Abstract
Bruton tyrosine kinase (Btk), a non-receptor-associated tyrosine kinase of the Tec family, appears to participate in many myeloid cell functions. We show that macrophages from X-linked immunodeficient (XID) mice lacking functional Btk cannot generate efficient bursts of reactive oxygen intermediates (ROIs). The induction of apoptotic cell death by inflammatory stimuli is also enhanced in XID macrophages. Phagocytosis of bacterial particles is only marginally affected in them. In vivo, XID mice show reduced severity of inflammatory diseases in models of experimental autoimmune encephalomyelitis (EAE), dextran sulfate sodium (DSS)-induced colitis, and carrageenan-induced acute edema. Also, polymorphonuclear neutrophil granulocytes (PMNs) in XID mice show poor ROI and nitric oxide (NO) induction, along with a reduction in PMN recruitment to peritoneal inflammation. XID mice show reduction in PMN numbers in peripheral blood, and their bone marrow shows a reduction in the numbers of both monocytic and granulocytic lineages, extending to the earliest progenitor populations. Thus, Btk is likely to play a significant role at multiple points during the development and functioning of the myeloid lineages, affecting the outcome of many infectious as well as noninfectious inflammatory events in vivo. (Blood. 2004;104:1191-1197)
Introduction
Non-receptor-associated tyrosine kinases function as major intermediates in signal transduction from a variety of cell-surface receptors to modulate both developmental and functional properties, and a variety of such kinases are implicated in controlling the complex development and function of hematopoietic cellular lineages. Bruton tyrosine kinase (Btk), a member of the Tec family of non-receptor-associated tyrosine kinases,1 has long been known to be crucial in controlling signaling through both the pre-B-cell receptor (BCR)2 and the BCR,3 and defects in Btk lead to X-linked agammaglobulinemia in humans4,5 and the related but less severe X-linked immunodeficiency defect in mice.6,7 In addition to the B-cell lineage, Btk is also expressed in the myeloid lineage,8,9 raising the possibility that it may have analogous functions in myeloid cells.
In recent years, several lines of evidence have begun to indicate that Btk deficiency may have functional consequences for myeloid lineage cells. Mast cell activation through immunoglobulin E (IgE) Fc receptors appears to be dependent on Btk functionality.10-12 Some platelet responses also appear to involve Btk.13,14 Previous work from us15-17 and other groups18,19 indicates that at least some macrophage functions are compromised in the absence of functional Btk, and there are recent data suggesting a functional role for Btk in some responses of polymophonuclear neutrophil granulocytes (PMNs).20,21 Also, there have been several instances where X-linked immunodeficient (XID) mice have been observed to be quantitatively less susceptible to the induction of many autoimmune inflammatory diseases.22-25 While these findings have mostly been ascribed to the B-cell deficits, particularly in the B-1 B-cell compartment, in these mice,22 it is possible to reinterpret them as indicative of a defect in myeloid cell function.
On this background, we have further characterized the functional deficits in XID macrophages and PMNs, examined the inflammatory response in XID mice in vivo, and identified differences in myeloid development in XID bone marrow. Together, our data indicate that Btk has a significant role at multiple points in the development and function of both macrophages and PMNs, and that its absence results in compromised inflammatory responses in vivo.
Materials and methods
Mice
XID CBA/N mice were used along with the wild-type (WT) strains CBA/J and CBA/CaJ at 6 to 12 weeks of age. Strains were obtained from the Jackson Laboratories (Bar Harbor, ME) and bred in the small-animal facility of the National Institute of Immunology (New Delhi, India). All experiments were done with the approval of the Institutional Animal Ethics Committee.
Cell isolation
Peritoneal exudate cells (PECs) were induced by intraperitoneal injection of thioglycollate broth (Himedia, Mumbai, India). At 72 hours after injection, cells were harvested from peritoneum and macrophages were isolated by plastic adherence. Purified populations were more than 90% CD11b+. Peritoneal granulocytes were induced by intraperitoneal injection of either thioglycollate broth (Himedia) or oyster glycogen (Sigma Chemical, St Louis, MO). At 12 to 18 hours after injection, cells harvested from the peritoneum were more than 90% Gr-1hi. These cells were more than 95% PMNs upon morphologic examination (data not shown). Bone marrow granulocytes were obtained from bone marrow cells by positive selection for high-level expression of the granulocytic cell-surface marker Gr-1 by magnetic cell sorting (Miltenyi Biotec, Bergisch Gladbach, Germany). Purified cell preparations were routinely more than 90% Gr-1hi.
Induction of reactive oxygen intermediates (ROIs) and NO
Purified macrophages or granulocytes were loaded with the indicator dye 2′7′-dichlorofluorescein diacetate (DCFH-DA; Sigma Chemical) at 5 μM for one hour at 37°C. Cells were washed and stimulated in triplicate cultures, and ROI induction detected at serial time points by fluorimetric measurements (Fluostar; BMG Labtechnologies, Mount Eliza, Australia).26 Data are expressed as arbitrary fluorescent units. For induction of nitric oxide (NO), cell preparations were stimulated in triplicate cultures for 36 to 48 hours, and NO production was detected by estimation of nitrite levels in the culture medium using the Griess reaction.15
Flow cytometry
For estimating macrophage apoptosis, peritoneal macrophages were stimulated with a combination of lipopolysaccharide (LPS) and recombinant interferon-gamma (IFNγ).27 After various times in culture, cells were stained with anti-CD11b-fluorescein (BD Pharmingen, San Diego, CA) followed by propidium iodide (PI), and analyzed flow cytometrically.
For measuring phagocytosis, macrophages or granulocytes were incubated with fluorescein-labeled heat-killed Escherichia coli for various times at either 4°C or 37°C. Bacteria were heat-killed at 60°C for 30 minutes and labeled with fluorescein isothiocyanate. The cells were then stained with anti-CD11b-phycoerythrin (PE) or with anti-Gr-1-PE (BD Pharmingen) and samples were analyzed flow cytometrically after addition of 0.1% crystal violet to quench extracellular fluorescence from cell-surface adherent bacterial particles.28
For analysis of bone marrow myeloid lineages, femoral bone marrow cells were harvested from individual WT or XID mice and stained with anti-CD11b-fluorescein, anti-CD54-PE, and anti-Gr-1-biotin followed by streptavidin-cychrome C (SA-CyC; BD Pharmingen).
Stained cells were analyzed flow cytometrically (BD-LSR; Becton Dickinson, San Jose, CA) and data were analyzed using FlowJo software (Treestar, San Carlos, CA).
Blood counts
For total leukocyte counts (TLCs), blood was diluted 1:20 in a leukocyte dilution fluid, mixed thoroughly, and counted in a hemocytometer. For differential leukocyte counts, blood smear slides were made, stained with Leishman stain, and a total of 400 leukocytes were classified morphologically.
Myeloid colony-forming assays
Nonadherent bone marrow cells were seeded in a semisolid medium (methylcellulose) containing recombinant growth factors, stem cell factor (SCF), interleukin 3 (IL-3), IL-6, and erythropoietin (StemCell Technologies, Vancouver, BC, Canada) as described.29 Colonies were scored by microscopy after 10 to 12 days of culture.
Induction and assessment of experimental autoimmune encephalitis (EAE)
For induction of EAE, mice were immunized subcutaneously with lyophilized mouse spinal cord homogenate (MSCH) in complete Freund adjuvant (CFA; 400 μg/mouse), and were given 1 × 1010 heat-killed Bordetella pertussis bacilli intramuscularly as described.30 Mice were examined daily for disease, with clinical severity being assessed on a semiquantitative scale (0: no disease; 1: tail paresis or hind-limb paresis; 2: tail paresis or paralysis and hind-limb paresis; 3: tail paralysis and hind-limb paresis or paralysis; 4: tail and hind-limb paralysis; 5: moribund state) as described.30
Induction and assessement of DSS-induced colitis
Mice were administered 3% dextran sulfate sodium (DSS; MW 36 000-50 000; ICN, Costa Mesa, CA) in drinking water ad libitum, and weight loss was scored as a measure for the induction of colitis as described.31
Induction of inflammatory response to carrageenan
Carrageenan-induced inflammation was induced by a subcutaneous injection of 0.5% iota-carrageenan (Sigma Chemical) in normal saline into one hind footpad and normal saline alone into the contralateral footpad (50 μL/footpad) as described.32 Footpad thickness was measured prior to injections (T0) and later at intervals of 3 hours using spring-loaded dial callipers (Mitutoyo, Kawasaki, Japan), and expressed as the increase in footpad thickness over T0 at various time points after injection.
Cell motility assays
Cell motility was assessed using peritoneal elicited cells (macrophages and granulocytes) as described.33 Briefly, a gap was created with a pipette tip in confluent cell monolayers in 1% bovine serum albumin (BSA)-coated wells, and the number of cells migrating into the open space was assessed microscopically serially over time in 3 designated microscopic fields.
Results
XID macrophages show alterations in induction of ROIs and apoptosis but not phagocytosis
Adherent PECs (> 90% CD11b+) were used as macrophages and were loaded with the ROI indicator dye DCFH-DA, stimulated with varying concentrations of bacterial LPS, and ROI generation was measured. Upon LPS activation, WT cells showed a dose-dependent induction of ROIs over time, whereas the responses of XID macrophages were substantially poorer at all LPS doses (Figure 1A). On the other hand, bypassing membrane-proximal signaling steps by the addition of phorbol myristate-acetate (PMA) led to equivalent induction of ROIs in WT and XID macrophages (Figure 1B).
Partial dysfunction in XID macrophages. (A) ROI responses of PEC macrophages from WT (white symbols) or XID (black symbols) mice to varying concentrations (μg/mL) of LPS over time as indicated (mean ± standard error [SE] of triplicate cultures). (B) ROI responses of PEC macrophages from WT or XID mice to PMA (30 ng/mL) over time (mean ± SE of triplicate cultures). (C) Phagocytosis of fluorescein-labeled, heat-killed bacteria by PEC macrophages from WT (thin lines) or XID (thick lines) mice, shown as histograms of fluorescein intensity in gated CD11b+ cells either not exposed to bacteria (-), or exposed to them at 4°C (4 C) or at 37°C (37 C) for 1 hour. Percentages of CD11b+ cells with associated bacterial fluorescence are indicated. (D) Time course of phagocytosis of fluorescein-labeled, heat-killed bacteria by PEC macrophages from WT or XID mice in gated CD11b+ cells exposed to labeled bacteria at 37°C for varying times as indicated. (E) Time course of motility of PEC macrophages from WT or XID mice in vitro. (F) Death of PEC macrophages from WT or XID mice upon exposure to medium alone (-), or the IFNγ (50 IU/mL) along with either low-dose LPS (3 μg/mL) or high-dose LPS (30 μg/mL). Death was scored over time flow cytometrically by PI staining of CD11b+ cells.
Partial dysfunction in XID macrophages. (A) ROI responses of PEC macrophages from WT (white symbols) or XID (black symbols) mice to varying concentrations (μg/mL) of LPS over time as indicated (mean ± standard error [SE] of triplicate cultures). (B) ROI responses of PEC macrophages from WT or XID mice to PMA (30 ng/mL) over time (mean ± SE of triplicate cultures). (C) Phagocytosis of fluorescein-labeled, heat-killed bacteria by PEC macrophages from WT (thin lines) or XID (thick lines) mice, shown as histograms of fluorescein intensity in gated CD11b+ cells either not exposed to bacteria (-), or exposed to them at 4°C (4 C) or at 37°C (37 C) for 1 hour. Percentages of CD11b+ cells with associated bacterial fluorescence are indicated. (D) Time course of phagocytosis of fluorescein-labeled, heat-killed bacteria by PEC macrophages from WT or XID mice in gated CD11b+ cells exposed to labeled bacteria at 37°C for varying times as indicated. (E) Time course of motility of PEC macrophages from WT or XID mice in vitro. (F) Death of PEC macrophages from WT or XID mice upon exposure to medium alone (-), or the IFNγ (50 IU/mL) along with either low-dose LPS (3 μg/mL) or high-dose LPS (30 μg/mL). Death was scored over time flow cytometrically by PI staining of CD11b+ cells.
Heat-killed E coli labeled with fluorescein were used as phagocytosis targets. PECs were coincubated with labeled bacteria for 1 hour either at 4°C or 37°C, and bacterial internalization into macrophages was assayed by 2-color flow cytometry using CD11b as a macrophage marker. Bacterial fluorescence associated with CD11b+ cells was confirmed to be intracellular by incubation of the samples in crystal violet (0.1%), which quenches the fluorescence of extracellular but not intracellular fluorescein.28 WT macrophages showed significant active internalization of labeled bacteria, and there was no substantial inhibition of phagocytosis in XID macrophages (Figure 1C). PECs were also coincubated with labeled bacteria for varying periods of time, and the resultant increase in phagocytosis over time did not show any substantial differences between WT and XID macrophages (Figure 1D). Similarly, when the undirected motility of macrophages was assessed in cultures in vitro over a period of time, XID macrophages showed only minor reduction in their motility compared with that of WT macrophages (Figure 1E).
Adherent PECs from WT or XID mice were subjected in vitro to treatment with LPS and IFNγ, which has been reported to induce macrophage apoptosis.34 After 24 or 48 hours in culture, death in CD11b+ cells was assayed by PI staining and flow cytometry. While WT macrophages showed significant apoptosis only at a high concentration of LPS and at 48 hours of culture, XID macrophages showed significant death earlier even at the lower concentration of LPS (Figure 1F).
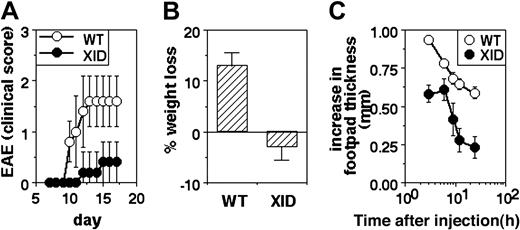
XID mice show poor induction of inflammatory responses in vivo
EAE was induced in WT and XID mice by immunization with MSCH in CFA along with killed B pertussis bacilli, and morbidity was quantified by clinical scoring. XID mice showed slower induction of EAE and milder clinical disease than did WT mice (Figure 2A).
Poor inflammatory responses in vivo in XID mice. (A) Induction of EAE in WT or XID mice by immunization with spinal cord homogenate. Clinical scores are shown (mean ± SE) at the indicated time points. (B) Induction of colitis in WT or XID mice by administration of DSS in drinking water. The extent of weight change with reference to starting weight is shown at day 9 of treatment in WT and XID mice (mean ± SE). (C) Induction of footpad edema in WT or XID mice by administration of carrageenan. The increase in footpad thickness is shown (mean ± SE) over time.
Poor inflammatory responses in vivo in XID mice. (A) Induction of EAE in WT or XID mice by immunization with spinal cord homogenate. Clinical scores are shown (mean ± SE) at the indicated time points. (B) Induction of colitis in WT or XID mice by administration of DSS in drinking water. The extent of weight change with reference to starting weight is shown at day 9 of treatment in WT and XID mice (mean ± SE). (C) Induction of footpad edema in WT or XID mice by administration of carrageenan. The increase in footpad thickness is shown (mean ± SE) over time.
DSS-induced colitis was triggered in groups of WT and XID mice by administration of 3% DSS in drinking water, and weight loss was monitored as a measure of colitis. While WT mice began showing significant weight loss by the tenth day of DSS treatment, XID mice showed no weight loss by this time (Figure 2B).
The third model tested was of acute inflammation in vivo, triggered by the injection of carrageenan in the footpad. WT or XID mice were given carrageenan subcutaneous, and the resultant increase in footpad thickness was monitored over time. The peak swelling was reached within hours, and was lower in XID mice in comparison to WT mice (Figure 2C).
NO and ROI induction is poor in XID PMNs but not phagocytosis or CD80/CD86 induction
Since poor induction of carrageenan edema usually indicates poor recruitment of PMNs, PMN function was next examined in XID mice. The source of PMNs was either bone marrow or peritoneum lavage fluid of mice treated with thiogycollate broth 18 hours earlier. PMNs were purified and populations more than 90% Gr-1hi were used for functional assessment.
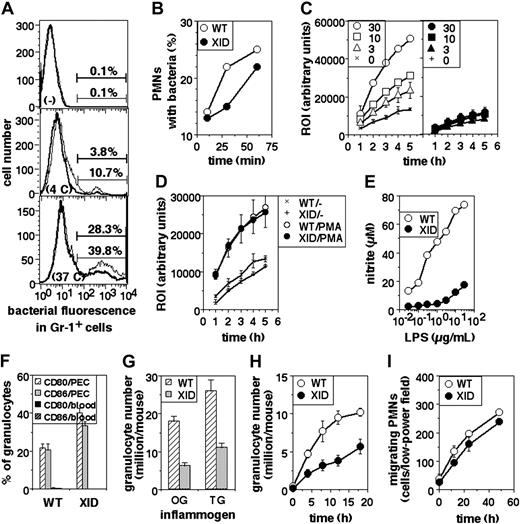
The phagocytic ability of XID PMNs was assayed. The frequency of phagocytosis seen in XID PMNs was somewhat lower than that seen in WT PMNs (Figure 3A). This difference was also seen in assays in which PMNs were coincubated with labeled bacteria for varying periods of time (Figure 3B).
Functional defects in XID PMNs. (A) Phagocytosis of fluorescein-labeled, heat-killed bacteria by PMNs from WT (thin lines) or XID (thick lines) mice, shown as histograms of fluorescein intensity in gated Gr-1+ cells either not exposed to bacteria (-), or exposed to them at 4°C (4C) or at 37°C (37C) for one hour. (B) Time course of phagocytosis of fluorescein-labeled, heat-killed bacteria by peritoneal PMNs from WT or XID mice in gated Gr-1+ cells exposed to labeled bacteria at 37°C for varying times as indicated. (C) ROI responses of PMNs from WT (hollow symbols) or XID (filled symbols) mice to varying concentrations (μg/mL) of LPS over time as indicated (mean ± SE of triplicate cultures). (D) ROI responses of PMNs from WT or XID mice to PMA (30 ng/mL) over time (mean ± SE of triplicate cultures). (E) Induction of NO in PMNs from WT or XID mice to varying concentrations of LPS as indicated (mean ± SE of triplicate cultures). (F) Frequency of PMNs, either from blood or from peritoneal exudates induced by thioglycollate broth, expressing high levels of cell-surface CD80 or CD86 as indicated (mean ± SE). (G) Induction of PMN recruitment in the peritoneum of WT or XID mice in response to injection of oyster glycogen (OG) or thioglycollate broth (TG) 6 hours (OG) or 18 hours (TG) earlier, shown as PMNs rcovered per peritoneum (mean ± SE). (H) Time course of induction of PMN recruitment in the peritoneum of WT or XID mice in response to injection of thioglycollate broth at varying time points after injection, shown as PMNs recovered per peritoneum (mean ± SE). (I) Time course of motility of PMNs from WT or XID mice in vitro.
Functional defects in XID PMNs. (A) Phagocytosis of fluorescein-labeled, heat-killed bacteria by PMNs from WT (thin lines) or XID (thick lines) mice, shown as histograms of fluorescein intensity in gated Gr-1+ cells either not exposed to bacteria (-), or exposed to them at 4°C (4C) or at 37°C (37C) for one hour. (B) Time course of phagocytosis of fluorescein-labeled, heat-killed bacteria by peritoneal PMNs from WT or XID mice in gated Gr-1+ cells exposed to labeled bacteria at 37°C for varying times as indicated. (C) ROI responses of PMNs from WT (hollow symbols) or XID (filled symbols) mice to varying concentrations (μg/mL) of LPS over time as indicated (mean ± SE of triplicate cultures). (D) ROI responses of PMNs from WT or XID mice to PMA (30 ng/mL) over time (mean ± SE of triplicate cultures). (E) Induction of NO in PMNs from WT or XID mice to varying concentrations of LPS as indicated (mean ± SE of triplicate cultures). (F) Frequency of PMNs, either from blood or from peritoneal exudates induced by thioglycollate broth, expressing high levels of cell-surface CD80 or CD86 as indicated (mean ± SE). (G) Induction of PMN recruitment in the peritoneum of WT or XID mice in response to injection of oyster glycogen (OG) or thioglycollate broth (TG) 6 hours (OG) or 18 hours (TG) earlier, shown as PMNs rcovered per peritoneum (mean ± SE). (H) Time course of induction of PMN recruitment in the peritoneum of WT or XID mice in response to injection of thioglycollate broth at varying time points after injection, shown as PMNs recovered per peritoneum (mean ± SE). (I) Time course of motility of PMNs from WT or XID mice in vitro.
Next, PMNs were stimulated with titrating concentrations of bacterial LPS and the induction of ROIs assayed. WT PMNs showed excellent induction of ROIs, whereas XID PMNs did not (Figure 3C), although PMA induced similar levels of ROIs from both cell populations (Figure 3D). When NO generation was assayed in cultures of PMNs upon stimulation with LPS, it was evident that XID PMNs produced far less NO than did WT PMNs (Figure 3E).
We next measured the induction of surface expression of CD80 and CD86 on PMNs by activation in vivo as another outcome of activation. WT or XID mice were given thioglycollate broth intraperitoneally; peritoneal cells were collected 18 hours later and stained for Gr-1 and surface CD80 or CD86 expression for 2-color flow cytometry. Splenic and peripheral blood cells were similarly stained as nonactivated controls. While splenic or blood granulocytes showed very low frequencies of surface CD80hi or CD86hi cells, granulocytes recruited to the peritoneum by inflammatory stimuli showed induced expression of these molecules, and the induction of these molecules was comparable between WT and XID cells (Figure 3F).
The efficiency of recruitment of PMNs to sites of inflammation in vivo was examined by determining the numbers of PMNs in the peritoneum upon intraperitoneal administration of inflammatory agents. The numbers of PMNs recruited to the peritoneum were estimated after administration of oyster glycogen (6 hours) or thioglycollate broth (18 hours), and were found to be substantially lower in XID than in WT mice (Figure 3G). The time course of PMN recruitment to the peritoneum was also examined using thioglycollate broth as the inducing agent, and showed consistent differences between WT and XID mice at all time points (Figure 3H). When the undirected motility of granulocytes was assessed in cultures in vitro over a period of time, XID cells showed only minor reduction in their motility compared with that of WT cells (Figure 3I).
XID mice show developmental abnormalities in the myeloid lineages
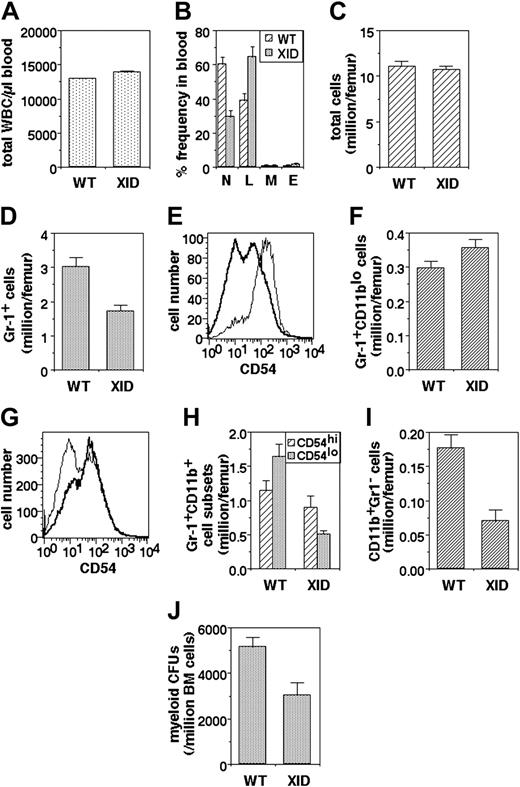
Since recruitment of PMNs to inflammatory sites would depend to some extent on their availability, we next examined the frequency of PMNs in peripheral blood. While total leukocyte numbers are not drastically altered in XID mice (Figure 4A), they show a significant reduction in peripheral PMN frequency (Figure 4B).
Myeloid developmental abnormalities in XID mice. (A) Total white blood cell (WBC) numbers in the blood of WT and XID mice (mean ± SE). (B) Differential WBC frequencies (N: PMNs; L: lymphocytes; M: monocytes; E: eosinophils) in the blood of WT and XID mice (mean ± SE). (C) Total bone marrow cell yields per femur from WT and XID mice (mean ± SE). (D) Granulocytic lineage (Gr-1+) cell numbers in bone marrow from WT and XID mice (mean ± SE). (E) CD54 intensity in Gr-1+CD11blo (thin line) and Gr-1+CD11bhi (thick line) cells from WT bone marrow. (F) Early granulocytic lineage (Gr-1+CD11blo) cell numbers in bone marrow from WT and XID mice (mean ± SE). (G) CD54 intensity in Gr-1+CD11bhi cells from WT (thin line) and XID (thick line) WT bone marrow. (H) Late granulocytic lineage (Gr-1+CD11bhi) cell numbers in the CD54hi and CD54lo subpopulations from bone marrow of WT and XID mice (mean ± SE). (I) Monocytic lineage (CD11b+Gr-1-) cell numbers in bone marrow from WT and XID mice (mean ± SE). (J) Myeloid colony-forming cell numbers in bone marrow from WT and XID mice (mean ± SE).
Myeloid developmental abnormalities in XID mice. (A) Total white blood cell (WBC) numbers in the blood of WT and XID mice (mean ± SE). (B) Differential WBC frequencies (N: PMNs; L: lymphocytes; M: monocytes; E: eosinophils) in the blood of WT and XID mice (mean ± SE). (C) Total bone marrow cell yields per femur from WT and XID mice (mean ± SE). (D) Granulocytic lineage (Gr-1+) cell numbers in bone marrow from WT and XID mice (mean ± SE). (E) CD54 intensity in Gr-1+CD11blo (thin line) and Gr-1+CD11bhi (thick line) cells from WT bone marrow. (F) Early granulocytic lineage (Gr-1+CD11blo) cell numbers in bone marrow from WT and XID mice (mean ± SE). (G) CD54 intensity in Gr-1+CD11bhi cells from WT (thin line) and XID (thick line) WT bone marrow. (H) Late granulocytic lineage (Gr-1+CD11bhi) cell numbers in the CD54hi and CD54lo subpopulations from bone marrow of WT and XID mice (mean ± SE). (I) Monocytic lineage (CD11b+Gr-1-) cell numbers in bone marrow from WT and XID mice (mean ± SE). (J) Myeloid colony-forming cell numbers in bone marrow from WT and XID mice (mean ± SE).
In order to explore the cause of reduced PMN frequencies in peripheral blood, the bone marrow was next examined with reference to the granulocytic lineage. Total cell numbers per femur in the XID bone marrow were not very different from those in WT mice (Figure 4C). However, the frequency of Gr-1-expressing cells, as identified by flow cytometry, was significantly lower in the XID bone marrow (Figure 4D).
The Gr-1+ bone marrow cells were further analyzed for the expression of CD11b, which is known to be low on early granulocytic stages and increases with maturation,35 and CD54, since its surface level is high in early immature granulocytes and decline during maturation.34 This was confirmed by a comparison of the levels of CD54 expression on the CD11blo and CD11bhi subsets of Gr-1+ cells from WT bone marrow, which showed that Gr-1+CD11blo cells uniformly expressed high levels of CD54, whereas the Gr-1+CD11bhi cells had a substantial population expressing relatively low CD54 levels (Figure 4E). The numbers of Gr-1+CD11blo cells were not significantly different between XID and WT bone marrow (Figure 4F). The expression pattern of CD54 on Gr-1+CD11bhi cells differs significantly on XID and WT mice (Figure 4G). While numbers of the less mature Gr-1+CD11bhiCD54hi cells show some reduction in XID marrow, the most marked reduction was seen in the more mature Gr-1+CD11bhiCD54lo cell population (Figure 4H). The Gr-1- cell population in the bone marrow was also examined with regard to the monocytic lineage. The frequency of monocytic lineage Gr-1-CD11b+ cells was also substantially decreased in the bone marrow of XID mice (Figure 4I).
We next examined the precursor frequency of granulocyticmonocytic myeloid progenitors in a functional assay. Nonadherent bone marrow cells from WT or XID mice were plated in limiting numbers on soft medium with appropriate growth factors, and the frequency and phenotypes of the resultant colonies was determined. The frequency of myeloid precursors was significantly reduced in XID bone marrow (Figure 4J).
Discussion
Our data indicate that Btk plays a significant role in myeloid cell development as well as in the transduction of some but not all functions of mature myeloid cells. We have previously reported that the induction of many crucial transcription factors by TLR ligation is poor in XID macrophages.16 Most of the proinflammatory functional responses of macrophages, which we have previously reported to be compromised in the absence of functional Btk, are transcriptionally mediated, such as the induction of tumor necrosis factor-alpha (TNFα) or inducible nitric oxide synthase (iNOS).15,16 We therefore examined if lack of Btk function also compromises inducible functions dependent on posttranslational mechanisms. One such major function crucial in conjunction with iNOS for bactericidal effector functions of macrophages is the induction of a respiratory burst leading to increased availability of ROI species.36 Our data show that ROI generation also requires Btk function.
Another crucial effector function of macrophages is phagocytosis, which is of particular interest in this context since Btk has been shown to associate with the Wiskott-Aldrich syndrome protein (WASP) and with cdc42, together necessary for the nucleation of actin polymerization.37-39 However, unlike many other functions of XID macrophages, phagocytosis is not substantially compromised in them, suggesting that the Btk-WASP-cdc42 connection may not be nonredundantly necessary for phagocytosis.
The potential role of Btk in macrophage apoptosis is interesting in a number of contexts. It has been previously shown that XID B cells show enhanced apoptosis through a variety of pathways,40-42 and an analogous role for Btk in macrophages was possible. Also, we have previously shown that a lack of functional Btk leads to poor induction of transcription factors of the nuclear factor κ B (NF-kB) family,15 which are known to regulate apoptosis. Finally, Btk appears to be involved in the induction of most antimicrobial and proinflammatory functions of macrophages,15-17,19 and it was possible that a resistance to apoptosis induced by TLR ligands and cytokines may also be coordinately regulated. Our data show that this is indeed the case, since XID macrophages die more rapidly in response to bacterial and cytokine ligands.
The major role of Btk in B cells is thought to be in inducing phosphorylation of phospholipase C-gamma, leading to the generation of diacylglycerol and inositol triphosphate, and subsequently to the induction of a sustained calcium flux, raising the question of whether Btk functions in an analogous manner in macrophage signaling. Preliminary experiments indicate that calcium flux induction in response to TLR ligation is compromised in XID macrophages (data not shown), supporting an analogous role for Btk in signaling downstream of TLRs in macrophages.
Since an absence of functional Btk thus leads to substantial reduction in practically all proinflammatory functions of macrophages coupled to an increased tendency to apoptosis, it appeared reasonable to examine if this had consequences on inflammation in vivo. Adaptive immune response-mediated autoimmune inflammation in vivo has been reported to be poorer in XID mice.30 However, XID macrophages show a complex functional phenotype with regard to their antigen-presenting cell (APC) functions. The relative lack of iNOS induction leads to increased induction of IL-12 from XID macrophages in response to TLR ligands, and this is correlated with a tendency in vivo of generating relatively IFNγ-dominated T-helper 1 (Th1) T-cell responses.15,16,43 In this light, we chose a model of autoimmune inflammation in vivo which depends on autoimmune Th1 responses, namely, EAE. Despite the Th1 bias of XID mice, induction of EAE was poor in XID mice.
We also examined models of inflammation in vivo that do not depend on adaptive immune responses. One such model is DSS-induced colitis, to which XID mice are less susceptible than are WT mice. While the manifestations of DSS-induced colitis are seen over a period of days, they are seen within hours in another more acute model, the induction of footpad edema by the injection of the polysaccharide carrageenan. Carrageenan-induced edema was also substantially lower in XID mice than in WT mice. Acute inflammatory models such as carrageean-induced edema depend significantly on PMN recruitment and function.44,45 We therefore tested the functional status of XID PMNs. Analogous to the data with macrophages, XID PMNs also showed deficient induction of both ROIs and NO. Activation of PMNs has been shown to lead to enhanced expression of the costimulatory molecules CD80 and CD86 on their surface.46,47 We have previously reported that the induction of these costimulatory molecules on macrophages by LPS is unaffected by the absence of functional Btk.15 Consistent with this, their induction on the PMN surface is also unaffected by the absence of Btk. However, the phagocytic ability of XID PMNs is somewhat lower than that of WT PMNs, possibly indicating differences between regulation of phagocytosis in the 2 phagocytic cell lineages. On the other hand, the undirected motility of cells appears to be substantially unaffected by the absence of functional Btk in both macrophages and PMNs.
Together, these data indicate that the presence of a Btk-dependent signaling pathway is required for some but not all functions of phagocytic cell lineages. Phagocytic cells do not show optimal induction of microbicidal products such as ROIs or NO and die more easily in the absence of Btk, and this is likely to lead to significant compromised innate immune responses responsible for clearing microbial infections.
The absence of Btk also has developmental consequences for the myeloid lineage. XID marrow shows a reduction in the frequency of granulocytic-myeloid progenitor cells capable of colony formation in vitro, indicating that Btk may be involved in the commitment to or expansion of the granulocyte-monocyte progenitor cell population. Monocytic lineage cells are fewer in the XID bone marrow than in WT marrow. The reduction in numbers of circulating peripheral PMNs is also accompanied by a reduction in the number of granulocytic lineage cells in the XID bone marrow. While there is little reduction at the earliest CD11blo stage of granulocytic differentiation, there is progressive reduction in each succeeding stage of granulocytic maturation as identified by progressive acquisition of CD11b and loss of CD54 expression, indicating that Btk is liable to play significant roles at multiple stages of granulocytic differentiation. While human patients suffering from X-linked agammaglobulinemia do not necessarily show an obvious neutropenia under normal conditions, infections frequently induce neutropenic states in them,48,49 and this may be a consequence of either enhanced apoptosis of myeloid cells, and/or reduced capability of the bone marrow in responding to the increased demand for PMNs.
Thus, Btk is likely to be playing a significant, although partially redundant role at multiple points during the development and functioning of the myeloid lineages, affecting the outcome of many infectious as well as noninfectious inflammatory events in vivo. This pleiotropy of functional roles raises the possibility that subtle mutations in Btk may contribute signifi-cantly to the susceptibility of carriers to both infectious and autoimmune diseases.
Supported in part by grants from the Departments of Science and Technology and Biotechnology, Government of India (A.G., S.R., and V.B.), the Indian Council of Medical Research (S.R., V.B., and B.R.), the Wellcome Trust (V.B.), and the Indo-US Vaccine Action Program (S.R.). The National Institute of Immunology is supported by the Department of Biotechnology, Government of India.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, April 29, 2004; DOI 10.1182/blood-2004-01-0207.

![Figure 1. Partial dysfunction in XID macrophages. (A) ROI responses of PEC macrophages from WT (white symbols) or XID (black symbols) mice to varying concentrations (μg/mL) of LPS over time as indicated (mean ± standard error [SE] of triplicate cultures). (B) ROI responses of PEC macrophages from WT or XID mice to PMA (30 ng/mL) over time (mean ± SE of triplicate cultures). (C) Phagocytosis of fluorescein-labeled, heat-killed bacteria by PEC macrophages from WT (thin lines) or XID (thick lines) mice, shown as histograms of fluorescein intensity in gated CD11b+ cells either not exposed to bacteria (-), or exposed to them at 4°C (4 C) or at 37°C (37 C) for 1 hour. Percentages of CD11b+ cells with associated bacterial fluorescence are indicated. (D) Time course of phagocytosis of fluorescein-labeled, heat-killed bacteria by PEC macrophages from WT or XID mice in gated CD11b+ cells exposed to labeled bacteria at 37°C for varying times as indicated. (E) Time course of motility of PEC macrophages from WT or XID mice in vitro. (F) Death of PEC macrophages from WT or XID mice upon exposure to medium alone (-), or the IFNγ (50 IU/mL) along with either low-dose LPS (3 μg/mL) or high-dose LPS (30 μg/mL). Death was scored over time flow cytometrically by PI staining of CD11b+ cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/4/10.1182_blood-2004-01-0207/6/m_zh80160465250001.jpeg?Expires=1769109414&Signature=MdlHaILNKIlHAW~Qf5rDit5kU5axJkCP-GEjLfGVTQbnfY5Zm-o5Gp0xOyRvI4Fc483y79BbozWXWC5aMN2gdnsVgJ6Otsj8mBsJkMln0eyafM4SIIM6EW4cPlBtlCrvdiRca6fQGc4GHcxY9B-8gLzxQilEsCJlrM8s~9mhPticYYx7uwOyIw2~BmI4gaqV6kxDOzjXakrgUpUyIDnx50ODjvPaHt-6ugbyWKd94A3IsdIyGw0bgU4JLhSc7L27G3cJOXKQq7dZEQaw-RPHfHFpuqnSvKDIpjOLR8YZrNpgftOvpMT9aow5S1f3KLCsn22QussmfgmkLYE9dETBHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal