformly in the cytoplasm in addition to its membrane expression (Figure 1C, right panel), while CD9 and CD81 are mostly expressed at the cell surface (data not shown). The intracellular expression of CD9, CD63, CD81, CD82, and CD151 was also analyzed by FACS on permeabilized DCs, confirming confocal microscopy results (data not shown).
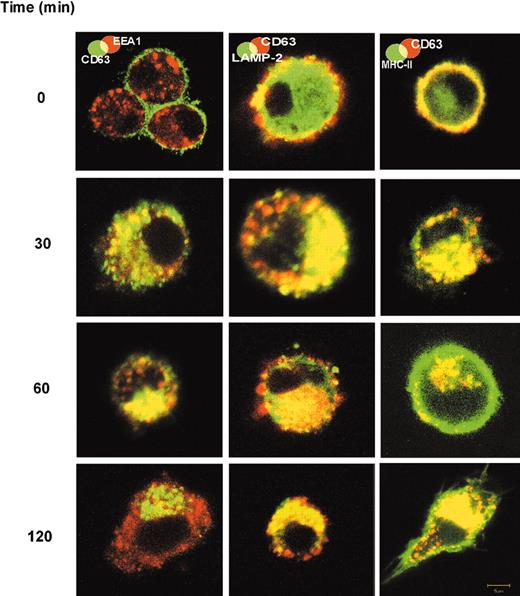
Translocation of FC-5.01/CD63 complexes from the cell surface to early endosomes, lysosomes, and MIICs of immature DCs. Double labeling to detect internalized FC-5.01 Mab and EEA1, LAMP-2, and MHC class II molecules was performed. Immature DCs were incubated with 10 μg/mL FC-5.01 Mab for one hour on ice. After washing to remove unbound FC-5.01, cells were further incubated in culture medium at 37°C for the indicated periods of time. FC-5.01 was stained with Cy5-GAM (green in EEA1 panel) or Cy3-RAM (red in LAMP-2 and MHC class II panels). Cells were then incubated with rabbit anti-EEA1 (red; secondary antibody Cy3-GAR), goat anti-LAMP-2 (green; secondary antibody Cy5-RAG), or anti-HLADR, -DP, -DQ FITC-labeled (green). Colocalization is visualized by yellow staining. Images are representative of at least 4 similar experiments. Objective lenses: Plan-Neofluar 100 ×/1.3 NA, oil; digital zoom: 2.
Translocation of FC-5.01/CD63 complexes from the cell surface to early endosomes, lysosomes, and MIICs of immature DCs. Double labeling to detect internalized FC-5.01 Mab and EEA1, LAMP-2, and MHC class II molecules was performed. Immature DCs were incubated with 10 μg/mL FC-5.01 Mab for one hour on ice. After washing to remove unbound FC-5.01, cells were further incubated in culture medium at 37°C for the indicated periods of time. FC-5.01 was stained with Cy5-GAM (green in EEA1 panel) or Cy3-RAM (red in LAMP-2 and MHC class II panels). Cells were then incubated with rabbit anti-EEA1 (red; secondary antibody Cy3-GAR), goat anti-LAMP-2 (green; secondary antibody Cy5-RAG), or anti-HLADR, -DP, -DQ FITC-labeled (green). Colocalization is visualized by yellow staining. Images are representative of at least 4 similar experiments. Objective lenses: Plan-Neofluar 100 ×/1.3 NA, oil; digital zoom: 2.
Surface FC-5.01/CD63 complexes are translocated to endosomes, lysosomes, and MIICs, and do not require crosslinking for internalization
We have previously shown that the binding of Mab FC-5.01 to CD63 expressed at the cell surface of IIB-BR-G human breast cancer cells is followed by a rapid internalization of the complex.20 Thus, we investigated in DCs whether Mab FC-5.01, after binding to surface CD63, was translocated into intracellular compartments of immature DCs. The Mab FC-5.01 was detected in various cellular compartments in parallel with the resident endosomal, lysosomal and MHC class II proteins. As shown in Figure 2, the anti-CD63 Mab FC-5.01 incubated at 4°C with immature DCs was found to be located exclusively at the cell surface, where it colocalized with MHC class II molecules, as expected (Figure 2, upper right).
After shifting to 37°C, the complex rapidly internalized, and, after 30 minutes, the Mab FC-5.01 was detected in early endosomes (demonstrated by colocalization with EEA1) and in lysosomes (demonstrated by colocalization with Lamp-2). At that time, some molecules had already reached the MIICs. After a 60-minute incubation, the Mab FC-5.01 localized in the lysosomes of every DC analyzed. Localization in the MIICs was also observed. By 120 minutes, the internalized Mab FC-5.01 had clearly left the early endosomal pool and was located mainly in lysosomes and MIICs, indicating that the internalized Mab FC-5.01 reaches the compartments where antigen processing and peptide loading takes place.
In order to rule out the possibility that some degree of the internalization process was due to FC-5.01 binding to Fcγ R present at the DC cell surface, the same assays were performed with FC-5.01-derived Fab fragments (Fab-5.01). These experiments also intended to investigate if CD63 crosslinking with a bivalent Mab was necessary for its internalization. Similar results to those observed with the whole FC-5.01 antibody were obtained, since at 30 minutes most Fab-5.01 was located in early endosomes. At 60 minutes, it was concentrated in lysosomes and at 120 minutes it was mostly found in MIICs (Figure S2). Thus, the FC-5.01 Fc region does not play a role in the internalization process and a bona fide CD63 internalization is observed in the experiments depicted for FC-5.01. In addition, it should be noted that CD63 crosslinking is not a prerequisite for internalization to occur. The internalization of other tetraspanins after binding with specific Mabs was also determined: CD81 and CD82 internalized, whereas CD9 did not (Table 1).
Expression and functional characteristics of tetraspanins in immature DCs
. | Expression . | . | Effect of Mab binding . | . | ||
|---|---|---|---|---|---|---|
| Tetraspanin . | Surface . | Cytoplasm . | Translocation . | Migration . | ||
| CD9 | ++* | No | No | ↑ | ||
| CD63 | ++* | Yes | Yes | ↑ | ||
| CD81 | ++* | +/−† | No | ↑ | ||
| CD82 | +‡ | Yes | Yes | ↑ | ||
| CD151 | +/−† | No | No | = | ||
. | Expression . | . | Effect of Mab binding . | . | ||
|---|---|---|---|---|---|---|
| Tetraspanin . | Surface . | Cytoplasm . | Translocation . | Migration . | ||
| CD9 | ++* | No | No | ↑ | ||
| CD63 | ++* | Yes | Yes | ↑ | ||
| CD81 | ++* | +/−† | No | ↑ | ||
| CD82 | +‡ | Yes | Yes | ↑ | ||
| CD151 | +/−† | No | No | = | ||
More than 90% labeled cells with an MFI range of 53 to 91.
35% labeled cells with a low MFI of 16.
70% labeled cells with an intermediate MFI of 27.
Mabs against tetraspanins increase immature DCs migration: association between CD63 and integrins
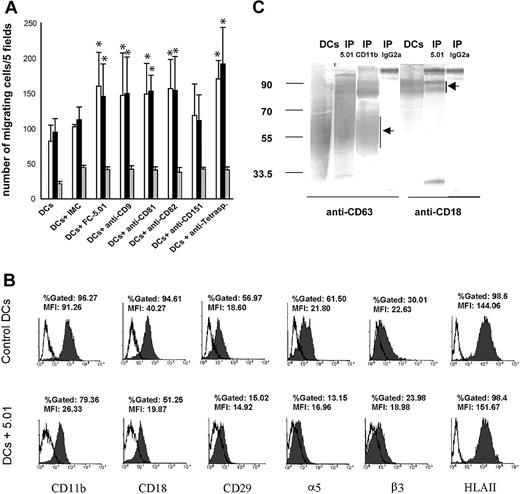
Tetraspanins have been previously shown to delay tumor cell migration,23,24 probably through interactions with integrins.28,29 To investigate if CD63 and other tetraspanins play a role in immature DC migration, a chemotaxis assay was performed using chemokines MIP-1α or MIP-5 (100 ng/mL) as chemoattractants. It was observed that Mabs directed against CD9, CD63, CD81, and CD82 stimulated migration induced by MIP-1α (Figure 3, open bars) and MIP-5 (Figure 3, black bars), whereas the Mab directed against CD151 had no significant effect on migration. When the Mabs against all tetraspanins studied (ie, CD9, CD63, CD81, CD82, and CD151) were added together, migration increased almost 100%. Migration against RPMI-0.1% BSA (Figure 3, black and white bars) was significantly lower than migration induced by MIP-1α and MIP-5 in all cases, showing that the Abs against tetraspanins exerted a specific effect on the cellular response to chemoattractants (Figure 3A).
Effect of antitetraspanin Mabs on the migration of immature DCs: association between CD63 and integrins. (A) Immature DCs were prepared as described in “Materials and methods,” and their migration in the presence of 100 ng/mL MIP-1α (□), MIP-5 (▪), and RPMI-0.1% BSA alone (▦) was performed as described in “Materials and methods.” An average of 5 experiments is shown. Error bars show standard deviation. Statistically significant differences between DCs incubated with the corresponding Mabs and DCs incubated with isotype-matched control immunoglobulins (IMCs) are indicated (*P < .05, according to a 2-tailed Student t test). (B) Expression of CD29, CD11b, CD18, α5, β3, and MHC class II before and after CD63 internalization (shaded histograms). Isotype-matched control immunoglobulin labeling is shown in open histograms. The histograms shown are representative of at least 3 experiments. (C) Immunoprecipitation of CD63 and CD11b was performed as described in “Materials and methods.” The immunoprecipitates were analyzed by 10% SDS-PAGE under nonreducing conditions and revealed with anti-CD63 (broad band between 40 kDa and 70 kDa19 ) and anti-CD18 Mabs followed by GAM-PA and BCIP/NBT. Lanes 1 and 5: DC extract. Lanes 2 and 6: CD63 immunoprecipitates (IP 5.01). Lane 3: CD11b immunoprecipitate (IP CD11b). Lanes 4 and 7: control immunoprecipitations performed using isotype-matched control IgG2a (IP IgG2a).
Effect of antitetraspanin Mabs on the migration of immature DCs: association between CD63 and integrins. (A) Immature DCs were prepared as described in “Materials and methods,” and their migration in the presence of 100 ng/mL MIP-1α (□), MIP-5 (▪), and RPMI-0.1% BSA alone (▦) was performed as described in “Materials and methods.” An average of 5 experiments is shown. Error bars show standard deviation. Statistically significant differences between DCs incubated with the corresponding Mabs and DCs incubated with isotype-matched control immunoglobulins (IMCs) are indicated (*P < .05, according to a 2-tailed Student t test). (B) Expression of CD29, CD11b, CD18, α5, β3, and MHC class II before and after CD63 internalization (shaded histograms). Isotype-matched control immunoglobulin labeling is shown in open histograms. The histograms shown are representative of at least 3 experiments. (C) Immunoprecipitation of CD63 and CD11b was performed as described in “Materials and methods.” The immunoprecipitates were analyzed by 10% SDS-PAGE under nonreducing conditions and revealed with anti-CD63 (broad band between 40 kDa and 70 kDa19 ) and anti-CD18 Mabs followed by GAM-PA and BCIP/NBT. Lanes 1 and 5: DC extract. Lanes 2 and 6: CD63 immunoprecipitates (IP 5.01). Lane 3: CD11b immunoprecipitate (IP CD11b). Lanes 4 and 7: control immunoprecipitations performed using isotype-matched control IgG2a (IP IgG2a).
The surface expression of integrins CD29, CD11b, CD18, α5, and β3 was analyzed by FACS after tetraspanin CD63 and CD82 (not shown) internalization. We observed that the surface expression of all integrins tested diminished except for β3, compared with their control expression at 4°C, whereas MHC class II expression remained unchanged (Figure 3B). Accordingly, when CD63 and CD11b were immunoprecipitated as described in “Materials and methods,” CD11b and CD18 were found to be associated with CD63, as it has been described in neutrophils30 (Figure 3C).
The results of tetraspanin expression at the cell surface and in the cytoplasm of immature DCs, and the effect of specific Mabs on tetraspanin translocation and DC migration are summarized in Table 1.
Phagocytosis of S cerevisiae yeasts, but not endocytosis of FITC-dextran, is accompanied by a decrease on surface expression of CD63, which is associated with β-glycan receptor dectin-1
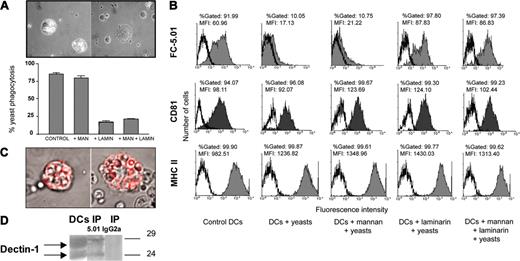
DCs possess distinct types of receptors for antigen uptake, among which are the β-1,3-glycan receptors and the MMR, that allow to phagocytose particles such as yeasts and to endocytose soluble sugar-containing antigens.31,32 Since CD63 is translocated into intracellular compartments following Mab binding, we investigated whether the engagement of DCs in phagocytosis or endocytosis could modify the location of this tetraspanin. First, the effect of S cerevisiae phagocytosis on CD63 expression at the cell surface was studied. After incubation of immature DCs with S cerevisiae (4 hours, 37°C), a high percentage of DCs (> 85%) with more than 5 yeasts per cell was observed (Figure 4A and left picture of the inset). In order to determine which types of receptors are involved, immature DCs were preincubated with α-mannan (a bacterial polysaccharide that binds with high affinity to the MMR), with laminarin (a β-1,3-glycan that resembles yeast glycan), or with both polyosides. The yeast phagocytosis assay was then performed. As shown in Figure 4A, the S cerevisiae uptake was not significantly inhibited by α-mannan (6.2% inhibition). In contrast, when laminarin was added to the cell culture (Figure 4A and right picture of the inset) a strong inhibition of phagocytosis was observed (80.4%). The addition of α-mannan did not modify the extent of the inhibition obtained with laminarin (73%; Figure 4A). Thus, β-1,3-glycan recognition appears to be essential for S cerevisiae phagocytosis, whereas this process involves MMR only marginally. FACS analysis of DCs after yeast phagocytosis demonstrated a strong decrease in CD63 surface expression as compared with control DCs (Figure 4B), a process that could be reverted by laminarin but not by α-mannan. Translocation of surface molecules after yeast phagocytosis was not general, since it did not occur with CD81 and MHC class II molecules (Figure 4B), as well as with CD9 and CD82 (data not shown).
Internalization of cell-surface CD63 accompaniesS cerevisiaeyeast phagocytosis in immature DCs: association of CD63 with dectin-1. (A) A yeast phagocytosis assay was performed as described in “Materials and methods” in the presence or absence of α-mannan, laminarin, or both polyosides. The percentage of phagocytic DCs was calculated in triplicate (at least 200 cells were counted per point). The top panel shows an example of DCs bearing phagocytic vacuoles containing yeasts (left) and of DCs incubated in the presence of laminarin (right); objective lens: Plan-Neofluar 100 ×/1.3 NA, oil. The results shown are representative of at least 5 experiments. (B) CD63 surface expression was evaluated by FACS analysis after yeast phagocytosis in the presence or absence of α-mannan, laminarin, or both polyosides and in control DCs (not incubated with yeasts). Ordinate indicates number of positive cells and abscissa indicates MFI. The histograms shown are representative of at least 5 experiments. (C) Example of FC-5.01/CD63 complexes surrounding internalized yeasts as described in “Results.” Objective lens: Plan-Neofluar 100 ×/1.3 NA, oil. (D) Immunoprecipitation of CD63 was performed as described in “Materials and methods.” The immunoprecipitates were analyzed by 10% SDS-PAGE under nonreducing conditions and revealed with anti-dectin-1 polyclonal antibody followed by HRP-GAR and chemiluminescence. The antibody detected a double band of approximately 27 kDa and 23 kDa, which corresponds to the estimated molecular weight for the dectin-1 full-length and 1b isoforms.25 Lane 1: DC extract. Lane 2: CD63 immunoprecipitate (IP 5.01). Lane 3: control immunoprecipitation performed using isotype-matched control IgG2a (IP IgG2a).
Internalization of cell-surface CD63 accompaniesS cerevisiaeyeast phagocytosis in immature DCs: association of CD63 with dectin-1. (A) A yeast phagocytosis assay was performed as described in “Materials and methods” in the presence or absence of α-mannan, laminarin, or both polyosides. The percentage of phagocytic DCs was calculated in triplicate (at least 200 cells were counted per point). The top panel shows an example of DCs bearing phagocytic vacuoles containing yeasts (left) and of DCs incubated in the presence of laminarin (right); objective lens: Plan-Neofluar 100 ×/1.3 NA, oil. The results shown are representative of at least 5 experiments. (B) CD63 surface expression was evaluated by FACS analysis after yeast phagocytosis in the presence or absence of α-mannan, laminarin, or both polyosides and in control DCs (not incubated with yeasts). Ordinate indicates number of positive cells and abscissa indicates MFI. The histograms shown are representative of at least 5 experiments. (C) Example of FC-5.01/CD63 complexes surrounding internalized yeasts as described in “Results.” Objective lens: Plan-Neofluar 100 ×/1.3 NA, oil. (D) Immunoprecipitation of CD63 was performed as described in “Materials and methods.” The immunoprecipitates were analyzed by 10% SDS-PAGE under nonreducing conditions and revealed with anti-dectin-1 polyclonal antibody followed by HRP-GAR and chemiluminescence. The antibody detected a double band of approximately 27 kDa and 23 kDa, which corresponds to the estimated molecular weight for the dectin-1 full-length and 1b isoforms.25 Lane 1: DC extract. Lane 2: CD63 immunoprecipitate (IP 5.01). Lane 3: control immunoprecipitation performed using isotype-matched control IgG2a (IP IgG2a).
In order to examine if surface CD63 accompanied phagocytosed yeasts, immature DCs were preincubated with Mab FC-5.01 at 4°C. After removing the supernatant, yeasts were added and incubated for 24 hours at 37°C. After washing and membrane permeabilization, DCs were analyzed by confocal microscopy as described in “Materials and methods.” A typical result is shown in Figure 4C, where the complex FC-5.01/CD63 was found surrounding phagocytosed yeasts. These results suggest that CD63 is translocated from the cell surface to the intracellular organelles containing phagocytosed yeasts.
In order to investigate if CD63 is associated with the human β-1,3 glycan receptor dectin-1, immunoprecipitation experiments were performed. As can be observed in Figure 4D, we have found that dectin-1 coimmunoprecipitated with CD63.
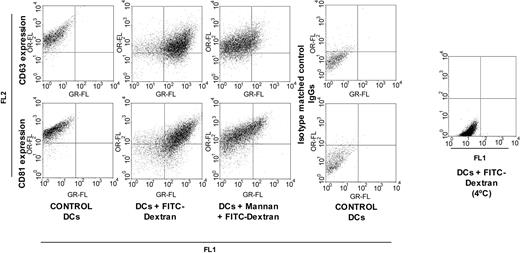
In another set of experiments, the effect of FITC-Dx endocytosis by immature DCs on CD63 surface expression was analyzed. As shown in Figure 5, 77% FITC-Dx uptake was obtained after a 30-minute incubation at 37°C. As expected, the presence of α-mannan significantly inhibited the uptake of FITC-Dx (60% inhibition), since the MMR has been identified as the major receptor for endocytosis of FITC-Dx.33 However, in contrast to what was observed after yeast phagocytosis, no significant decrease of CD63 surface expression was detected. The same results were observed after latex bead endocytosis (data not shown). In all these experiments, the surface expression of CD81 (Figure 4B and Figure 5), CD9, CD82, MHC class I and MHC class II (data not shown), was not modified by antigen uptake as well.
Internalization of cell surface CD63 does not accompany FITC-Dx endocytosis. Endocytosis of FITC-Dx (one mg/mL) and CD63 cell-surface expression were assessed as described in “Materials and methods.” Anti-CD63 FC-5.01 and anti-CD81 Mabs binding to control DCs (FL2: phycoerythrin fluorescence) is shown in the left panels. Binding of anti-CD63 Mab and of anti-CD81 Mab (FL2) to DCs incubated with FITC-Dx (FL1: FITC fluorescence) in the absence or presence of α-mannan is shown on center right and center left panels, respectively. Binding of isotype-matched control immunoglobulins are indicated in the upper and lower right panels. Control DCs were not incubated with FITC-Dx. Binding of FITC-Dx at 4°C is indicated in the right panel. In total, 10 000 events were analyzed. FITC-Dx endocytosis was confirmed by confocal microscopy (data not shown). The dot plots shown are representative of at least 5 experiments.
Internalization of cell surface CD63 does not accompany FITC-Dx endocytosis. Endocytosis of FITC-Dx (one mg/mL) and CD63 cell-surface expression were assessed as described in “Materials and methods.” Anti-CD63 FC-5.01 and anti-CD81 Mabs binding to control DCs (FL2: phycoerythrin fluorescence) is shown in the left panels. Binding of anti-CD63 Mab and of anti-CD81 Mab (FL2) to DCs incubated with FITC-Dx (FL1: FITC fluorescence) in the absence or presence of α-mannan is shown on center right and center left panels, respectively. Binding of isotype-matched control immunoglobulins are indicated in the upper and lower right panels. Control DCs were not incubated with FITC-Dx. Binding of FITC-Dx at 4°C is indicated in the right panel. In total, 10 000 events were analyzed. FITC-Dx endocytosis was confirmed by confocal microscopy (data not shown). The dot plots shown are representative of at least 5 experiments.
Discussion
CD63 is a member of the tetraspanin family to which no function has been yet unambiguously assigned. The first conclusive evidence for a pivotal role played by a tetraspanin was the finding that female knockout mice lacking CD9 displayed a severe reduction in fertility due to a failure in the sperm-egg fusion.34 Recently, the ligand for CD9 in mice has been shown to be the pregnancy-specific glycoprotein 17.35 With respect to the role of tetraspanins in tumor growth regulation, there is growing evidence that CD9, CD63, and CD82 could act as tumor-suppressor proteins. CD63 has been found to be expressed in all breast and lung tumors examined, whereas CD9 and CD82 were expressed only in some tumors, confering better prognosis.10,36,37 Interestingly, the role that tetraspanins might play in the immune system is still elusive. In this paper, we have analyzed in detail the role of CD63 and other tetraspanins in DCs. Using subcellular fractionation and immunoprecipitation techniques, other authors18 reported that in immature DCs CD63 is found in the lysosomal-endosomal compartment but not on the plasma membrane; besides, they could not detect CD82. Using FACS and confocal microscopy, we have shown that (1) CD9, CD63, CD81, and CD82 are expressed on the cell surface of immature DCs; (2) CD9 and CD81, which are poorly glycosylated tetraspanins, are predominantly expressed at the DC cell surface; and (3) CD63 and CD82 are also expressed in lysosomes and/or MIICs. Our results are supported by other reports stating that in lymphoblastoid B-cell lines CD82 is found in MIICs.13 It is also noteworthy that it has been recently found that CD63 subcellular localization remains unaltered after maturation.12 Coincidentally, we have not found significant differences on CD63 (as well as the other tetraspanins studied) expression on immature and mature DCs.
First, we have analyzed the effect of antitetraspanin Mabs on the migration of immature DCs. Experiments were performed using MIP-5 and MIP-1α, chemoattractants that act preferentially on immature DCs.38,39 Mabs directed to CD9, CD63, CD81, and CD82 increased migration between 50% and 70%. Mab against CD151 did not significantly affect migration. When Mabs against all the tetraspanins were added, migration increased almost 100%. The mechanisms of this increase may be different for CD9, CD63, CD81, and CD82, since Mab binding induced internalization of CD63 and CD82, but not of CD9 and CD81. Anyhow, a common explanation can be offered, since the association between tetraspanins and integrins has been well demonstrated.28 It has been shown in mutant Chinese hamster ovary (CHO) cells (D14) that the effect of CD82 on laminin- or fibronectin-induced motility is based on its ability to associate with α3 or α5 integrin.40 The expression of a conformational epitope on the CD9 tetraspanin depends on CD9 association with α6β1 integrin and it is functionally relevant in β1 integrin-mediated cellular processes such as cell migration.41 Regarding our migration experiments, when we analyzed the surface expression of integrins CD29, CD11b, CD18, and α5, we observed that the expression of these integrins diminished after Mab-induced internalization of CD63 and CD82. This could be due either to a concomitant internalization of the associated integrins or to a conformational change which caused diminished affinity of the anti-integrin Mabs. Furthermore, we have confirmed the association of CD63 with CD11b and CD18 by immunoprecipitation.
The relationship between tetraspanins and integrins could also explain why CD63, CD9, or CD82 would act as a tumor suppressor, as their diminished expression would increase the metastatic capacity of tumor cells and therefore worsen the prognosis.36,37 In fact, the stable transfection of CD9 into a small cell lung cancer (SCLC) line reduced cell motility on fibronectin, suggesting that CD9 modifies beta1 integrin function to reduce cell motility.36
We have demonstrated that after binding a specific Mab, CD63 also internalizes in DCs, similarly to what takes place in tumor cells.19 In addition, our results also indicate that CD63 follows the early endosomes-lysosomes-MIICs route, and that this internalization does not need previous crosslinking of CD63, since a Fab fragment induced exactly the same effect. The translocation of CD63 toward the endosome-lysosome route may be linked to antigen uptake by DCs. The description of CD63 ligand(s) should allow a better understanding of the possible functional effect of translocation of CD63 into early endosomes, lysosomes, and MIICs. In the present work, we have demonstrated that CD63 is also internalized after yeast phagocytosis, colocalizing intracellularly with phagocytosed yeasts. This internalization appeared to be dependent on the β-glycan receptor but not on the MMR, since it was inhibited by laminarin but not by α-mannan. These results confirm previous data where the β-glycan receptor was also suggested to mediate phagocytosis of cell wall derivatives of S cerevisiae by human cells lacking MMR.42 Furthermore, we have demonstrated by immunoprecipitation the association of CD63 with the β-glycan receptor dectin-1. Dectin-1 is a novel C-type lectinlike receptor preferentially expressed in DCs. It has been identified as a β-glycan receptor and recognizes a variety of β-1,3- and/or β-1,6-linked glycans as well as intact yeast. The receptor mRNA is alternatively spliced, resulting in 2 major (A and B) and 6 minor isoforms. The 2 major isoforms are the only isoforms that are functional for β-glycan binding.43 By contrast, CD63 internalization is not associated with the MMR, since it was not internalized after FITC-Dx uptake. CD63 did not internalize after latex bead phagocytosis either.
To summarize our findings, we have demonstrated that tetraspanins CD9, CD63, CD81, and CD82 are expressed at the immature DC cell surface. This location could have an important role in down-modulating DC migratory capacity, since addition of Mab antitetraspanins CD63, CD9, CD81, and CD82 increased DC migration by 50%. Whereas CD9 and CD81 appear to be exclusively located at the cell surface, the other tetraspanins are also distributed intracellularly. CD63 has been shown to be located in the storage granules of hematopoietic cells, such as neutrophils,30,44 basophils,21 eosinophils,22 cytotoxic T lymphocytes,45 and platelets,46 and to accompany these granules to the plasma membrane upon activation. We have observed by Western blot that FC-5.01 detected a broad band, ranging from 40 kDa to 70 kDa, which is characteristic of the CD63 antigen.47 On previous work, we and others have also detected this smear on melanoma cell lines and several melanoma tumors.12,19,47 This is due to CD63 variable N-glycosylation, which depends on the cell type and its state of maturation. Moreover, it has been suggested that CD63 posttranslational modification during maturation of DCs could be involved in the modulation of class II peptide loading events.12 Due to its strategic location in the plasma membrane and in intracellular compartments, to its ability to form complexes with phagocytic receptors, and to its predisposition to internalize and follow the endosomal-lysosomal-MIICs route, not only by Mab FC-5.01, but also by pathogens, as is the case for yeast phagocytosis, it would be tempting to propose that, in human DCs, CD63 is involved in antigen capture and its subsequent processing and/or presentation in the context of MHC class II molecules.
Prepublished online as Blood First Edition Paper, May 6, 2004; DOI 10.1182/blood-2004-01-0104.
Supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), the Fundación Sales (grant R1883 Sales-CONICET), the Fundación para la Investigación y Prevención del Cáncer (FUCA), the Fundación Pedro F. Mosoteguy and the Fundación María Calderón de la Barca of Argentina and the Institut Curie and the INSERM of France, and by an ECOS-Sud/SEPCyT Contract (A98S02) from the Argentine SEPCyT and the French Ministère des Affaires Etrangères.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Elvira Pastorino Aubry, Dr Miriam Bascoy, and all the members of the Hemotherapy Division, Department of Biochemistry, Hospital Naval Pedro Mallo, Argentina, for the provision of blood from healthy volunteers; Dr Tomás Santa Coloma, Licenciado Michele Bianchini and Bioquímica Beatriz Reyes for their expert technical assistance in confocal microscopy and FACScan; and Dr Jean Salamero and Dr Vaclav Horejsi for the generous gifts of the antibodies against EEA-1, CD11b, and CD18.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal